Evaluating the Anticarcinogenic Activity of Surface Modified/Functionalized Nanochitosan: The Emerging Trends and Endeavors
Abstract
:1. Introduction
2. Antioxidant Activity of Chitosan
3. Surface Modifications of Chitosan
4. Nanochitosan and Its Modifications
4.1. Methods Used to Prepare Nanochitosan
4.2. Chitosan/Functionalized Chitosan Nanocarriers—A Snap Shot of Biomedical Achievements
5. Anticarcinogenic/Antitumour Activity of Chitosan Nanomaterials
5.1. Chitosan Nanocarriers—Anticancer Impacts
5.2. Surface Modified/Functionalized Chitosan Nanocarriers—Anticarcinogenic Impacts
6. Limitations and Future Endeavors
6.1. Toxicity Aspects of Chitosan
6.2. Inadequate Clinical Testings
6.3. Unexplored Arenas
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2016, 25, 854–866. [Google Scholar] [CrossRef]
- Park, B.K.; Kim, M.-M. Applications of Chitin and Its Derivatives in Biological Medicine. Int. J. Mol. Sci. 2010, 11, 5152–5164. [Google Scholar] [CrossRef] [Green Version]
- Aranaz, I.; Mengíbar, M.; Harris, R.; Panos, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, A. Functional characterization of chitin and chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [Green Version]
- Venkatesan, J.; Lowe, B.; Anil, S.; Manivasagan, P.; Al Kheraif, A.A.; Kang, K.-H.; Kim, S.-K. Seaweed polysaccharides and their potential biomedical applications. Starch-Stärke 2015, 67, 381–390. [Google Scholar] [CrossRef]
- Aranaz, I.; Harris, R.; Heras, A. Chitosan amphiphilic derivatives. Chemistry and applications. Curr. Org. Chem. 2010, 14, 308–330. [Google Scholar] [CrossRef]
- Grenha, A.; Al-Qadi, S.; Seijo, B.; Remuñán-López, C. The potential of chitosan for pulmonary drug delivery. J. Drug Deliv. Sci. Technol. 2010, 20, 33–43. [Google Scholar] [CrossRef]
- Kim, S.; Requejo, K.I.; Nakamatsu, J.; Gonzales, K.N.; Torres, F.G.; Cavaco-Paulo, A. Modulating antioxidant activity and the controlled release capability of laccase mediated catechin grafting of chitosan. Process. Biochem. 2017, 59, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Samar, M.M.; El-Kalyoubi, M.; Khalaf, M.; El-Razik, M.A. Physicochemical, functional, antioxidant and antibacterial properties of chitosan extracted from shrimp wastes by microwave technique. Ann. Agric. Sci. 2013, 58, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Qinna, N.A.; Karwi, Q.G.; Al-Jbour, N.; Al-Remawi, M.A.; Alhussainy, T.M.; Al-So’Ud, K.A.; Al Omari, M.M.H.; Badwan, A.A. Influence of Molecular Weight and Degree of Deacetylation of Low Molecular Weight Chitosan on the Bioactivity of Oral Insulin Preparations. Mar. Drugs 2015, 13, 1710–1725. [Google Scholar] [CrossRef] [Green Version]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Chesnutt, B.M.; Haggard, W.O.; Bumgardner, J.D. Deacetylation of Chitosan: Material Characterization and in vitro Evaluation via Albumin Adsorption and Pre-Osteoblastic Cell Cultures. Materials 2011, 4, 1399–1416. [Google Scholar] [CrossRef] [Green Version]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, X.; Gong, Y.; Zhao, N.; Zhang, X. Properties and biocompatibility of chitosan films modified by blending with PEG. Biomaterials 2001, 23, 2641–2648. [Google Scholar] [CrossRef]
- Ahmed, S.; Ikram, S. Chitosan Based Scaffolds and Their Applications in Wound Healing. Achiev. Life Sci. 2016, 10, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Sun, T.; Jiang, C. Biomacromolecules as carriers in drug delivery and tissue engineering. Acta Pharm. Sin. B 2017, 8, 34–50. [Google Scholar] [CrossRef]
- Bakar, L.M.; Abdullah, M.Z.; Doolaanea, A.A.; Ichwan, S.J.A. PLGA-Chitosan nanoparticle-mediated gene delivery for oral cancer treatment: A brief review. J. Phys. Conf. Ser. 2017, 884, 12117. [Google Scholar] [CrossRef]
- Kumirska, J.; Weinhold, M.X.; Thöming, J.; Stepnowski, P. Biomedical Activity of Chitin/Chitosan Based Materials—Influence of Physicochemical Properties Apart from Molecular Weight and Degree of N-Acetylation. Polymers 2011, 3, 1875–1901. [Google Scholar] [CrossRef]
- Keawchaoon, L.; Yoksan, R. Preparation, characterization and in vitro release study of carvacrol-loaded chitosan nanoparticles. Colloids Surf. B Biointerfaces 2011, 84, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Ajun, W.; Yan, S.; Li, G.; Huili, L. Preparation of aspirin and probucol in combination loaded chitosan nanoparticles and in vitro release study. Carbohydr. Polym. 2009, 75, 566–574. [Google Scholar] [CrossRef]
- Arya, N.; Chakraborty, S.; Dube, N.; Katti, D.S. Electrospraying: A facile technique for synthesis of chitosan-based mi-cro/nanospheres for drug delivery applications. J. Biomed. Mater. Res. Part B 2009, 88, 17–31. [Google Scholar] [CrossRef]
- Hu, B.; Pan, C.; Sun, Y.; Hou, Z.; Ye, H.; Hu, B.; Zeng, X. Optimization of fabrication parameters to produce chitosan− tripolyphosphate nanoparticles for delivery of tea catechins. J. Agric. Food Chem. 2008, 56, 7451–7458. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhang, B.; Whent, M.; Yu, L.L.; Wang, Q. Preparation and characterization of zein/chitosan complex for encap-sulation of α-tocopherol, and its in vitro controlled release. Colloids Surf. B Biointerfaces 2011, 85, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.T.; Chang, C.-H.; Lin, Y.Y.; Wu, M.F.; Han, J.L.; Hsieh, K.H. Kinetic study of acid depolymerization of chitosan and effects of low molecular weight chitosan on erythrocyte rouleaux formation. Carbohydr. Res. 2011, 346, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.R.; Zhou, M.Q.; Wu, S.J.; Pan, S.K. Depolymerization of chitosan by enzymes from the digestive tract of sea cucumber Stichopus japonicus. Afr. J. Biotechnol. 2012, 11, 423–428. [Google Scholar]
- Kumar, A.B.; Tharanathan, R.N. A comparative study on depolymerization of chitosan by proteolytic enzymes. Carbohydr. Polym. 2004, 58, 275–283. [Google Scholar]
- Novikov, V.Y.; Mukhin, V. Chitosan Depolymerization by Enzymes from the Hepatopancreas of the Crab Paralithodes camtschaticus. Appl. Biochem. Microbiol. 2003, 39, 464–468. [Google Scholar] [CrossRef]
- Muanprasat, C.; Chatsudthipong, V. Chitosan oligosaccharide: Biological activities and potential therapeutic applications. Pharmacol. Ther. 2017, 170, 80–97. [Google Scholar] [CrossRef]
- Enescu, D.; Olteanu, C.E. Functionalized chitosan and its use in pharmaceutical, biomedical, and biotechnological research. Chem. Eng. Commun. 2008, 195, 1269–1291. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [Green Version]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [Green Version]
- Yoo, S.-J.; Go, E.; Kim, Y.-E.; Lee, S.; Kwon, J. Roles of Reactive Oxygen Species in Rheumatoid Arthritis Pathogenesis. J. Rheum. Dis. 2016, 23. [Google Scholar] [CrossRef] [Green Version]
- Abbas, M.; Monireh, M. The role of reactive oxygen species in immunopathogenesis of rheumatoid arthritis. Iran. J. Allergy Asthma Immunol. 2008, 7, 195–202. [Google Scholar]
- Rahman, T.; Hosen, I.; Islam, M.M.T.; Shekhar, H.U. Oxidative stress and human health. Adv. Biosci. Biotechnol. 2012, 3, 997–1019. [Google Scholar] [CrossRef] [Green Version]
- Xie, W.; Xu, P.; Liu, Q. Antioxidant activity of water-soluble chitosan derivatives. Bioorg. Med. Chem. Lett. 2001, 11, 1699–1701. [Google Scholar] [CrossRef]
- Hajji, S.; Younes, I.; Rinaudo, M.; Jellouli, K.; Nasri, M. Characterization and in vitro evaluation of cytotoxicity, antimi-crobial and antioxidant activities of chitosans extracted from three different marine sources. Appl. Biochem. Biotechnol. 2015, 177, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Thomas, R. Antioxidative activity of chitosans with varying molecular weights. Food Chem. 2007, 101, 308–313. [Google Scholar] [CrossRef]
- Sun, T.; Zhou, D.; Xie, J.; Mao, F. Preparation of chitosan oligomers and their antioxidant activity. Eur. Food Res. Technol. 2006, 225, 451–456. [Google Scholar] [CrossRef]
- Chang, S.-H.; Wu, C.-H.; Tsai, G.-J. Effects of chitosan molecular weight on its antioxidant and antimutagenic properties. Carbohydr. Polym. 2018, 181, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, Q.; Chen, Y.; Wan, A. Effect of concentration and molecular weight of chitosan and its derivative on the free radical scavenging ability. J. Biomed. Mater. Res. Part A 2013, 102, 911–916. [Google Scholar] [CrossRef]
- Božič, M.; Gorgieva, S.; Kokol, V. Laccase-mediated functionalization of chitosan by caffeic and gallic acids for mod-ulating antioxidant and antimicrobial properties. Carbohydr. Polym. 2012, 87, 2388–2398. [Google Scholar] [CrossRef]
- Shukla, S.K.; Mishra, A.K.; Arotiba, O.; Mamba, B. Chitosan-based nanomaterials: A state-of-the-art review. Int. J. Biol. Macromol. 2013, 59, 46–58. [Google Scholar] [CrossRef]
- Park, S.; Jun, S.; Marsh, K. Physical properties of PVOH/chitosan-blended films cast from different solvents. Food Hydrocoll. 2001, 15, 499–502. [Google Scholar] [CrossRef]
- Mima, S.; Miya, M.; Iwamoto, R.; Yoshikawa, S. Highly deacetylated chitosan and its properties. J. Appl. Polym. Sci. 1983, 28, 1909–1917. [Google Scholar] [CrossRef]
- Risbud, M.V.; Hardikar, A.A.; Bhat, S.V.; Bhonde, R.R. pH-sensitive freeze-dried chitosan–polyvinyl pyrrolidone hydrogels as controlled release system for antibiotic delivery. J. Control. Release 2000, 68, 23–30. [Google Scholar] [CrossRef]
- Strobl, G.R. The Physics of Polymers; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 978-3-540-25278-8. [Google Scholar]
- Dambies, L.; Vincent, .T.; Domard, .A.A.; Guibal, .E. Preparation of Chitosan Gel Beads by Ionotropic Molybdate Gelation. Biomacromolecules 2001, 2, 1198–1205. [Google Scholar] [CrossRef]
- Al-Qadi, S.; Grenha, A.; Carrión-Recio, D.; Seijo, B.; Remuñán-López, C. Microencapsulated chitosan nanoparticles for pulmonary protein delivery: In vivo evaluation of insulin-loaded formulations. J. Control. Release 2011, 157, 383–390. [Google Scholar] [CrossRef]
- Thanou, M.; Kotzé, A.; Scharringhausen, T.; Lueßen, H.; de Boer, A.; Verhoef, J.; Junginger, H. Effect of degree of quaternization of N-trimethyl chitosan chloride for enhanced transport of hydrophilic compounds across intestinal Caco-2 cell monolayers. J. Control. Release 2000, 64, 15–25. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Hornof, M.; Zoidl, T. Thiolated polymers—Thiomers: Synthesis and in vitro evaluation of chitosan–2-iminothiolane conjugates. Int. J. Pharm. 2003, 260, 229–237. [Google Scholar] [CrossRef]
- Sadeghi, A.; Dorkoosh, F.; Avadi, M.; Weinhold, M.; Bayat, A.; Delie, F.; Gurny, R.; Larijani, B.; Rafieetehrani, M.; Junginger, H. Permeation enhancer effect of chitosan and chitosan derivatives: Comparison of formulations as soluble polymers and nanoparticulate systems on insulin absorption in Caco-2 cells. Eur. J. Pharm. Biopharm. 2008, 70, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.; Guebitz, G.M.; Kokol, V. Antimicrobial and antioxidant properties of chitosan enzymatically functionalized with flavonoids. Process. Biochem. 2009, 44, 749–756. [Google Scholar] [CrossRef]
- Aytekin, A..; Morimura, S.; Kida, K. Synthesis of chitosan–caffeic acid derivatives and evaluation of their antioxidant activities. J. Biosci. Bioeng. 2011, 111, 212–216. [Google Scholar] [CrossRef]
- Božič, M.; Štrancar, J.; Kokol, V. Laccase-initiated reaction between phenolic acids and chitosan. React. Funct. Polym. 2013, 73, 1377–1383. [Google Scholar] [CrossRef]
- Aljawish, A.; Chevalot, I.; Piffaut, B.; Rondeau-Mouro, C.; Girardin, M.; Jasniewski, J.; Scher, J.; Muniglia, L. Functionalization of chitosan by laccase-catalyzed oxidation of ferulic acid and ethyl ferulate under heterogeneous reaction conditions. Carbohydr. Polym. 2012, 87, 537–544. [Google Scholar] [CrossRef]
- Silva, C.; Matamá, T.; Kim, S.; Padrão, J.; Prasetyo, E.N.; Kudanga, T.; Nyanhongo, G.S.; Guebitz, G.M.; Casal, M.; Cavaco-Paulo, A. Antimicrobial and antioxidant linen via laccase-assisted grafting. React. Funct. Polym. 2011, 71, 713–720. [Google Scholar] [CrossRef] [Green Version]
- Munawar, A.M.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar]
- Zhao, K.; Shi, X.; Zhao, Y.; Wei, H.; Sun, Q.; Huang, T.; Zhang, X.; Wang, Y. Preparation and immunological effectiveness of a swine influenza DNA vaccine encapsulated in chitosan nanoparticles. Vaccine 2011, 29, 8549–8556. [Google Scholar] [CrossRef]
- Zhuo, Y.; Han, J.; Tang, L.; Liao, N.; Gui, G.-F.; Chai, Y.-Q.; Yuan, R. Quenching of the emission of peroxydisulfate system by ferrocene functionalized chitosan nanoparticles: A sensitive “signal off” electrochemiluminescence immunosensor. Sens. Actuators B Chem. 2013, 192, 791–795. [Google Scholar] [CrossRef]
- Nagpal, K.; Singh, S.; Mishra, D.N. Chitosan Nanoparticles: A Promising System in Novel Drug Delivery. Chem. Pharm. Bull. 2010, 58, 1423–1430. [Google Scholar] [CrossRef] [Green Version]
- Hembram, K.C.; Prabha, S.; Chandra, R.; Ahmed, B.; Nimesh, S. Advances in preparation and characterization of chitosan nanoparticles for therapeutics. Artif. Cells Nanomed. Biotechnol. 2014, 44, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Tiyaboonchai, W. Chitosan Nanoparticles: A Promising System for Drug Delivery. Naresuan Univ. J. 2003, 11, 51–66. [Google Scholar]
- Wang, Y.; Wang, X.; Luo, G.; Dai, Y. Adsorption of bovin serum albumin (BSA) onto the magnetic chitosan nanoparticles prepared by a microemulsion system. Bioresour. Technol. 2008, 99, 3881–3884. [Google Scholar] [CrossRef]
- El-Shabouri, M. Positively charged nanoparticles for improving the oral bioavailability of cyclosporin-A. Int. J. Pharm. 2002, 249, 101–108. [Google Scholar] [CrossRef]
- Niwa, T.; Takeuchi, H.; Hino, T.; Kunou, N.; Kawashima, Y. Preparations of biodegradable nanospheres of water-soluble and insoluble drugs with D,L-lactide/glycolide copolymer by a novel spontaneous emulsification solvent diffusion method and the drug release behavior. J. Control Release 1993, 25, 89–98. [Google Scholar] [CrossRef]
- Banerjee, T.; Mitra, S.; Singh, A.K.; Sharma, R.K.; Maitra, A. Preparation, characterization and biodistribution of ultrafine chitosan nanoparticles. Int. J. Pharm. 2002, 243, 93–105. [Google Scholar] [CrossRef]
- Dube, A.; Nicolazzo, J.A.; Larson, I. Chitosan nanoparticles enhance the intestinal absorption of the green tea catechins (+)-catechin and (−)-epigallocatechin gallate. Eur. J. Pharm. Sci. 2010, 41, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, S.; Buttini, F.; Rossi, A.; Bettini, R.; Colombo, G.; Ponchel, G.; Sonvico, F. Ex vivo permeation of tamoxifen and its 4-OH metabolite through rat intestine from lecithin/chitosan nanoparticles. Int. J. Pharm. 2015, 491, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Wang, Z.; Jiang, C.; Kong, M.; Zhou, X.; Li, Y.; Cheng, X.; Chen, X. Chitosan/o-carboxymethyl chitosan nanoparticles for efficient and safe oral anticancer drug delivery: In vitro and in vivo evaluation. Int. J. Pharm. 2013, 457, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.J.; Sangeetha, D.; Gomathi, T. Sunitinib loaded chitosan nanoparticles formulation and its evaluation. Int. J. Biol. Macromol. 2016, 82, 952–958. [Google Scholar] [CrossRef]
- Diop, M.; Auberval, N.; Viciglio, A.; Langlois, A.; Bietiger, W.; Mura, C.; Peronet, C.; Bekel, A.; David, D.J.; Zhao, M.; et al. Design, characterisation, and bioefficiency of insulin–chitosan nanoparticles after stabilisation by freeze-drying or cross-linking. Int. J. Pharm. 2015, 491, 402–408. [Google Scholar] [CrossRef]
- Xue, M.; Hu, S.; Lu, Y.; Zhang, Y.; Jiang, X.; An, S.; Guo, Y.; Zhou, X.; Hou, H.; Jiang, C. Development of chitosan nanoparticles as drug delivery system for a prototype capsid inhibitor. Int. J. Pharm. 2015, 495, 771–782. [Google Scholar] [CrossRef]
- Saboktakin, M.R.; Tabatabaie, R.M.; Maharramov, A.; Ramazanov, M. Synthesis and in vitro evaluation of carboxymethyl starch–chitosan nanoparticles as drug delivery system to the colon. Int. J. Biol. Macromol. 2011, 48, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, S.; Ho, P.C. Intranasal administration of carbamazepine-loaded carboxymethyl chitosan nanoparticles for drug delivery to the brain. Asian J. Pharm. Sci. 2017, 13, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Rawal, T.; Parmar, R.; Tyagi, R.K.; Butani, S. Rifampicin loaded chitosan nanoparticle dry powder presents an improved therapeutic approach for alveolar tuberculosis. Colloids Surf. B Biointerfaces 2017, 154, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Jafarinejad, S.; Gilani, K.; Moazeni, E.; Ghazi-Khansari, M.; Najafabadi, A.R.; Mohajel, N. Development of chitosan-based nanoparticles for pulmonary delivery of itraconazole as dry powder formulation. Powder Technol. 2012, 222, 65–70. [Google Scholar] [CrossRef]
- Giovino, C.; Ayensu, I.; Tetteh, J.; Boateng, J. An integrated buccal delivery system combining chitosan films impregnated with peptide loaded PEG-b-PLA nanoparticles. Colloids Surf. B Biointerfaces 2013, 112, 9–15. [Google Scholar] [CrossRef]
- Mazzarino, L.; Travelet, C.; Ortega-Murillo, S.; Otsuka, I.; Pignot-Paintrand, I.; Lemos-Senna, E.; Borsali, R. Elaboration of chitosan-coated nanoparticles loaded with curcumin for mucoadhesive applications. J. Colloid Interface Sci. 2012, 370, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Nayak, D.; Minz, A.P.; Ashe, S.; Rauta, P.R.; Kumari, M.; Chopra, P.; Nayak, B. Synergistic combination of antioxidants, silver nanoparticles and chitosan in a nanoparticle based formulation: Characterization and cytotoxic effect on MCF-7 breast cancer cell lines. J. Colloid Interface Sci. 2016, 470, 142–152. [Google Scholar] [CrossRef]
- Shah, B.R.; Zhang, C.; Li, Y.; Li, B. Bioaccessibility and antioxidant activity of curcumin after encapsulated by nano and Pickering emulsion based on chitosan-tripolyphosphate nanoparticles. Food Res. Int. 2016, 89, 399–407. [Google Scholar] [CrossRef]
- Kaur, R.; Rajput, R.; Nag, P.; Kumar, S.; Rachana; Singh, M. Synthesis, characterization and evaluation of antioxidant properties of catechin hydrate nanoparticles. J. Drug Deliv. Sci. Technol. 2017, 39, 398–407. [Google Scholar] [CrossRef] [Green Version]
- Nallamuthu, I.; Devi, A.; Khanum, F. Chlorogenic acid loaded chitosan nanoparticles with sustained release property, retained antioxidant activity and enhanced bioavailability. Asian J. Pharm. Sci. 2015, 10, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Zhao, Y.; Guan, L.; Zhang, Y.; Dang, Q.; Dong, P.; Li, J.; Liang, X. Preparation of astaxanthin-loaded DNA/chitosan nanoparticles for improved cellular uptake and antioxidation capability. Food Chem. 2017, 227, 9–15. [Google Scholar] [CrossRef]
- Cerchiara, T.; Abruzzo, A.; di Cagno, M.; Bigucci, F.; Bauer-Brandl, A.; Parolin, C.; Vitali, B.; Gallucci, M.; Luppi, B. Chitosan based micro- and nanoparticles for colon-targeted delivery of vancomycin prepared by alternative processing methods. Eur. J. Pharm. Biopharm. 2015, 92, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Coco, R.; Plapied, L.; Pourcelle, V.; Jérôme, C.; Brayden, D.; Schneider, Y.-J.; Préat, V. Drug delivery to inflamed colon by nanoparticles: Comparison of different strategies. Int. J. Pharm. 2013, 440, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.A.; Chaves, P.S.; D’Amore, C.M.; Contri, R.V.; Frank, A.G.; Beck, R.; Pohlmann, A.R.; Buffon, A.; Guterres, S.S. The use of chitosan as cationic coating or gel vehicle for polymeric nanocapsules: Increasing penetration and adhesion of imiquimod in vaginal tissue. Eur. J. Pharm. Biopharm. 2017, 114, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Xia, G.; Bao, Z.; Feng, C.; Cheng, X.; Kong, M.; Liu, Y.; Chen, X. Chitosan based nanoparticles as protein carriers for efficient oral antigen delivery. Int. J. Biol. Macromol. 2016, 91, 716–723. [Google Scholar] [CrossRef] [Green Version]
- Miladi, K.; Sfar, S.; Fessi, H.; Elaissari, A. Enhancement of alendronate encapsulation in chitosan nanoparticles. J. Drug Deliv. Sci. Technol. 2015, 30, 391–396. [Google Scholar] [CrossRef]
- Bagre, A.P.; Jain, K.; Jain, N.K. Alginate coated chitosan core shell nanoparticles for oral delivery of enoxaparin: In vitro and in vivo assessment. Int. J. Pharm. 2013, 456, 31–40. [Google Scholar] [CrossRef]
- Wang, J.; Tan, J.; Luo, J.; Huang, P.; Zhou, W.; Chen, L.; Long, L.; Zhang, L.-M.; Zhu, B.; Yang, L.; et al. Enhancement of scutellarin oral delivery efficacy by vitamin B12-modified amphiphilic chitosan derivatives to treat type II diabetes induced-retinopathy. J. Nanobiotechnol. 2017, 15, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.-C.; Chen, J.-K.; Lam, U.-I.; Chen, S.-Y. Preparing, characterizing, and evaluating chitosan/fucoidan nanoparticles as oral delivery carriers. J. Polym. Res. 2014, 21, 1–9. [Google Scholar] [CrossRef]
- Shi, Y.; Xue, J.; Jia, L.; Du, Q.; Niu, J.; Zhang, D. Surface-modified PLGA nanoparticles with chitosan for oral delivery of tol-butamide. Colloids Surf. B Biointerfaces 2018, 161, 67–72. [Google Scholar] [CrossRef]
- Derakhshandeh, K.; Fathi, S. Role of chitosan nanoparticles in the oral absorption of Gemcitabine. Int. J. Pharm. 2012, 437, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Mukhopadhyay, P.; Kundu, P.P.; Chakraborti, A.S. Alginate coated chitosan core-shell nanoparticles for efficient oral delivery of naringenin in diabetic animals—An in vitro and in vivo approach. Carbohydr. Polym. 2017, 170, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Yan, H.; Yang, H.-J.; Kim, H.W.; Wan, X.; Lee, J.; Ko, S. Synthesis and controlled-release properties of chi-tosan/β-Lactoglobulin nanoparticles as carriers for oral administration of epigallocatechin gallate. Food Sci. Biotechnol. 2016, 25, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Aluani, D.; Tzankova, V.; Kondeva-Burdina, M.; Yordanov, Y.; Nikolova, E.; Odzhakov, F.; Apostolov, A.; Markova, T.; Yoncheva, K. Evaluation of biocompatibility and antioxidant efficiency of chitosan-alginate nanoparticles loaded with quer-cetin. Int. J. Biol. Macromol. 2017, 103, 771–782. [Google Scholar] [CrossRef]
- Ni, S.; Liu, Y.; Tang, Y.; Chen, J.; Li, S.; Pu, J.; Han, L. GABA B receptor ligand-directed trimethyl chitosan/tripolyphosphate nanoparticles and their pMDI formulation for survivin siRNA pulmonary delivery. Carbohydr. Polym. 2017, 179, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Trapani, A.; Di Gioia, S.; Ditaranto, N.; Cioffi, N.; Goycoolea, F.M.; Carbone, A.; Garcia-Fuentes, M.; Conese, M.; Alonso, M.J. Systemic heparin delivery by the pulmonary route using chitosan and glycol chitosan nanoparticles. Int. J. Pharm. 2013, 447, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-W.; Shirley, S.A.; Lockey, R.F.; Mohapatra, S.S. Thiolated chitosan nanoparticles enhance anti-inflammatory effects of intranasally delivered theophylline. Respir. Res. 2006, 7, 112. [Google Scholar] [CrossRef] [Green Version]
- Lytting, E.; Nguyen, J.; Wang, X.; Kissel, T. Biodegradable polymeric nanocarriers for pulmonary drug delivery Biodegradable polymeric nanocarriers for pulmonary drug delivery. Expert Opin. Drug Deliv. 2008, 56, 629–639. [Google Scholar] [CrossRef]
- Wang, X.; Chi, N.; Tang, X. Preparation of estradiol chitosan nanoparticles for improving nasal absorption and brain targeting. Eur. J. Pharm. Biopharm. 2008, 70, 735–740. [Google Scholar] [CrossRef]
- Vila, A.; Sánchez, A.; Janes, K.; Behrens, I.; Kissel, T.; Jato, J.L.V.; Alonso, M.J. Low molecular weight chitosan nanoparticles as new carriers for nasal vaccine delivery in mice. Eur. J. Pharm. Biopharm. 2004, 57, 123–131. [Google Scholar] [CrossRef]
- Soares, P.I.P.; Isabel, A.; Carvalho, J.; Ferreira, I.M.M.; Novo, C.M.M.; Paulo, J. Chitosan-based nanoparticles as drug delivery systems for doxorubicin: Optimization and modelling. Carbohydr. Polym. 2016, 147, 304–312. [Google Scholar] [CrossRef]
- Yostawonkul, J.; Surassmo, S.; Iempridee, T.; Pimtong, W.; Suktham, K.; Sajomsang, W.; Gonil, P.; Ruktanonchai, U.R. Surface modification of nanostructure lipid carrier (NLC) by oleoyl-quaternized-chitosan as a mucoadhesive nanocarrier. Colloids Surf. B Biointerfaces 2016, 149, 301–311. [Google Scholar] [CrossRef]
- Yu, X.; Mu, Y.; Xu, M.; Xia, G.; Wang, J.; Liu, Y.; Chen, X. Preparation and characterization of mucosal adhesive and two-step drug releasing cetirizine-chitosan nanoparticle. Carbohydr. Polym. 2017, 173, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Costa, I.D.S.M.; Abranches, R.P.; Garcia, M.T.J.; Pierre, M.B.R. Chitosan-based mucoadhesive films containing 5-aminolevulinic acid for buccal cancer’s treatment. J. Photochem. Photobiol. B Biol. 2014, 140, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Mazzarino, L.; Borsali, R.; Lemos-Senna, E. Mucoadhesive Films Containing Chitosan-Coated Nanoparticles: A New Strategy for Buccal Curcumin Release. J. Pharm. Sci. 2014, 103, 3764–3771. [Google Scholar] [CrossRef] [PubMed]
- Al-Kassas, R.; Wen, J.; Cheng, A.E.-M.; Kim, A.M.-J.; Liu, S.S.M.; Yu, J. Transdermal delivery of propranolol hydrochloride through chitosan nanoparticles dispersed in mucoadhesive gel. Carbohydr. Polym. 2016, 153, 176–186. [Google Scholar] [CrossRef]
- dos Santos, T.C.; Rescignano, N.; Boff, L.; Reginatto, F.H.; Simões, C.M.O.; de Campos, A.M.; Mijangos, C.U. Manufacture and characterization of chitosan/PLGA nanoparticles nanocomposite buccal films. Carbohydr. Polym. 2017, 173, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Pistone, S.; Goycoolea, F.M.; Young, A.; Smistad, G.; Hiorth, M. Formulation of polysaccharide-based nanoparticles for local administration into the oral cavity. Eur. J. Pharm. Sci. 2017, 96, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Giovino, C.; Ayensu, I.; Tetteh, J.; Boateng, J. Development and characterisation of chitosan films impregnated with insulin loaded PEG-b-PLA nanoparticles (NPs): A potential approach for buccal delivery of macromolecules. Int. J. Pharm. 2012, 428, 143–151. [Google Scholar] [CrossRef]
- Suvannasara, P.; Juntapram, K.; Praphairaksit, N.; Siralertmukul, K.; Muangsin, N. Mucoadhesive 4-carboxybenzenesulfonamide-chitosan with antibacterial properties. Carbohydr. Polym. 2013, 94, 244–252. [Google Scholar] [CrossRef]
- Baptista da Silva, S.; Ferreira, D.; Pintado, M.; Sarmento, B. Chitosan-based nanoparticles for rosmarinic acid ocular delivery—In vitro tests. Int. J. Biol. Macromol. 2016, 84, 112–120. [Google Scholar] [CrossRef]
- Sarjono, P.R.; Putri, L.D.; Budiarti, C.E.; Mulyani, N.S.; Kusrini, D.; Prasetya, N.B. Antioxidant and antibacterial activities of secondary metabolite endophytic bacteria from papaya leaf (Carica papaya L.). IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 012112. [Google Scholar] [CrossRef]
- Zhou, Y.S.; Yang, D.Z.; Chen, X.M.; Xu, Q.; Lu, F.M.; Nie, J. Electrospun water-soluble carboxyethyl chitosan/poly(vinyl al-cohol) nanofibrous membrane as potential wound dressing for skin regeneration. Biomacromolecules 2008, 9, 349–354. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, H.; Liu, X.; Mao, J.; Gu, S.; Xu, W. Electrospinning of carboxyethyl chitosan/poly(vinyl alcohol)/silk fibroin nanoparticles for wound dressings. Int. J. Biol. Macromol. 2012, 53, 88–92. [Google Scholar] [CrossRef]
- Toshkova, R.; Manolova, N.; Gardeva, E.; Ignatova, M.; Yossifova, L.; Rashkov, I.; Alexandrov, M. Antitumor activity of quaternized chitosan-based electrospun implants against Graffi myeloid tumor. Int. J. Pharm. 2010, 400, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Alipour, S.M.; Nouri, M.; Mokhtari, J.; Bahrami, S.H. Electrospinning of poly(vinyl alcohol)–water-soluble quaternized chitosan derivative blend. Carbohydr. Res. 2009, 344, 2496–2501. [Google Scholar] [CrossRef]
- Ignatova, M.; Starbova, K.; Markova, N.; Manolova, N.; Rashkov, I. Electrospun nano-fibre mats with antibacterial properties from quaternised chitosan and poly(vinyl alcohol). Carbohydr. Res. 2006, 341, 2098–2107. [Google Scholar] [CrossRef] [PubMed]
- Ignatova, M.; Manolova, N.; Rashkov, I. Novel antibacterial fibers of quaternized chitosan and poly(vinyl pyrrolidone) pre-pared by electrospinning. Eur. Polym. J. 2007, 43, 1112–1122. [Google Scholar] [CrossRef]
- Bai, B.; Mi, X.; Xiang, X.; Heiden, P.A.; Heldt, C.L. Non-enveloped virus reduction with quaternized chitosan nanofibers containing graphene. Carbohydr. Res. 2013, 380, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Ignatova, M.; Manolova, N.; Markova, N.; Rashkov, I. Electrospun Non-Woven Nanofibrous Hybrid Mats Based on Chitosan and PLA for Wound-Dressing Applications. Macromol. Biosci. 2009, 9, 102–111. [Google Scholar] [CrossRef]
- Chen, H.L.; Huang, J.; Yu, J.H.; Liu, S.Y.; Gu, P. Electrospun chitosan-graft-poly (epsilon-caprolactone)/poly (epsi-lon-caprolactone) cationic nanofibrous mats as potential scaffolds for skin tissue engineering. Int. J. Biol. Macromol. 2011, 48, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Razi, M.A.; Wakabayashi, R.; Goto, M.; Kamiya, N. Self-Assembled Reduced Albumin and Glycol Chitosan Nanoparticles for Paclitaxel Delivery. Langmuir 2019, 35, 2610–2618. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.S.; Morris, A.; Billa, N.; Leong, C.-O. An Evaluation of Curcumin-Encapsulated Chitosan Nanoparticles for Transdermal Delivery. AAPS PharmSciTech 2019, 20, 69. [Google Scholar] [CrossRef]
- Wang, T.; Hou, J.; Su, C.; Zhao, L.; Shi, Y. Hyaluronic acid-coated chitosan nanoparticles induce ROS-mediated tumor cell apoptosis and enhance antitumor efficiency by targeted drug delivery via CD44. J. Nanobiotechnol. 2017, 15, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Esfandiari, F.; Motazedian, M.; Asgari, Q.; Morowvat, M.H.; Molaei, M.; Heli, H. Paromomycin-loaded mannosylated chitosan nanoparticles: Synthesis, characterization and targeted drug delivery against leishmaniasis. Acta Trop. 2019, 197, 105045. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Madni, A.; Torchilin, V.; Filipczak, N.; Pan, J.; Tahir, N.; Shah, H. Lipid-chitosan hybrid nanoparticles for controlled delivery of cisplatin. Drug Deliv. 2019, 26, 765–772. [Google Scholar] [CrossRef] [Green Version]
- Piazzini, V.; Landucci, E.; D’Ambrosio, M.; Fasiolo, L.T.; Cinci, L.; Colombo, G.; Pellegrini-Giampietro, D.E.; Bilia, A.R.; Luceri, C.; Bergonzi, M.C. Chitosan coated human serum albumin nanoparticles: A promising strategy for nose-to-brain drug delivery. Int. J. Biol. Macromol. 2019, 129, 267–280. [Google Scholar] [CrossRef]
- Gomillion, C.T. Assessing the potential of chitosan/polylactide nanoparticles for delivery of therapeutics for tri-ple-negative breast cancer treatment. Regener. Eng. Transl. Med. 2019, 5, 61–73. [Google Scholar]
- Abdelhamid, H.N.; El-Bery, H.M.; Metwally, A.A.; Elshazly, M.; Hathout, R.M. Synthesis of CdS-modified chitosan quantum dots for the drug delivery of Sesamol. Carbohydr. Polym. 2019, 214, 90–99. [Google Scholar] [CrossRef]
- Chen, C.; Yao, W.; Sun, W.; Guo, T.; Lv, H.; Wang, X.; Ying, H.; Wang, Y.; Wang, P. A self-targeting and controllable drug delivery system constituting mesoporous silica nanoparticles fabricated with a multi-stimuli responsive chitosan-based thin film layer. Int. J. Biol. Macromol. 2018, 122, 1090–1099. [Google Scholar] [CrossRef]
- Mohammed, M.; Mansell, H.; Shoker, A.; Wasan, K.M.; Wasan, E.K. Development and in vitro characterization of chi-tosan-coated polymeric nanoparticles for oral delivery and sustained release of the immunosuppressant drug mycophenolate mofetil. Drug Dev. Ind. Pharm. 2019, 45, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mukherjee, S.; Yi, J.; Banerjee, P.; Chen, Q.; Zhou, S. Biocompatible Chitosan–Carbon Dot Hybrid Nanogels for NIR-Imaging-Guided Synergistic Photothermal–Chemo Therapy. ACS Appl. Mater. Interfaces 2017, 9, 18639–18649. [Google Scholar] [CrossRef]
- Belabassi, Y.; Moreau, J.; Gheran, V.; Henoumont, C.; Robert, A.; Callewaert, M.; Rigaux, G.; Cadiou, C.; Elst, L.V.; Laurent, S.; et al. Synthesis and Characterization of PEGylated and Fluorinated Chitosans: Application to the Synthesis of Targeted Nanoparticles for Drug Delivery. Biomacromolecules 2017, 18, 2756–2766. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Zhang, Z.; Luo, Z.; Yu, J.; Liang, R.; Li, X.; Chen, H. Chitosan grafted methoxy poly(ethylene gly-col)-poly(epsilon-caprolactone) nanosuspension for ocular delivery of hydrophobic diclofenac. Sci. Rep. 2015, 5, 11337–11348. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Li, J.; Zhang, C.; Xie, Y.; Qiao, H.; Su, Z.; Oupicky, D.; Ping, Q. Arginine-Modified Nanostructured Lipid Carriers with Charge-Reversal and pH-Sensitive Membranolytic Properties for Anticancer Drug Delivery. Adv. Healthc. Mater. 2017, 6, 1600693–1600705. [Google Scholar] [CrossRef]
- Marques, J.; Valle-Delgado, J.J.; Urban, P.; Baró, E.; Prohens, R.; Mayor, A.; Cisteró, P.; Delves, M.; Sinden, R.E.; Grandfils, C.; et al. Adaptation of targeted nanocarriers to changing requirements in antimalarial drug delivery. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 515–525. [Google Scholar] [CrossRef] [Green Version]
- Luo, L.; Bian, Y.; Liu, Y.; Zhang, X.; Wang, M.; Xing, S.; Li, L.; Gao, D. Combined Near Infrared Photothermal Therapy and Chemotherapy Using Gold Nanoshells Coated Liposomes to Enhance Antitumor Effect. Small 2016, 12, 4103–4112. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Crayton, S.H.; Thawani, J.P.; Amirshaghaghi, A.; Tsourkas, A.; Cheng, Z. A pH-Responsive Drug-Delivery Platform Based on Glycol Chitosan-Coated Liposomes. Small 2015, 11, 4870–4874. [Google Scholar] [CrossRef] [Green Version]
- Romainor, A.N.B.; Chin, S.F.; Pang, S.C.; Bilung, L.M. Preparation and Characterization of Chitosan Nanoparticles-Doped Cellulose Films with Antimicrobial Property. J. Nanomater. 2014, 2014, 710459. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wu, F.; Li, Y.; Yang, X.; Huang, J.; Lv, T.; Zhang, Y.; Chen, J.; Chen, H.; Gao, Y.; et al. Chitosan-based nanoparticles for improved anticancer efficacy and bioavailability of mifepristone. Beilstein J. Nanotechnol. 2016, 7, 1861–1870. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Shen, Y.; Zhao, L. Chitosan nanoparticles loaded with aspirin and 5-fluororacil enable synergistic antitumour activity through the modulation of NF-κB/COX-2 signalling pathway. IET Nanobiotechnol. 2020, 14, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Abu-Zaied, M.A.; Loutfy, S.A.; Hassan, A.E.; Elgemeie, G.H. Novel purine thioglycoside analogs: Synthesis, nanoformulation and biological evaluation in in vitro human liver and breast cancer models. Drug Des. Dev. Ther. 2019, 13, 2437–2457. [Google Scholar] [CrossRef] [Green Version]
- Deepa, G.; Sivakumar, K.C.; Sajeevan, T.P. Molecular simulation and in vitro evaluation of chitosan nanoparticles as drug delivery systems for the controlled release of anticancer drug cytarabine against solid tumours. 3 Biotech 2018, 8, 493. [Google Scholar] [CrossRef]
- Cavalli, R.; Leone, F.; Minelli, R.; Fantozzi, R.; Dianzani, C. New Chitosan Nanospheres for the Delivery of 5-Fluorouracil: Preparation, Characterization and in vitro Studies. Curr. Drug Deliv. 2014, 11, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Sahu, P.; Kashaw, S.K.; Sau, S.; Kushwah, V.; Jain, S.; Agrawal, R.K.; Iyer, A.K. pH Responsive 5-Fluorouracil Loaded Biocompatible Nanogels For Topical Chemotherapy of Aggressive Melanoma. Colloids Surf. B Biointerfaces 2018, 174, 232–245. [Google Scholar] [CrossRef]
- Keerthikumarc, W.; Jalalpure, S.; Mallashwara Rao, P.V.S. Chitosan encapsulated Curcumin nanoparticles as an effective drug delivery system for oral cancer treatment. Indian Drugs. 2015, 52, 40–48. [Google Scholar]
- Shahiwala, A.; Shehab, N.; Khider, M.; Khan, R. Chitosan nanoparticles as a carrier for indigofera intricata plant extract: Prepa-ration, characterization and anticancer activity. Curr. Cancer Ther. Rev. 2019, 15, 162–169. [Google Scholar] [CrossRef]
- Alipour, M.; Bigdeli, M.; Aligholi, H.; Rasoulian, B.; Khaksarian, M. Sustained release of silibinin-loaded chitosan nanoparticle in-duced apoptosis in glioma cells. J. Biomed. Mater. Res. A 2020, 3, 458–469. [Google Scholar] [CrossRef]
- Xu, Y.; Wen, Z.; Xu, Z. Chitosan Nanoparticles Inhibit the Growth of Human Hepatocellular Carcinoma Xenografts through an Antiangiogenic Mechanism. Anticancer Res. 2009, 29, 5103–5109. [Google Scholar] [PubMed]
- Sharifi-Rad, J.; Quispe, C.; Butnariu, M.; Rotariu, L.S.; Sytar, O.; Sestito, S.; Rapposelli, S.; Akram, M.; Iqbal, M.; Krishna, A.; et al. Chitosan nanoparticles as a promising tool in nanomedicine with particular emphasis on oncological treatment. Cancer Cell Int. 2021, 21, 1–21. [Google Scholar] [CrossRef]
- Santoso, A.V.; Susanto, A.; Irawaty, W.; Hadisoewignyo, L.; Hartono, S.B. Chitosan modified mesoporous silica nanoparticles as a versatile drug carrier with pH dependent properties. AIP Conf. Proc. 2019, 2114, 20011. [Google Scholar] [CrossRef]
- Lv, Y.; Li, K.; Li, Y. Surface modification of quantum dots and magnetic nanoparticles with PEG-conjugated chi-tosan derivatives for biological applications. Chem. Pap. 2013, 67, 1404–1413. [Google Scholar] [CrossRef]
- Ai, J.-w.; Liao, W.; Ren, Z.-L. Enhanced anticancer effect of copper-loaded chitosan nanoparticles against osteosarcoma. RSC Adv. 2017, 7, 15971–15977. [Google Scholar] [CrossRef] [Green Version]
- Mazzotta, E.; De Benedittis, S.; Qualtieri, A.; Muzzalupo, R. Actively Targeted and Redox Responsive Delivery of Anticancer Drug by Chitosan. Nanoparticles Pharm. 2020, 12, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, M.; Li, Y.; Yang, X.; Huang, Y.; Wu, H.; Huang, Y.; Lin, J.; Li, Y.; Hou, Z.; Zhang, Q. Development of Both Methotrexate and Mitomycin C Loaded PEGylated Chitosan Nanoparticles for Targeted Drug Codelivery and Synergistic Anticancer Effect. ACS Appl. Mater. Interfaces 2014, 6, 11413–11423. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V. Anti-breast cancer activity of folate-chitosan nanoparticles loaded with irinotecan. Ann. Oncol. 2017, 28, x2–x3. [Google Scholar] [CrossRef]
- Liu, M.; Chang, Y.; Yang, J.; You, Y.; He, R.; Chen, T.; Zhou, C. Functionalized halloysite nanotube by chitosan grafting for drug de-livery of curcumin to achieve enhanced anticancer efficac. J. Mater. Chem. B 2016, 4, 2253–2263. [Google Scholar] [CrossRef]
- Abbas, Y.; Azzazy, H.M.; Tammam, S.; Lamprecht, A.; Ali, M.E.; Schmidt, A.; Sollazzo, S.; Mathur, S. Development of an inhalable, stimuli-responsive particulate system for delivery to deep lung tissue. Colloids Surf. B Biointerfaces 2016, 146, 19–30. [Google Scholar] [CrossRef]
- Almutairi, F.; Abd-Rabou, A.; Mohamed, M.S. Raloxifene-encapsulated hyaluronic acid-decorated chitosan nanoparticles selec-tively induce apoptosis in lung cancer cells. Bioorg. Med. Chem. 2019, 27, 1629–1638. [Google Scholar] [CrossRef]
- Bae, M.S.; Kwon, I.C.; Jeong, S.Y.; Lee, K.Y. Nano-structured chitosan self-aggregates as a drug delivery carrier. In Proceedings of the NSTI Nanotechnology Conference and Trade Show—NSTI Nanotech 2005, Anaheim, CA, USA, 8–12 May 2005. [Google Scholar]
- Wu, S.; Yang, X.; Lu, Y.; Fan, Z.; Li, Y.; Jiang, Y.; Hou, Z. A green approach to dual-drug nanoformulations with targeting and syn-ergistic effects for cancer therapy. Drug Deliv. 2017, 24, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Yang, F.; Yang, R. Synthesis and characterization of chitosan derivatives with dual-antibacterial functional groups. Int. J. Biol. Macromol. 2015, 75, 378–387. [Google Scholar] [CrossRef]
- Roy, K.; Kanwar, R.K.; Kanwar, J.R. Corrigendum to “LNA aptamer based multi-modal, Fe3O4-saturated lactoferrin (Fe3O4-bLf) nanocarriers for triple positive (EpCAM, CD133, CD44) colon tumour targeting and NIR, MRI and CT imaging” [Biomaterials 71C (2015) 84–99]. Biomaterials 2017, 138, 118–120. [Google Scholar] [CrossRef]
- Arunkumar, P.; Indulekha, S.; Vijayalakshmi, S.; Srivastava, R. In vitro comparative studies of Zein nanoparticles and composite Chitosan thermogels based injectable formulation of Doxorubicin. J. Drug Deliv. Sci. Technol. 2017, 40, 116–124. [Google Scholar] [CrossRef]
- Hwang, H.-Y.; Kim, I.-S.; Kwon, I.C.; Kim, Y.-H. Tumor targetability and antitumor effect of docetaxel-loaded hydrophobically modified glycol chitosan nanoparticles. J. Control. Release 2008, 128, 23–31. [Google Scholar] [CrossRef]
- Gomathi, T.; Sudha, P.; Florence, J.A.K.; Venkatesan, J.; Anil, S. Fabrication of letrozole formulation using chitosan nanoparticles through ionic gelation method. Int. J. Biol. Macromol. 2017, 104, 1820–1832. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, W. Optimized preparation of gefitinib chitosan protamine nanoparticles by central composite design-response surface method. Chin. J. New Drugs 2016, 25, 807–812. [Google Scholar]
- Koo, H.; Min, K.; Lee, S.C.; Park, J.; Park, K.; Jeong, S.; Choi, K.; Kwon, I.C.; Kim, K. Enhanced drug-loading and therapeutic efficacy of hydrotropic oligomer-conjugated glycol chitosan nanoparticles for tumor-targeted paclitaxel deliver. J. Control Release 2013, 172, 823–831. [Google Scholar] [CrossRef]
- Maya, S.; Kumar, L.G.; Sarmento, B.; Rejinold, N.S.; Menon, D.; Nair, S.V.; Jayakumar, R. Cetuximab conjugated O-carboxymethyl chitosan nanoparticles for targeting EGFR overexpressing cancer cells. Carbohydr. Polym. 2013, 93, 661–669. [Google Scholar] [CrossRef]
- Al-Musawi, S.; Jawad, A.; Hadi, S.; Hindi, N. Preparation and characterization of folated chitosan/magnetic nanocarrier for 5-fluorouracil drug delivery and studying its effect in bladder cancer therapy. J. Glob. Pharm. Technol. 2019, 11, 628–637. [Google Scholar]
- Anitha, A.; Gopal, D.; Rani, V.V.; Menon, D. Preparation, characterization, in vitro drug release and biological studies of curcumin loaded dextran sulphate-chitosan nanoparticles. Carbohydr. Polym. 2011, 84, 1158–1164. [Google Scholar] [CrossRef]
- Baghbani, F.; Chegeni, M.; Moztarzadeh, F.; Hadian-Ghazvini, S.; Raz, M. Novel ultrasound-responsive chitosan/perfluorohexane nanodroplets for image-guided smart delivery of an anticancer agent: Curcumin. Mater. Sci. Eng. C 2017, 74, 186–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajan, S.S.; Pandian, A.; Palaniappan, T. Curcumin loaded in bovine serum albumin–chitosan derived nanoparticles for targeted drug delivery. Bull. Mater. Sci. 2016, 39, 811–817. [Google Scholar] [CrossRef] [Green Version]
- George, D.; Maheswari, P.U.; Begum, K.M.S. Chitosan-cellulose hydrogel conjugated with L-histidine and zinc oxide nanoparticles for sustained drug delivery: Kinetics and in-vitro biological studies. Carbohydr. Polym. 2020, 236, 116101. [Google Scholar] [CrossRef] [PubMed]
- Chaichanasak, N.; Rojanapanthu, P.; Yoon, Y.; Gritsanapan, W.; Chirachanchai, S.; Sathirakul, K.; Nualsanit, T.; Seong, J.K.; Baek, S.J. Chitosan-based nanoparticles with damnacanthal suppress CRM1 expression. Oncol. Lett. 2018, 16, 7029–7034. [Google Scholar] [CrossRef] [PubMed]
- Sivanesan, I.; Muthu, M.; Gopal, J.; Hasan, N.; Ali, S.K.; Shin, J.; Oh, J.-W. Nanochitosan: Commemorating the Metamorphosis of an ExoSkeletal Waste to a Versatile Nutraceutical. Nanomaterials 2021, 11, 821. [Google Scholar] [CrossRef]
- Sivanesan, I.; Gopal, J.; Muthu, M.; Shin, J.; Mari, S.; Oh, J. Green Synthesized Chitosan/Chitosan Nanoforms/Nanocomposites for Drug Delivery Applications. Polymers 2021, 13, 2256. [Google Scholar] [CrossRef]
- Adhikari, H.S.; Yadav, P.S. Anticancer Activity of Chitosan, Chitosan Derivatives, and Their Mechanism of Action. Int. J. Biomater. 2018, 2018, 2952085. [Google Scholar] [CrossRef] [Green Version]
- Gavhane, Y.N.; Gurav, A.S.; Yadav, A.V. Chitosan and Its Applications: A Review of Literature. Int. J. Res. Pharm. Biomed. Sci. 2013, 4, 312–331. [Google Scholar]
- Nam, K.-S.; Shon, Y.-H. Suppression of metastasis of human breast cancer cells by chitosan oligosaccharides. J. Microbiol. Biotechnol. 2009, 19, 629–633. [Google Scholar]
- Kuppusamy, S.; Karuppaiah, J. Screening of Antiproliferative Effect of Chitosan on Tumor Growth and Metastasis in T24 Urinary Bladder Cancer Cell Line. Austrl-Asian J. Cancer 2013, 12, 145–149. [Google Scholar]
- Hasegawa, M.; Yagi, K.; Iwakawa, S.; Hirai, M. Chitosan Induces Apoptosis via Caspase-3 Activation in Bladder Tumor Cells. Jpn. J. Cancer Res. 2001, 92, 459–466. [Google Scholar] [CrossRef]
- Jiang, Z.; Han, B.; Li, H.; Yang, Y.; Liu, W. Carboxymethyl chitosan represses tumor angiogenesis in vitro and in vivo. Carbohydr. Polym. 2015, 129, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Atyabi, F.; Dinarvand, R.; Ostad, S.N. Chitosan-Pluronic nanoparticles as oral delivery of anticancer gemcitabine: Preparation and in vitro study. Int. J. Nanomed. 2012, 7, 1851–1863. [Google Scholar]
- Qi, L.; Xu, Z.; Chen, M. In vitro and in vivo suppression of hepatocellular carcinoma growth by chitosan nanoparticles. Eur. J. Cancer 2007, 43, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, T.; Ruttala, H.B.; Chitrapriya, N.; Poudal, B.K.; Choi, J.Y.; Kim, S.T.; Youn, Y.S.; Ku, S.K.; Choi, H.-G.; Yong, C.S.; et al. Engineering of cell microenvironment-responsive polypeptide nanovehicle co-encapsulating a synergistic combination of small molecules for effective chemotherapy in solid tumors. Acta Biomater. 2017, 48, 131–143. [Google Scholar] [CrossRef]
- Zeng, Z.W.; Wang, J.J.; Xiao, R.Z.; Xie, T.; Zhou, G.L.; Zhan, X.R.; Wang, S.L. Recent advances of chitosan nanoparticles as drug carriers. Int. J. Nanomed. 2011, 6, 765–774. [Google Scholar] [CrossRef] [Green Version]
- Casettari, L.; Illum, L. Chitosan in nasal delivery systems for therapeutic drugs. J. Control. Release 2014, 190, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.-Q.; Hu, Y.-L.; Wang, Q.; Han, F.; Shao, J.Z. Toxicity evaluation of biodegradable chitosan nanoparticles using a zebrafish embryo model. Int. J. Nanomed. 2011, 6, 3351–3359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, K.H.G.; Cosgrove, C.; McNeela, E.A.; Sexton, A.; Giemza, R.; Jabbal-Gill, I.; Church, A.; Lin, W.; Illum, L.; Podda, A.; et al. Protective Levels of Diphtheria-Neutralizing Antibody Induced in Healthy Volunteers by Unilateral Priming-Boosting Intranasal Immunization Associated with Restricted Ipsilateral Mucosal Secretory Immunoglobulin A. Infect. Immun. 2003, 71, 726–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Kamary, S.S.; Pasetti, M.F.; Mendelman, P.M.; Frey, S.E.; Bernstein, D.I.; Treanor, J.J.; Ferreira, J.; Chen, W.H.; Sublett, R.; Richardson, C.; et al. Adjuvanted Intranasal Norwalk Virus-Like Particle Vaccine Elicits Antibodies and Antibody-Secreting Cells That Express Homing Receptors for Mucosal and Peripheral Lymphoid Tissues. J. Infect. Dis. 2010, 202, 1649–1658. [Google Scholar] [CrossRef]
- Ramirez, K.; Wahid, R.; Richardson, C.; Bargatze, R.F.; El-Kamary, S.S.; Sztein, M.B.; Pasetti, M.F. Intranasal vaccination with an adjuvanted Norwalk virus-like particle vaccine elicits antigen-specific B memory responses in human adult volunteers. Clin. Immunol. 2012, 144, 98–108. [Google Scholar] [CrossRef]
- Islam, N.; Ferro, V. Recent advances in chitosan-based nanoparticulate pulmonary drug delivery. Nanoscale 2016, 8, 14341–14358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aragao-Santiago, L.; Hillaireau, H.; Grabowski, N.; Mura, S.; Nascimento, T.L.; Dufort, S.; Coll, J.-L.; Tsapis, N.; Fattal, E. Compared in vivo toxicity in mice of lung delivered biodegradable andnon-biodegradable nanoparticles. Nanotoxicology 2016, 10, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Marks, E.; Schneider, J.J.; Keely, S. Advances in oral nano-delivery systems for colon targeted drug delivery in in-flammatory bowel disease: Selective targeting to diseased versus healthy tissue. Nanomedicine 2015, 11, 1117–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grenha, A.; Grainger, C.I.; Dailey, L.A.; Seijo, B.; Martin, G.P.; Remuñán-López, C.; Forbes, B. Chitosan nanoparticles are compatible with respiratory epithelial cells in vitro. Eur. J. Pharm. Sci. 2007, 31, 73–84. [Google Scholar] [CrossRef]
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesàro, A. “The good, the bad and the ugly” of chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef] [Green Version]
- Key, J.; Park, K. Multicomponent, Tumor-Homing Chitosan Nanoparticles for Cancer Imaging and Therapy. Int. J. Mol. Sci. 2017, 18, 594. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, X.; Ji, J.; Liu, A.; Zhai, G. Tumor targeting strategies for chitosan-based nanoparticles. Colloids Surfaces B: Biointerfaces 2016, 148, 460–473. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Aljaeid, B.M. Preparation, characterization and potential application of chitosan, chitosan derivatives and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Devel. Ther. 2016, 10, 483–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swierczewska, M.; Han, H.; Kim, K.; Park, J.; Lee, S. Polysaccharide-based nanoparticles for theranostic nanomedicine. Adv. Drug Deliv. Rev. 2015, 99, 70–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-Based Nanomaterials for Drug Delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, I.; Yehye, W.A.; Etxeberria, A.E.; Alhadi, A.A.; Dezfooli, S.M.; Julkapli, N.B.M.; Basirun, W.J.; Seyfoddin, A. Nanoantioxidants: Recent Trends in Antioxidant Delivery Applications. Antioxidants 2019, 9, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
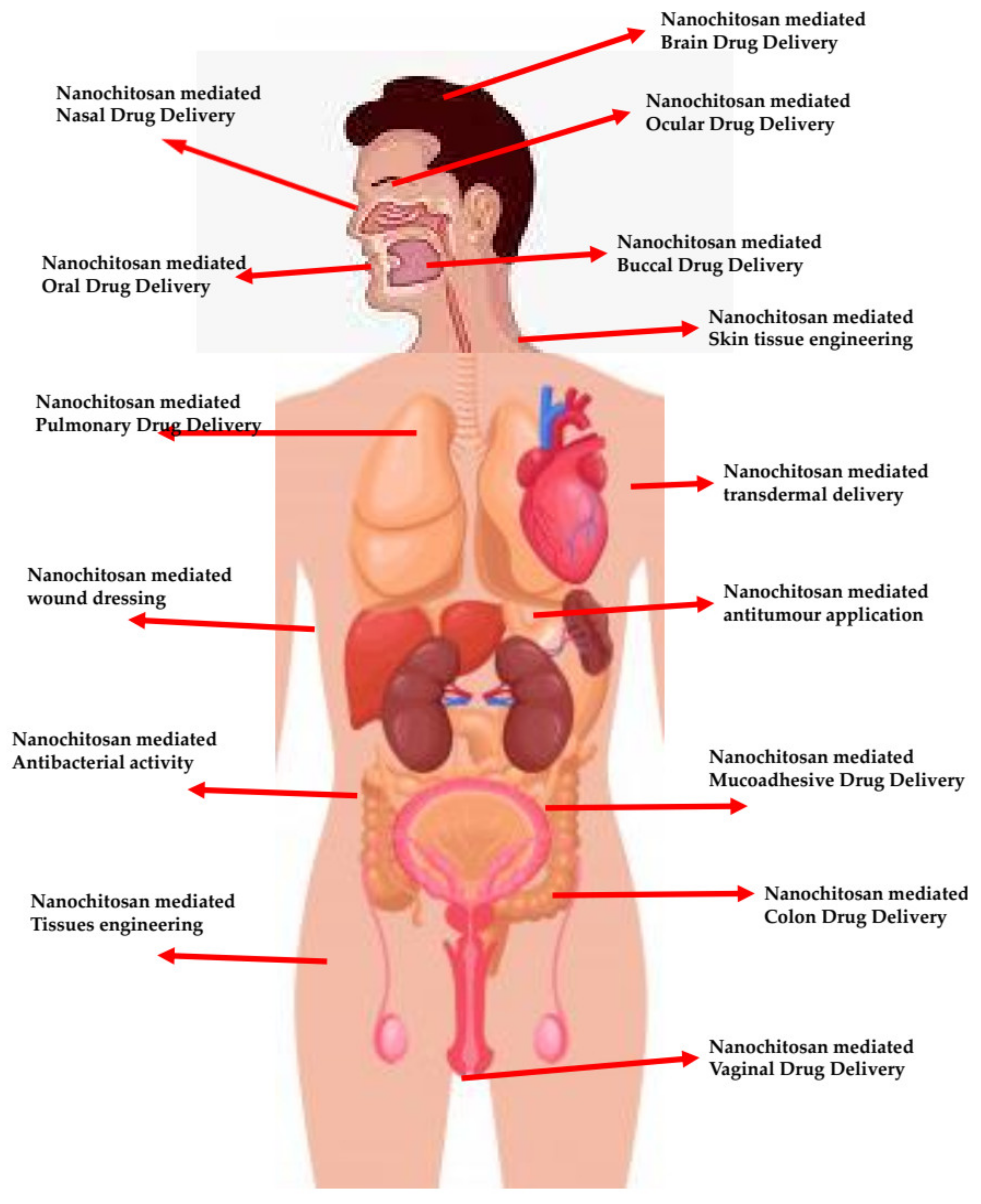
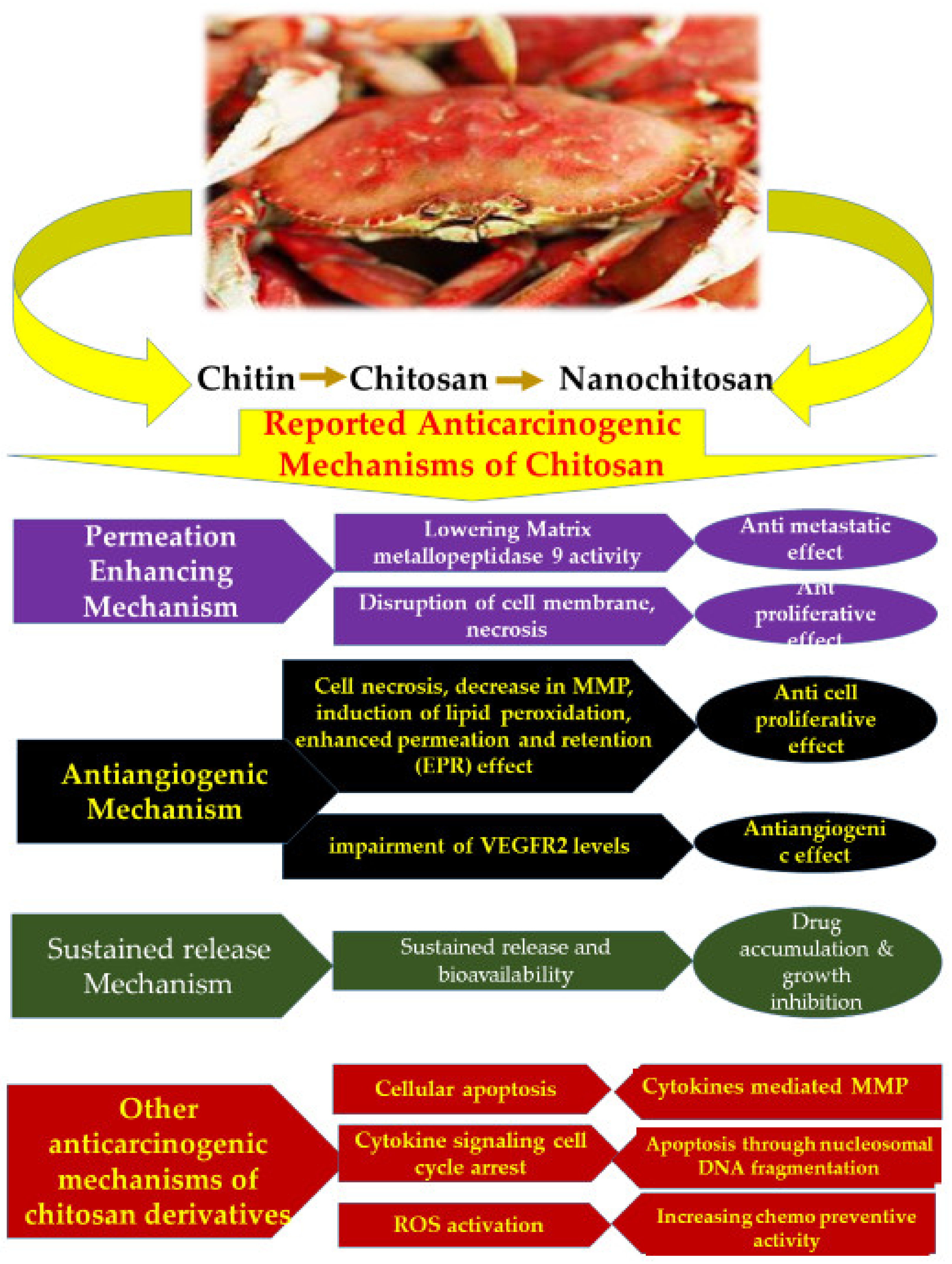
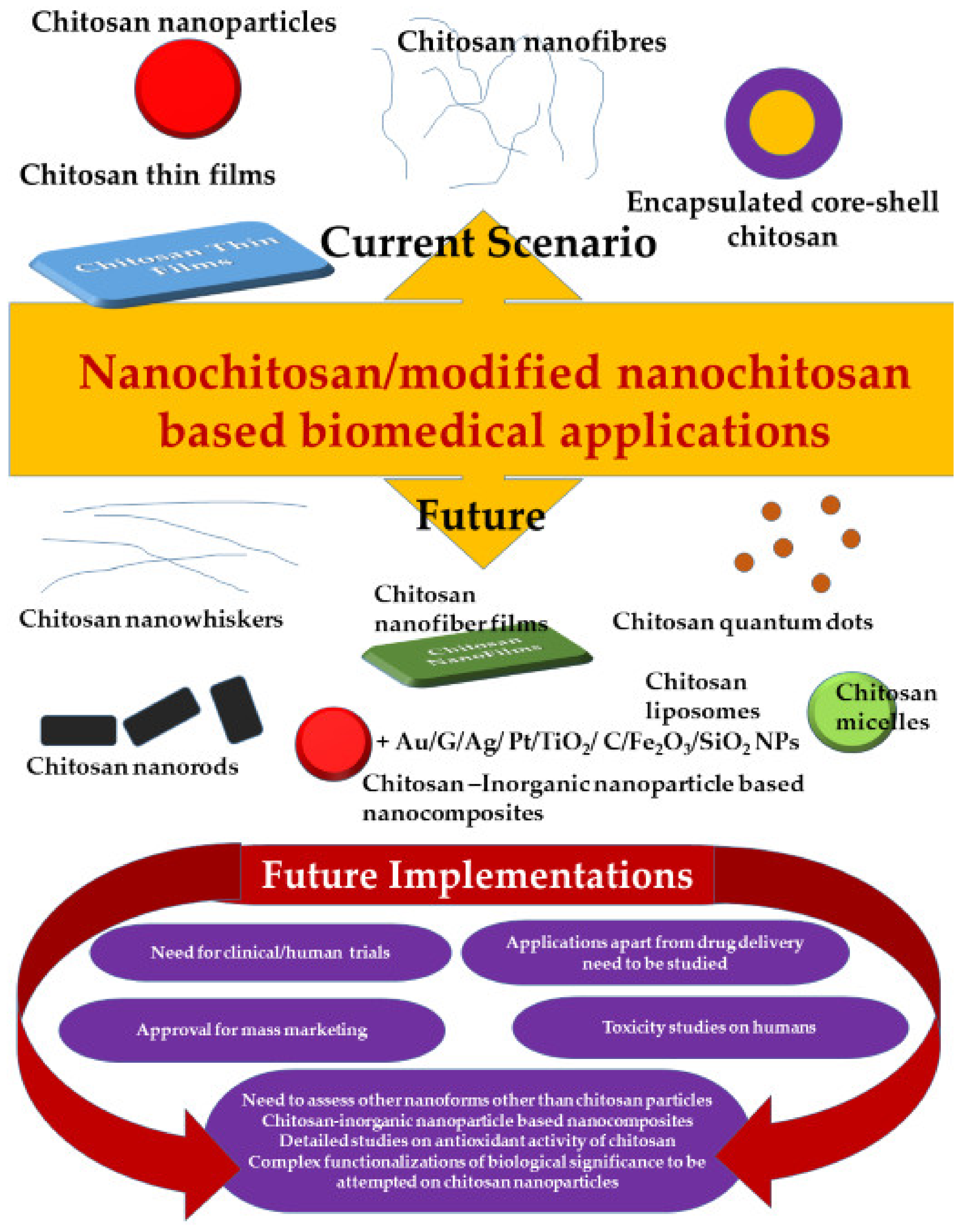
| Functionalized/Surface Modified/Encapsulated Chitosan Nanomaterial | Purpose | Application | Reference |
|---|---|---|---|
| Chitosan, soybean lecithin nanoparticles | Oral drug delivery | Intestinal permeation of tamoxifen through the rat intestinal wall | [68] |
| Chitosan nanoparticles | Oral drug delivery | Sunitinib drug delivery | [70] |
| LMW chitosan NP | Oral drug delivery | Solubility and bioavailability of Hydrophobic Bay41-4109 in rats | [72] |
| Chitosan, TPP | Oral delivery of insulin | Decreased glycaemia in diabetic rats after administering insulin-chitosan nanoparticles, in vivo | [71] |
| Chitosan HCl, Poloxamer 188, sodium glycolate, gelatin, soya lecithin | Oral delivery of Cyclosporin-A | Beagle dogs showed relative bioavailability of Cy-A was significantly increased by chitosan nanoparticles, in vivo. | [64] |
| Chitosan carboxymethyl chitosan | oral antigen delivery in fish vaccination | Extra cellular products (ECPs) of Vibrio anguillarum | [87] |
| Chitosan LMW, sodium tripolyphosphate (TPP), fluorenyl-methyloxycarbonyl chloride (FMOC) | Oral drug delivery | Chitosan nanoparticles released Alendronate sodium faster in 0.1 N HCl compared to PBS | [88] |
| Chitosan LMW, sodium tripolyphos-phate, tris[2-carboxyethyl] phosphine hydrochloride (TCEP) | Oral drug delivery | Enhanced intestinal absorption of catechins | [67] |
| Chitosan, STPP, sodium alginate | Oral drug delivery | Alginate coated chitosan nanoparticles containing enoxaparin for oral controlled release | [89] |
| Chitosan, deoxycholic acid, vitamin B12 | Oral drug delivery | Enhancement of scutellarin oral delivery | [90] |
| Chitosan, Tc-methylene di-phosphonate | Oral drug delivery | Chitosan nanoparticles/F nanoparticles stable in the stomach and decompose in the intestine | [91] |
| Chitosan, PLGA, streptozotocin | Oral drug delivery of Tolbutamide | PLGA nanoparticles modified with chitosan to form TOL-CS-PLGA NPs to improve bioavailability and reduce dose frequency | [92] |
| Chitosan LMW, penta sodium tripolyphos-phate | Oral drug release | Gemcitabine-loaded chitosan nanoparticles (Gem- Chitosan nanoparticles) for oral bio-availability enhancement | [93] |
| Sodium alginate, chitosan, streptozotocin | Naringenin nanoparticles have better efficacy in lowering blood glucose levels compare to free drug | Alginate coated chitosan core shell nanoparticles for effective oral delivery | [94] |
| N-carboxymethyl chitosan, chitosan hydrochloride | Oral drug delivery | EGCG-chitosan/β-Lg NPs to achieve prolonged release during oral administration in gastrointestinal tract | [95] |
| Chitosan, sodium alginate, sodium pyruvate, l-glutamine | Oral drug delivery | Quercetin-chitosan/alginate nanoparticles high antioxidant property no systemic toxicity | [96] |
| Chitosan nanoparticles -TPP, lactose, Tween 80 | Oral drug delivery | 90% release of RFM from Chitosan nanoparticles within 24 h, in vitro | [75] |
| Hydroxypropyl-beta-cyclodextrin (HPβCD), mannitol, lactose, TPP, l-leucine | Pulmonary delivery | Chitosan nanoparticles for pulmonary delivery of itraconazole as a dry powder formulation | [76] |
| N,N,N-tri-methyl chitosan, TPP | Pulmonary drug delivery | Cellular uptake of Bac-TMC3/TPP/siRNA nananoparticles greatly enhanced by clathrin -mediated cellular uptake pathway | [97] |
| Chitosan, lipoid S100, glycol chitosan | Pulmonary drug delivery | LMWH chitosan and glycol Chitosan nanoparticles for enhancing pulmonary absorption of LMW heparin | [98] |
| Chitosan thioglycolic acid, TPP | Pulmonary drug delivery | Theophylline-thiolated Chitosan nanoparticles enhances theophylline’s capacity to alleviate allergic asthma | [99] |
| Thiolated chitosan | Pulmonary drug delivery | In vitro slow and sustained release of leuprolide from thiolated chitosan about 43% in 2 h | [100] |
| Chitosan, methylated β-cyclodextrin, TPP | Intranasal administration | Estradiol-chitosan nanoparticles for improving nasal absorption and brain targeting | [101] |
| LMW Chitosan, TPP, trehalose | Intranasal immunization | Tetanus toxoid chitosan nanoparticles (TT-CS NPs) as a new long-term nasal vaccine delivery vehicle | [102] |
| Chitosan, 4-CBS, TPP, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide HCl (EDAC) | mucoadhesive drug delivery | In vitro drug release of DOX loaded 4-CBS-chitosan/PLA nanoparticles showed sustained release up to 26 days | [103] |
| Chitosan (MW = 600 kDa), methane-sulfonic acid, oleoyl chloride, sodium bicarbonate, glycidyl-trimethyl ammonium chloride | oral administration with enhanced mucoadhesion | In vivo toxicology study was performed in zebrafish embryos | [104] |
| Chitosan, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC. HCl), N-hydroxyl succinimide | mucoadhesive drug delivery | Mucosal adhesion and drug release of cetirizine-chitosan | [105] |
| Chitosan, lactic acid | mucoadhesive drug delivery | Chitosan-based 5-ALA mucoadhesive film to enhance its retention in oral mucosa | [106] |
| Chitosan Low, polycaprolactone, glycerol nanoparticle | mucoadhesive drug delivery | Mucoadhesive films containing curcumin-loaded nanoparticles to prolong the residence time in the oral cavity and to increase drug absorption through the buccal mucosa | [107] |
| Chitosan, TPP, Carbopol 940, poloxamer 407 | Drug delivery | Propranolol-chitosan nanoparticles of transdermal gels to improve the systemic bioavailability of the drug | [108] |
| Resomer PLGA, ploxamer 188, sorbitan monoaleate, chitosan | flavonoid enriched cytotoxic film | EFF-Cg nanocomposites chitosan film containing PLGA NPs, showed low toxicity | [109] |
| Chitosan, TPP, Triton X-100 | Oral drug delivery of alginate and pectin | Preparation of alginate and pectin chitosan nanoparticles for oral drug delivery | [110] |
| Chitosan MMW, PEG, PVP, trehalose | Insulin release | Chitosan films with insulin loaded PEG-b-PLA nanoparticles with sustained release | [111] |
| Chitosan buccal films of insulin loaded poly (ethylene glycol) methyl ether-block-polylactide (PEG-b-PLA) NP | Insulin release | Excellent mucoadhesive properties and insulin release | [77] |
| Polycaprolactone nanoparticles coated with chitosan | Buccal delivery | Delivery of curcumin | [57,78] |
| EFF-Cg loaded PLGA nanoparticles as chitosan films. | Buccal delivery | The bioavailability of EFF-Cg was improved and no signs of cytotoxicity were seen | [78] |
| Conjugating C2-N position of chitosan with aromatic sulfonamide, 4-carboxybenzenesulfonamide-chitosan (4-CBS-chitosan) | drug release in small intestine | Mucoadhesive property of chitosan in stomach acidic environment increased | [57] |
| Entrapping ovalbumin (OVA) into Eudragit S 100, trimethylchitosan, PLGA, PEG-PLGA and PEG-PCL | inflamed colon drug delivery | Nanoparticles with trimethyl chitosan have shown the highest permeability of OVA. And high permeability | [84] |
| chitosan-carboxymethyl starch nanoparticles of 5-aminosalicylic acid | Drug delivery for inflammatory bowel disease | Controlled drug release | [85] |
| Rosmarinic acid loaded chitosan nanoparticles | ocular delivery | The nanoparticles showed no cytotoxicity against the retinal pigment epithelium nor the human cornea cell line. | [112] |
| Chitosan coated PCL nanocapsules embedded in hydroethylcellulose gel | vaginal delivery to treat human papillomavirus infection | Imiquimod formulated chitosan coated PCL nanocapsules embedded in hydroethylcellulose gel | [86] |
| PCL nanocapsules embedded in chitosan hydrogel | vaginal delivery to treat human papillomavirus infection | Imiquimod delivery to vagina | [113] |
| Limonene coated in chitosan | Enhancing antioxidant activity | Limonene-chitosan encapsulation has antioxidant activity with IC50 value of 116 ppm | [114] |
| Carboxymethyl chitosan nanofibres PEO and PVA-Ag | Biomedical application | Antibacterial | [115] |
| Carboxymethyl chitosan nanofibres—PVA\PVA\silk fibroin | Biomedical application | Wound dressing | [116] |
| Quaternized chitosan nanofibres-coPLA/DOX/PLA | Biomedical application | Antitumor | [117] |
| Quaternized chitosan nanofibres—PVA/PVP | Biomedical application | Antibacterial | [118,119,120] |
| Quaternized chitosan nanofibres—graphene | Biomedical application | Virus removal | [121] |
| Quaternized chitosan nanofibres—PLA | Biomedical application | Wound dressing | [122] |
| Poly-3-caprolactonegra chitosan nanofibres | Biomedical application | Skin tissue engineering | [123] |
| Chitosan/Albumin Nanoparticles | Drug delivery | Used as a hydrophobic drug nanocarrier in pharmaceutical and medical applications | [124] |
| Chitosan/Curcumin nanoparticles | Drug delivery | Transdermal delivery | [125] |
| Chitosan/Sodium Nitrate nanoparticle | Drug delivery | Delivery of DOX | [103] |
| Chitosan/HA nanoparticle | Drug encapsulation | Used to encapsulate a chemotherapeutic drug | [126] |
| Chitosan/Paromomycin nanoparticle | Anti leishmaniasis | Treatment of leishmaniasis, especially when the current drugs are impaired by resistance | [127] |
| Chitosan/Lipid Hybrid nanoparticles | Drug delivery | Controlled delivery of cisplatin | [128] |
| Chitosan/Human serum albumin nanoparticle | Drug delivery | Nose-to-brain drug delivery | [129] |
| Chitosan/Polylactide nanoparticle | Drug delivery | Delivery of therapeutics for triple-negative breast cancer treatment | [130] |
| Chitosan/Cadmium Quantum Dots | Drug delivery | Drug delivery of Sesamol | [131] |
| Chitosan/Silica Nanoparticles Thin Film | Drug delivery | DOX delivery | [132] |
| Chitosan/PVA nanoparticle | Oral delivery | Sustained release of the immunosuppressant drug mycophenolate mofetil | [133] |
| Chitosan-carbon dot hybrid nanogel | Anticancer activity | Photothermal therapy-chemo | [134] |
| PEGylated and fluorinated chitosan nanogel | Drug delivery | Targeted drug delivery | [135] |
| Chitosan grafted MPEG-PCL micelles | Drug delivery | Ocular delivery of hydrophobic drug | [136] |
| Arginine-modified nanostructured lipid carriers | Drug delivery | Anticancer drug delivery | [137] |
| Glycosaminoglycan modified chitosan liposome | Drug delivery | Antimalarial | [138] |
| Gold nanoshell-coated liposomes | Anticancer | Photothermal and chemotherapy | [139] |
| Glycol chitosan-coated liposomes | Drug delivery | pH-responsive drug-delivery | [140] |
| Chitosan nanoparticles-doped cellulose films | Antibacterial activity | Inhibition of Escherichia coli | [141] |
| Test | Chitosan Form | Target Cell Line | Mode of Action | Reference |
|---|---|---|---|---|
| In vitro and in vivo | Chitosan | MDA-MB-231 | Permeation enhancement, lowering of matrix metallopeptidase 9 activity leading to antimetastatic effect | [180,181] |
| In vitro | Chitosan | T24 urinary bladder cell lines | Disruption of cell membrane, necrosis resulting in antiproliferative effect | [182,183] |
| In vitro | Chitosan nano particles | Human hepato carcinoma | Antiangiogenic effect through, antiangiogenic action of chitosan nanoparticles and impairment of vascular endothelial growth factor (VEGFR) 2 levels | [184,185] |
| In vitro | Chitosan nano particles | BEL7402, HT-29 | Cell necrosis, lipid peroxidation, decrease in MMP, enhanced permeation and retention (EPR) effect, resulting in inhibition of cellular proliferation | [155,186,187,188,189] |
| In vivo | Mifepristone (MIF) loaded chitosan nano particles | Solid tumor | Sustained release and enhancement of bioavailability of drug. Drug accumulation and growth inhibition | [190] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.-W.; Shin, J.; Chun, S.; Muthu, M.; Gopal, J. Evaluating the Anticarcinogenic Activity of Surface Modified/Functionalized Nanochitosan: The Emerging Trends and Endeavors. Polymers 2021, 13, 3138. https://doi.org/10.3390/polym13183138
Oh J-W, Shin J, Chun S, Muthu M, Gopal J. Evaluating the Anticarcinogenic Activity of Surface Modified/Functionalized Nanochitosan: The Emerging Trends and Endeavors. Polymers. 2021; 13(18):3138. https://doi.org/10.3390/polym13183138
Chicago/Turabian StyleOh, Jae-Wook, Juhyun Shin, Sechul Chun, Manikandan Muthu, and Judy Gopal. 2021. "Evaluating the Anticarcinogenic Activity of Surface Modified/Functionalized Nanochitosan: The Emerging Trends and Endeavors" Polymers 13, no. 18: 3138. https://doi.org/10.3390/polym13183138
APA StyleOh, J.-W., Shin, J., Chun, S., Muthu, M., & Gopal, J. (2021). Evaluating the Anticarcinogenic Activity of Surface Modified/Functionalized Nanochitosan: The Emerging Trends and Endeavors. Polymers, 13(18), 3138. https://doi.org/10.3390/polym13183138








