Synergies of Human Umbilical Vein Endothelial Cell-Laden Calcium Silicate-Activated Gelatin Methacrylate for Accelerating 3D Human Dental Pulp Stem Cell Differentiation for Endodontic Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Extracts of CS Powders and Si-Contained FGelMa Hydrogels
2.2. Alg/FGel Hydrogel Preparation for 3D Cell Block
2.3. Hydrogel Characterization
2.4. Weight Loss/Degradation and Release Profile of the Hydrogels
2.5. In Vitro Biological Tests
2.6. Western Blot
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. hDPSCs and HUVEC Cell Co-Culture Analysis
2.9. Odontogenesis Differentiation Assay
2.10. Mineralization
2.11. Statistical Analysis
3. Results and Discussion
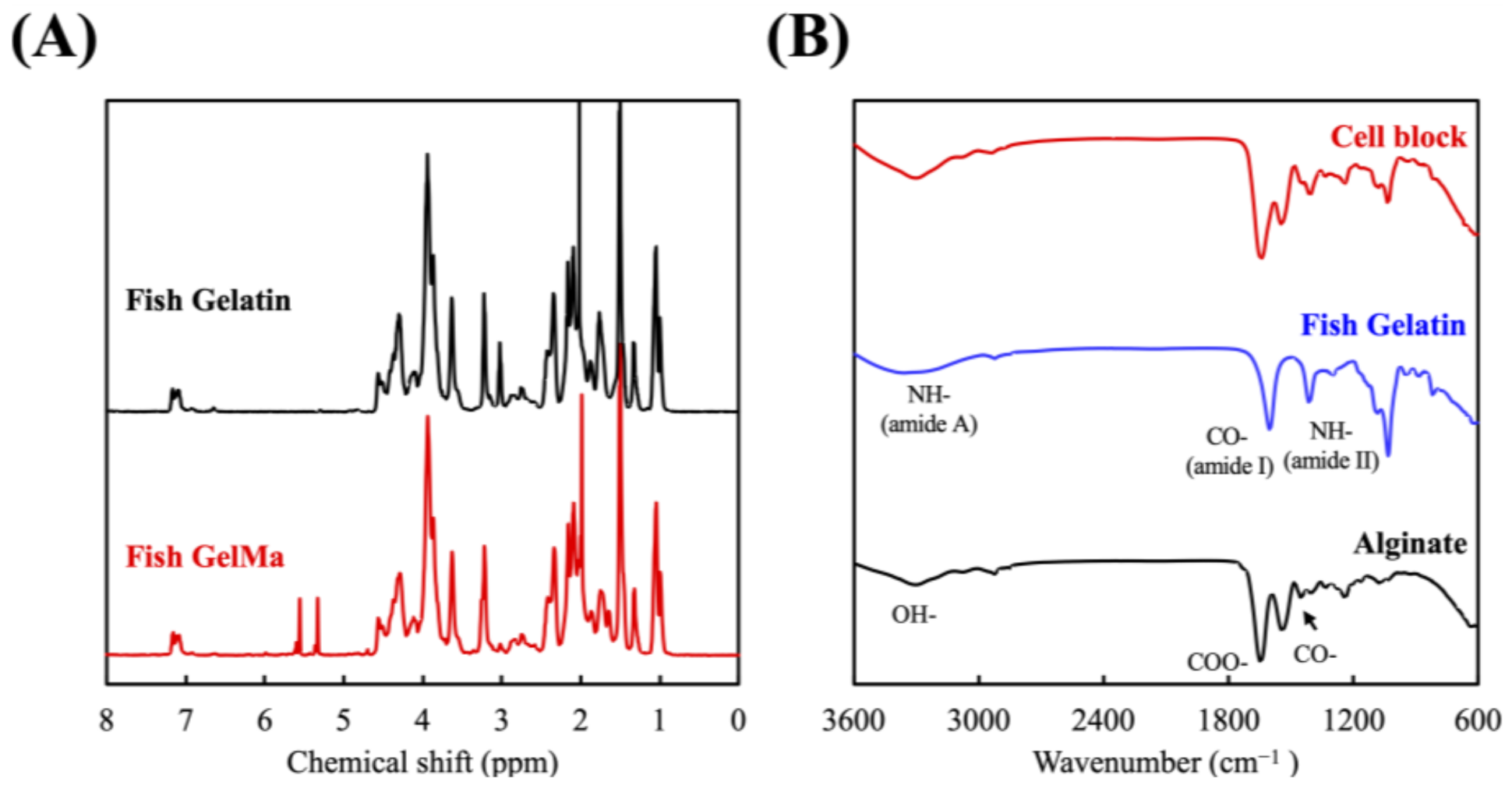
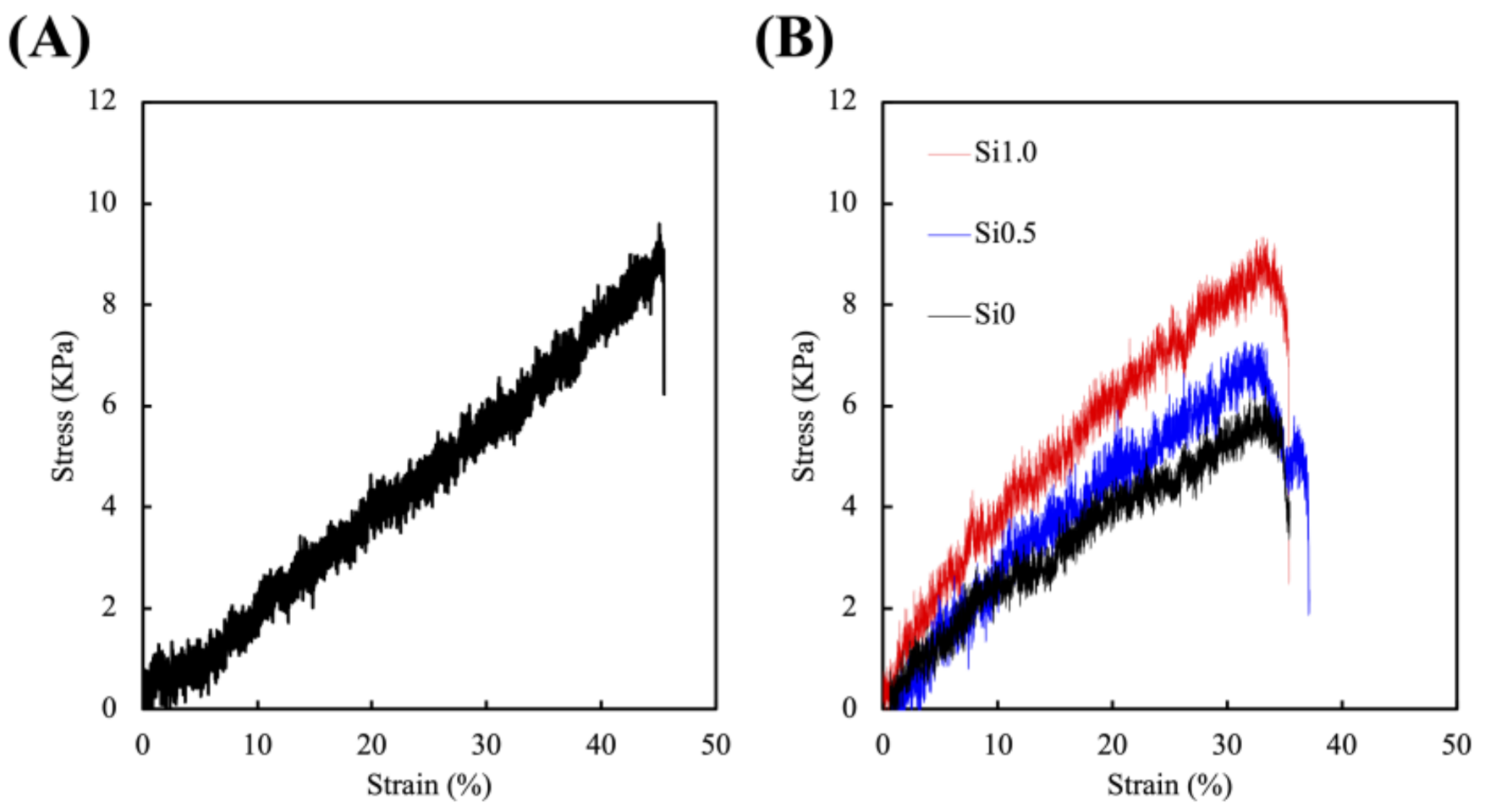
3.1. Synthesis and Characterization of the FGelMa and Alg/Gel Hydrogels
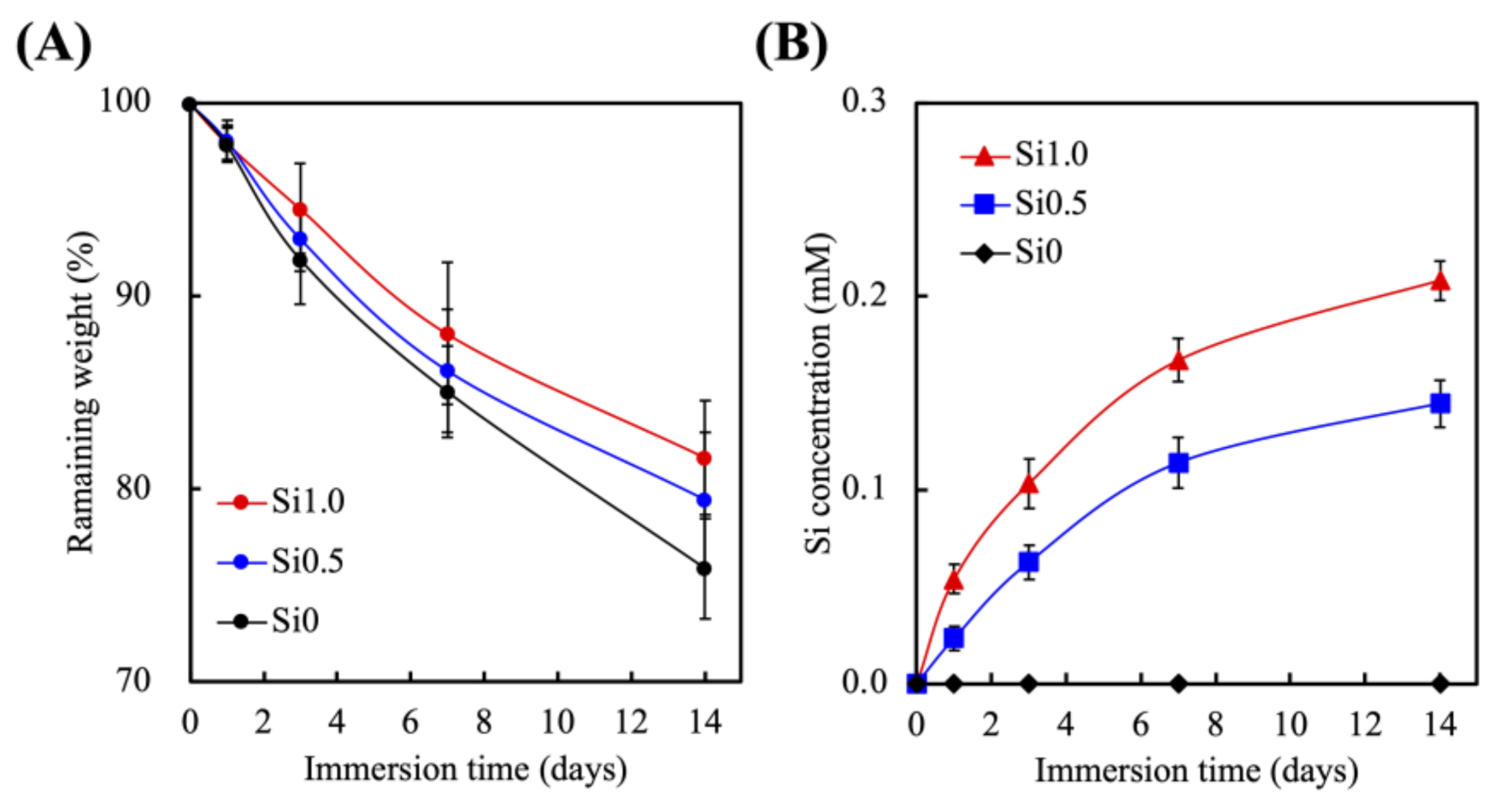
3.2. Effects of Degradation Profiles in the Immersion Experiments
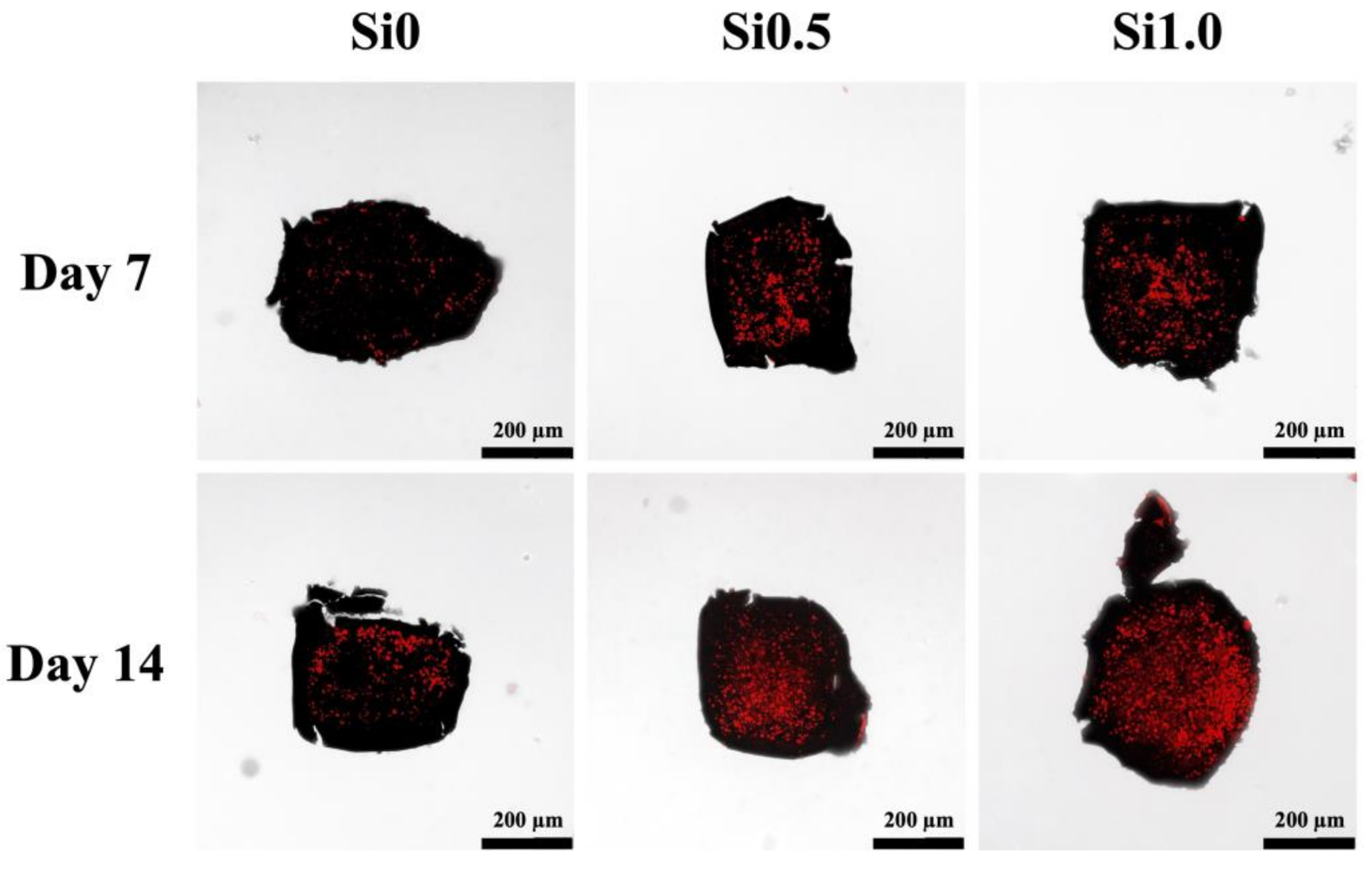
3.3. In Vitro hDPSCs Culture
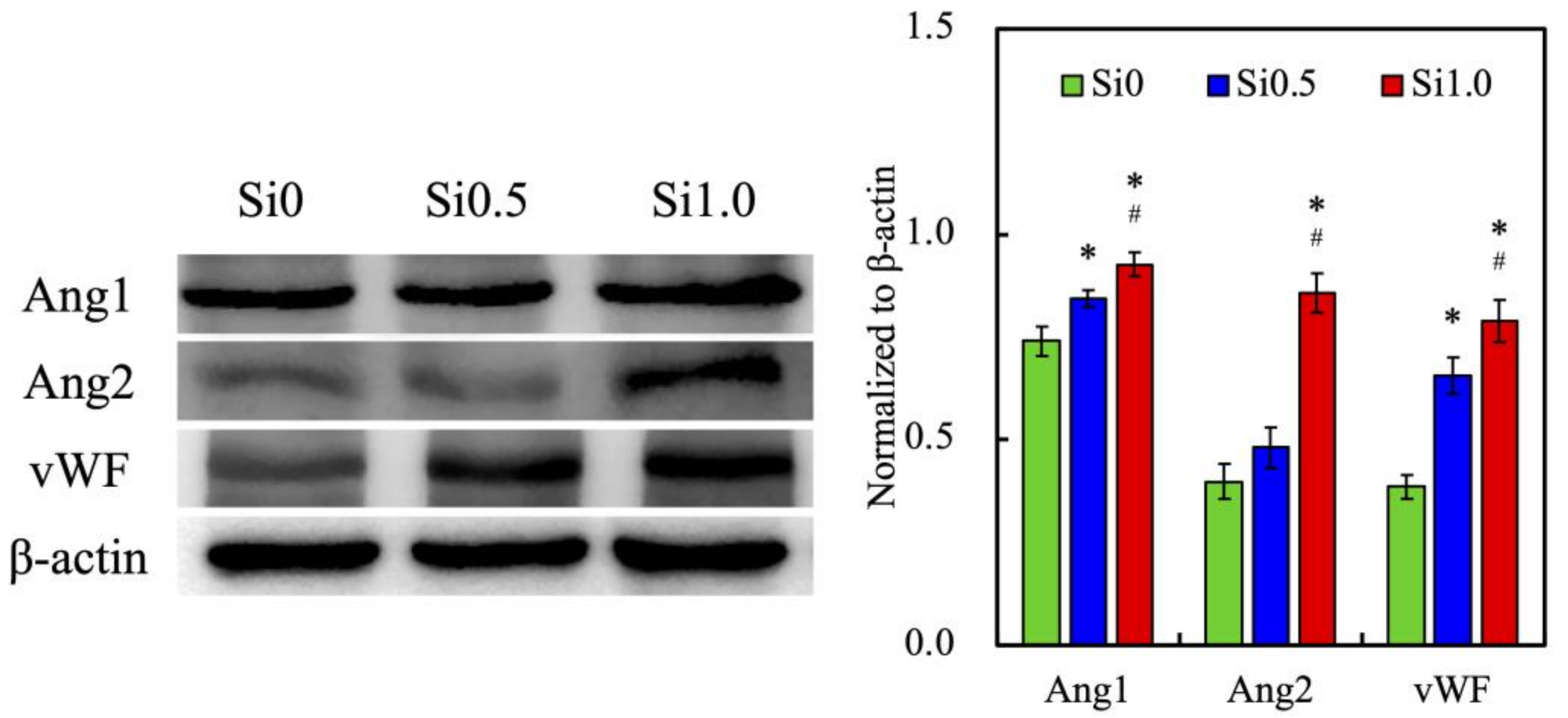
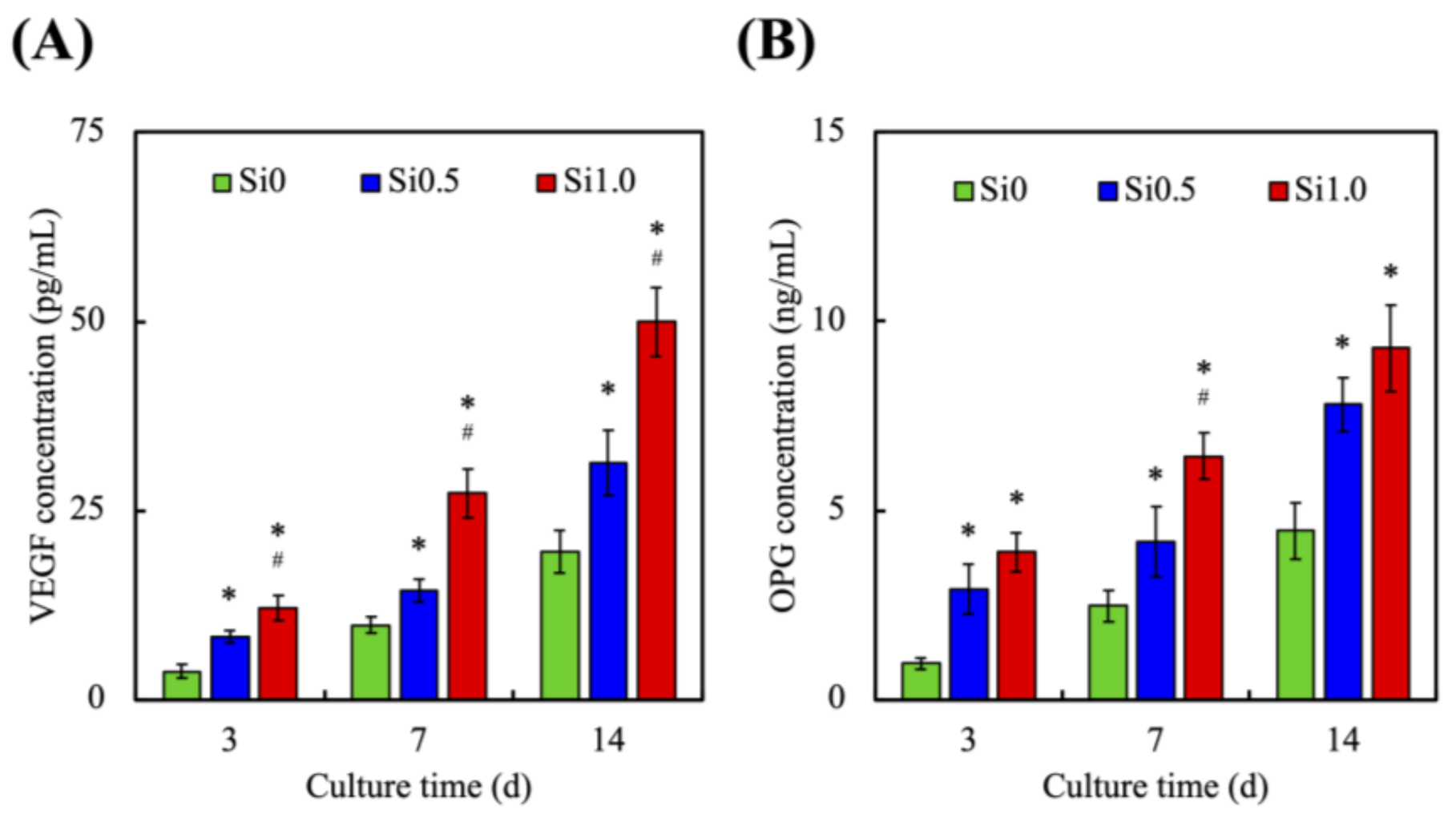
3.4. In Vitro HUVEC Culture
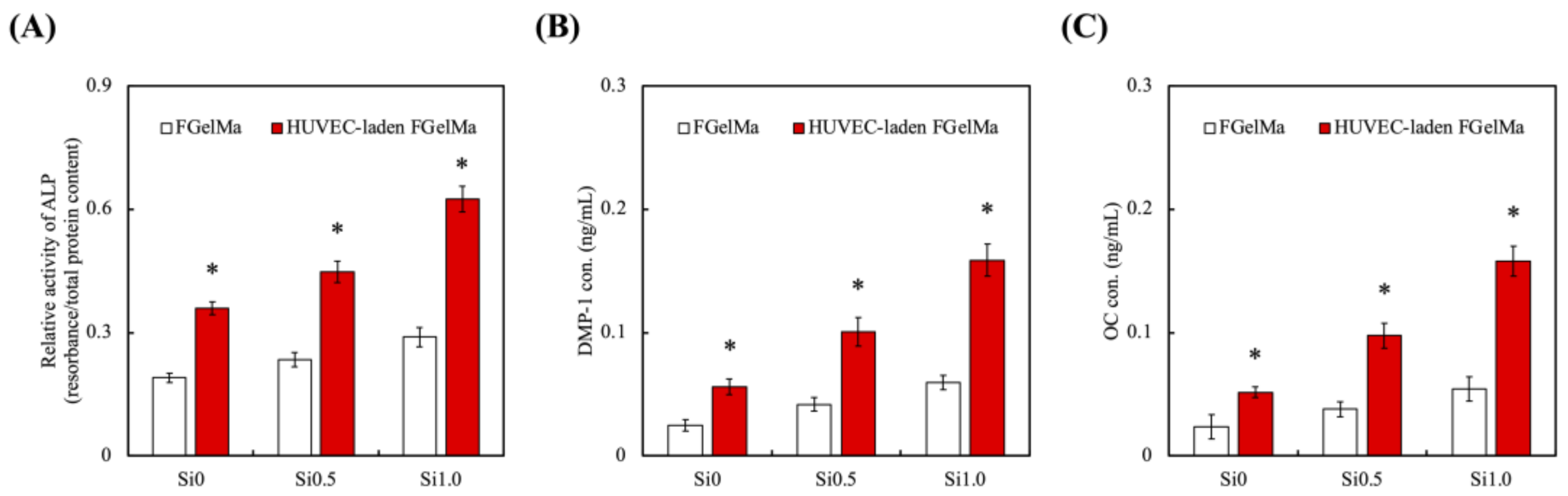
3.5. Odontogenic Behaviors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental caries. Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef]
- Xu, X.; Liang, C.; Gao, X.; Huang, H.; Xing, X.; Tang, Q.; Yang, J.; Wu, Y.; Li, M.; Li, H.; et al. Adipose tissue–derived microvascular fragments as vascularization units for dental pulp regeneration. J. Endod. 2021, 47, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Rao, Z.; Zhao, Y.; Xu, Y.; Chen, L.; Shen, Z.; Bai, Y.; Lin, Z.; Huang, Q. A decellularized matrix hydrogel derived from human dental pulp promotes dental pulp stem cell proliferation, migration, and induced multidirectional differentiation in vitro. J. Endod. 2020, 46, 1438–1447. [Google Scholar] [CrossRef] [PubMed]
- Nedunchezian, S.; Banerjee, P.; Lee, C.Y.; Lee, S.S.; Lin, C.W.; Wu, C.W.; Wu, S.C.; Chang, J.K.; Wang, C.K. Generating adipose stem cell-laden hyaluronic acid-based scaffolds using 3D bioprinting via the double crosslinked strategy for chondrogenesis. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 124, 112072. [Google Scholar] [CrossRef]
- Dou, Y.; Huang, J.; Xia, X.; Wei, J.; Zou, Q.; Zuo, Y.; Li, J.; Li, Y. A hierarchical scaffold with a highly pore-interconnective 3D printed PLGA/n-HA framework and an extracellular matrix like gelatin network filler for bone regeneration. J. Mater. Chem. B 2021, 9, 4488–4501. [Google Scholar] [CrossRef]
- Nulty, J.; Freeman, F.E.; Browe, D.C.; Burdis, R.; Ahern, D.P.; Pitacco, P.; Lee, Y.B.; Alsberg, E.; Kelly, D.J. 3D bioprinting of prevascularised implants for the repair of critically-sized bone defects. Acta Biomater. 2021, 126, 154–169. [Google Scholar] [CrossRef]
- Vagropouloua, G.; Trentsioua, M.; Georgopoulou, A.; Papachristou, E.; Prymak, O.; Kritis, A.; Epple, M.; Chatzinikolaidou, M.; Bakopoulou, A.; Koidis, P. Hybrid chitosan/gelatin/nanohydroxyapatite scaffolds promote odontogenic differentiation of dental pulp stem cells and in vitro biomineralization. Dent. Mater. 2021, 37, e23–e36. [Google Scholar] [CrossRef]
- Chen, W.J.; Xie, J.; Lin, X.; Ou, M.H.; Zhou, J.; Wei, X.L.; Chen, W.X. The role of small extracellular vesicles derived from lipopolysaccharide-preconditioned human dental pulp stem cells in dental pulp regeneration. J. Endod. 2021, 47, 961–969. [Google Scholar] [CrossRef]
- Fantini, V.; Bordoni, M.; Scocozza, F.; Conti, M.; Scarian, E.; Carelli, S.; Di Giulio, A.M.; Marconi, S.; Pansarasa, O.; Auricchio, F.; et al. Bioink composition and printing parameters for 3D modeling neural tissue. Cells 2019, 8, 830. [Google Scholar] [CrossRef] [Green Version]
- Dabaghi, M.; Saraei, N.; Carpio, M.B.; Nanduri, V.; Ungureanu, J.; Babi, M.; Chandiramohan, A.; Noble, A.; Revill, S.D.; Zhang, B.; et al. A robust protocol for decellularized human lung bioink generation amenable to 2D and 3D lung cell culture. Cells 2021, 10, 1538. [Google Scholar] [CrossRef]
- Horder, H.; Guaza Lasheras, M.; Grummel, N.; Nadernezhad, A.; Herbig, J.; Ergün, S.; Teßmar, J.; Groll, J.; Fabry, B.; Bauer-Kreisel, P.; et al. Bioprinting and differentiation of adipose-derived stromal cell spheroids for a 3D breast cancer-adipose tissue model. Cells 2021, 10, 803. [Google Scholar] [CrossRef]
- Wszoła, M.; Nitarska, D.; Cywoniuk, P.; Gomółka, M.; Klak, M. Stem cells as a source of pancreatic cells for production of 3D bioprinted bionic pancreas in the treatment of type 1 diabetes. Cells 2021, 10, 1544. [Google Scholar] [CrossRef]
- Messaoudi, O.; Henrionnet, C.; Bourge, K.; Loeuille, D.; Gillet, P.; Pinzano, A. Stem cells and extrusion 3D printing for hyaline cartilage engineering. Cells 2020, 10, 2. [Google Scholar] [CrossRef]
- Aldana, A.A.; Valente, F.; Dilley, R.; Doyle, B. Development of 3D bioprinted GelMA-alginate hydrogels with tunable mechanical properties. Bioprinting 2021, 21, e00105. [Google Scholar] [CrossRef]
- Schmidt, S.K.; Schmid, R.; Arkudas, A.; Kengelbach-Weigand, A.; Bosserhoff, A.K. Tumor cells develop defined cellular phenotypes after 3D-bioprinting in different bioinks. Cells 2019, 8, 1295. [Google Scholar] [CrossRef] [Green Version]
- Ansari, S.; Sarrion, P.; Hasani-Sadrabadi, M.M.; Aghaloo, T.; Wu, B.M.; Moshaverinia, A. Regulation of the fate of dental-derived mesenchymal stem cells using engineered alginate-GelMA hydrogels. J. Biomed. Mater. Res. Part A 2017, 92, 367. [Google Scholar] [CrossRef]
- Chen, K.Y.; Yao, C.H. Repair of bone defects with gelatin-based composites: A review. BioMedicine 2011, 1, 29–32. [Google Scholar] [CrossRef]
- Lee, J.H.; Parthiban, P.; Jin, G.Z.; Knowles, J.C.; Kim, H.W. Materials roles for promoting angiogenesis in tissue regeneration. Prog. Mater. Sci. 2021, 117, 100732. [Google Scholar] [CrossRef]
- Zhang, R.; Xie, L.; Wu, H.; Yang, T.; Zhang, Q.; Tian, Y.; Liu, Y.; Han, X.; Guo, W.; He, M.; et al. Alginate/laponite hydrogel microspheres co-encapsulating dental pulp stem cells and VEGF for endodontic regeneration. Acta Biomater. 2020, 113, 305–316. [Google Scholar] [CrossRef]
- Lin, F.S.; Lee, J.J.; Lee, K.X.A.; Ho, C.C.; Liu, Y.T.; Shie, M.Y. Calcium silicate-activated gelatin methacrylate hydrogel for accelerating human dermal fibroblast proliferation and differentiation. Polymers 2021, 13, 70. [Google Scholar] [CrossRef]
- Lin, Y.T.; Shie, M.Y.; Lin, Y.H.; Ho, C.C.; Kao, C.T.; Huang, T.H. The development of light-curable calcium-silicate-containing composites used in odontogenic regeneration. Polymers 2021, 13, 3107. [Google Scholar] [CrossRef]
- Wang, C.Y.; Chiu, Y.C.; Lee, K.X.; Lin, Y.A.; Lin, P.Y.; Shie, M.Y. Biofabrication of gingival fibroblast cell-laden collagen/strontium-doped calcium silicate 3D-printed bi-layered scaffold for osteoporotic periodontal regeneration. Biomedicines 2021, 9, 431. [Google Scholar] [CrossRef]
- Kao, C.T.; Chen, Y.J.; Huang, T.H.; Lin, Y.H.; Hsu, T.T.; Ho, C.C. Assessment of the release profile of fibroblast growth factor-2-load mesoporous calcium silicate/poly-ε-caprolactone 3D scaffold for regulate bone regeneration. Processes 2020, 8, 1249. [Google Scholar] [CrossRef]
- Lai, W.Y.; Chen, Y.J.; Lee, K.X.A.; Lin, Y.H.; Liu, Y.W.; Shie, M.Y. Therapeutic effects of the addition of fibroblast growth factor-2 to biodegradable gelatin/magnesium-doped calcium silicate hybrid 3D-printed scaffold with enhanced osteogenic capabilities for critical bone defect restoration. Biomedicines 2021, 9, 712. [Google Scholar] [CrossRef]
- Chen, C.Y.; Shie, M.Y.; Lee, K.X.; Chou, Y.T.; Chiang, C.; Lin, C.P. 3D-printed ginsenoside Rb1-Loaded mesoporous calcium silicate/calcium sulfate scaffolds for inflammation inhibition and bone regeneration. Biomedicines 2021, 9, 907. [Google Scholar] [CrossRef]
- Dashnyam, K.; El-Fiqi, A.; Buitrago, J.O.; Perez, R.A.; Knowles, J.C.; Kim, H.W. A mini review focused on the proangiogenic role of silicate ions released from silicon-containing biomaterials. J. Tissue Eng. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Wang, C.; Lin, K.; Chang, J.; Sun, J. Osteogenesis and angiogenesis induced by porous β-CaSiO3/PDLGA composite scaffold via activation of AMPK/ERK1/2 and PI3K/Akt pathways. Biomaterials 2013, 34, 64–77. [Google Scholar] [CrossRef]
- Kao, C.T.; Chiu, Y.C.; Lee, K.X.; Lin, Y.H.; Huang, T.H.; Liu, Y.C.; Shie, M.Y. The synergistic effects of Xu Duan combined Sr-contained calcium silicate/poly-ε-caprolactone scaffolds for the promotion of osteogenesis marker expression and the induction of bone regeneration in osteoporosis. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111629. [Google Scholar] [CrossRef]
- Shie, M.Y.; Lee, J.J.; Ho, C.C.; Yen, S.Y.; Ng, H.Y.; Chen, Y.W. Effects of gelatin methacrylate bio-ink concentration on mechano-physical properties and human dermal fibroblast behavior. Polymers 2020, 12, 1930. [Google Scholar] [CrossRef]
- Lecarpentier, Y.; Kindler, V.; Krokidis, X.; Bochaton-Piallat, M.-L.; Claes, V.; Hébert, J.-L.; Vallée, A.; Schussler, O. Statistical mechanics of non-muscle myosin IIA in human bone marrow-derived mesenchymal stromal cells seeded in a collagen scaffold: A thermodynamic near-equilibrium linear system modified by the tripeptide Arg-Gly-Asp (RGD). Cells 2020, 9, 1510. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, H.; Qiao, P.; Liu, Z. Development and properties of fish gelatin/oxidized starch double network film catalyzed by thermal treatment and schiff’ base reaction. Polymers 2019, 11, 2065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreller, T.; Distler, T.; Heid, S.; Gerth, S.; Detsch, R.; Boccaccini, A.R. Physico-chemical modification of gelatine for the improvement of 3D printability of oxidized alginate-gelatine hydrogels towards cartilage tissue engineering. Mater. Design 2021, 208, 109877. [Google Scholar] [CrossRef]
- Mehta, V.; Pang, K.-L.; Givens, C.S.; Chen, Z.; Huang, J.; Sweet, D.T.; Jo, H.; Reader, J.S.; Tzima, E. Mechanical forces regulate endothelial-to-mesenchymal transition and atherosclerosis via an Alk5-Shc mechanotransduction pathway. Sci. Adv. 2021, 7, eabg5060. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, X.; Cong, X. Spatial micro-variation of 3D hydrogel stiffness regulates the biomechanical properties of hMSCs. Biofabrication 2021, 13, 035051. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Enhe, J.; Yao, B.; Wang, Y.; Zhu, D.; Li, Z.; Song, W.; Duan, X.; Yuan, X.; et al. Bioactive nanoparticle reinforced alginate/gelatin bioink for the maintenance of stem cell stemness. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 126, 112193. [Google Scholar] [CrossRef]
- Yu, C.T.; Wang, F.M.; Liu, Y.T.; Lee, K.X.A.; Lin, T.L.; Chen, Y.W. Enhanced proliferation and differentiation of human mesenchymal stem cell-laden recycled fish gelatin/strontium substitution calcium silicate 3D scaffolds. Appl. Sci. 2020, 10, 2168. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H.J.; Shin, S.R.; Cha, J.M.; Lee, S.H.; Kim, J.H.; Do, J.T.; Song, H.; Bae, H. Cold water fish gelatin methacryloyl hydrogel for tissue engineering application. PLoS ONE 2016, 11, 0163902. [Google Scholar] [CrossRef] [Green Version]
- Ding, Z.; Xi, W.; Ji, M.; Chen, H.; Zhang, Q.; Yan, Y. Developing a biodegradable tricalcium silicate/glucono-delta-lactone/calcium sulfate dihydrate composite cement with high preliminary mechanical property for bone filling. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111621. [Google Scholar] [CrossRef]
- Liu, W.C.; Hu, C.C.; Tseng, Y.Y.; Sakthivel, R.; Fan, K.-S.; Wang, A.N.; Wang, Y.M.; Chung, R.J. Study on strontium doped tricalcium silicate synthesized through sol-gel process. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110431. [Google Scholar] [CrossRef]
- Liao, F.; Peng, X.Y.; Yang, F.; Ke, Q.F.; Zhu, Z.H.; Guo, Y.P. Gadolinium-doped mesoporous calcium silicate/chitosan scaffolds enhanced bone regeneration ability. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109999. [Google Scholar] [CrossRef]
- Hu, S.; Martinez-Garcia, F.D.; Moeun, B.N.; Burgess, J.K.; Harmsen, M.C.; Hoesli, C.; de Vos, P. An immune regulatory 3D-printed alginate-pectin construct for immunoisolation of insulin producing β-cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 123, 112009. [Google Scholar] [CrossRef]
- Leite, M.L.; Soares, D.G.; Anovazzi, G.; Anselmi, C.; Hebling, J.; de Souza Costa, C.A. Fibronectin-loaded collagen/gelatin hydrogel Is a potent signaling biomaterial for dental pulp regeneration. J. Endod. 2021, 47, 1110–1117. [Google Scholar] [CrossRef]
- Fathi, E.; Farahzadi, R.; Valipour, B. Alginate/gelatin encapsulation promotes NK cells differentiation potential of bone marrow resident C-kit+ hematopoietic stem cells. Int. J. Biol. Macromol. 2021, 177, 317–327. [Google Scholar] [CrossRef]
- Shie, M.Y.; Chiang, W.H.; Chen, I.W.P.; Liu, W.Y.; Chen, Y.W. Synergistic acceleration in the osteogenic and angiogenic differentiation of human mesenchymal stem cells by calcium silicate–graphene composites. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 73, 726–735. [Google Scholar] [CrossRef]
- Lin, Y.H.; Chuang, T.Y.; Chiang, W.H.; Chen, I.W.P.; Wang, K.; Shie, M.Y.; Chen, Y.W. The synergistic effects of graphene-contained 3D-printed calcium silicate/poly-ε-caprolactone scaffolds promote FGFR-induced osteogenic/angiogenic differentiation of mesenchymal stem cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109887. [Google Scholar] [CrossRef]
- Shie, M.Y.; Ding, S.J. Integrin binding and MAPK signal pathways in primary cell responses to surface chemistry of calcium silicate cements. Biomaterials 2013, 34, 6589–6606. [Google Scholar] [CrossRef]
- Wu, X.; Tang, Z.; Wu, K.; Bai, Y.; Lin, X.; Yang, H.; Yang, Q.; Wang, Z.; Ni, X.; Liu, H.; et al. Strontium-calcium phosphate hybrid cement with enhanced osteogenic and angiogenic properties for vascularised bone regeneration. J. Mater. Chem. B 2021, 9, 5982–5997. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Y.; Cao, Q.; Yu, T.; Zhang, J.; Liu, Q.; Yang, X. Growth factors enhanced angiogenesis and osteogenesis on polydopamine coated titanium surface for bone regeneration. Mater. Des. 2020, 196, 109162. [Google Scholar] [CrossRef]
- Huang, S.; Ninivaggi, M.; Chayoua, W.; de Laat, B. VWF, platelets and the antiphospholipid syndrome. Int. J. Mol. Sci. 2021, 22, 4200. [Google Scholar] [CrossRef]
- Wu, J.; Qin, C.; Ma, J.; Zhang, H.; Chang, J.; Mao, L.; Wu, C. An immunomodulatory bioink with hollow manganese silicate nanospheres for angiogenesis. Appl. Mater. Today 2021, 23, 101015. [Google Scholar] [CrossRef]
- Kao, C.T.; Hsu, T.T.; Huang, T.H.; Wu, Y.T.; Chen, Y.W.; Shie, M.Y. The synergistic effect on osteogenic differentiation of human mesenchymal stem cell by diode laser-treated stimulating human umbilical vein endothelial cell. Laser Phys. Lett. 2016, 13, 025604. [Google Scholar] [CrossRef]
- Kazemzadeh Narbat, M.; Rouwkema, J.; Annabi, N.; Cheng, H.; Ghaderi, M.; Cha, B.H.; Aparnathi, M.; Khalilpour, A.; Byambaa, B.; Jabbari, E.; et al. Engineering photocrosslinkable bicomponent hydrogel constructs for creating 3D vascularized bone. Adv. Healthc. Mater. 2017, 6, 1601122. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Teoh, S.H.; Chong, M.S.K.; Lee, E.S.M.; Mattar, C.N.Z.; Randhawa, N.K.; Zhang, Z.Y.; Medina, R.J.; Kamm, R.D.; Fisk, N.M.; et al. Vasculogenic and osteogenesis-enhancing potential of human umbilical cord blood endothelial colony-forming cells. Stem Cells 2012, 30, 1911–1924. [Google Scholar] [CrossRef]
- Jin, R.; Song, G.; Chai, J.; Gou, X.; Yuan, G.; Chen, Z. Effects of concentrated growth factor on proliferation, migration, and differentiation of human dental pulp stem cells in vitro. J. Tissue Eng. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, K.G.; Hwang, J.H.; Cho, Y.S.; Lee, K.S.; Jeong, H.J.; Park, S.H.; Park, Y.; Cho, Y.S.; Lee, B.K. Evaluation of mechanical strength and bone regeneration ability of 3D printed kagome-structure scaffold using rabbit calvarial defect model. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 949–959. [Google Scholar] [CrossRef]
- Qiao, Y.; Liu, X.; Zhou, X.; Zhang, H.; Zhang, W.; Xiao, W.; Pan, G.; Cui, W.; Santos, H.A.; Shi, Q. Gelatin templated polypeptide co-cross-linked hydrogel for bone regeneration. Adv. Healthc. Mater. 2020, 9, 1901239. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, W.-Y.; Lee, T.-H.; Chen, J.-X.; Ng, H.-Y.; Huang, T.-H.; Shie, M.-Y. Synergies of Human Umbilical Vein Endothelial Cell-Laden Calcium Silicate-Activated Gelatin Methacrylate for Accelerating 3D Human Dental Pulp Stem Cell Differentiation for Endodontic Regeneration. Polymers 2021, 13, 3301. https://doi.org/10.3390/polym13193301
Lai W-Y, Lee T-H, Chen J-X, Ng H-Y, Huang T-H, Shie M-Y. Synergies of Human Umbilical Vein Endothelial Cell-Laden Calcium Silicate-Activated Gelatin Methacrylate for Accelerating 3D Human Dental Pulp Stem Cell Differentiation for Endodontic Regeneration. Polymers. 2021; 13(19):3301. https://doi.org/10.3390/polym13193301
Chicago/Turabian StyleLai, Wei-Yun, Tzu-Hsin Lee, Jian-Xun Chen, Hooi-Yee Ng, Tsui-Hsien Huang, and Ming-You Shie. 2021. "Synergies of Human Umbilical Vein Endothelial Cell-Laden Calcium Silicate-Activated Gelatin Methacrylate for Accelerating 3D Human Dental Pulp Stem Cell Differentiation for Endodontic Regeneration" Polymers 13, no. 19: 3301. https://doi.org/10.3390/polym13193301
APA StyleLai, W.-Y., Lee, T.-H., Chen, J.-X., Ng, H.-Y., Huang, T.-H., & Shie, M.-Y. (2021). Synergies of Human Umbilical Vein Endothelial Cell-Laden Calcium Silicate-Activated Gelatin Methacrylate for Accelerating 3D Human Dental Pulp Stem Cell Differentiation for Endodontic Regeneration. Polymers, 13(19), 3301. https://doi.org/10.3390/polym13193301






