Oxidative Stress and Inflammation, MicroRNA, and Hemoglobin Variations after Administration of Oxygen at Different Pressures and Concentrations: A Randomized Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Selections of Participants
- aged between 18 and 50 years;
- active recreationally athlete: subject involved in a programmed training routine in different mixed sports requiring 3/4 training sessions/week at a medium intensity of 70% of maximal heart rate (calculated with 211 − 0.64 × age [33]) measured by a commercial heart rate monitor (Polar M430, Polar Electro Inc., Kempele, Finland);
- non-smoker.
2.2. Experimental Protocol
- Arm 1 (control): no intervention;
- Arm 2 (30% O2): breathing normobaric air mixture with 30% oxygen for 40 min (at rest);
- Arm 3 (50% O2): breathing normobaric air mixture with 50% oxygen for 40 min (at rest);
- Arm 4: treated with 100% oxygen at 1.45 atmosphere absolute (ATA) (hereafter: 1.5 ATA) for 60 min (2 periods of 25 min each, separated by air breaks of 5 min; inclusive of compression and decompression times);
- Arm 5: treated with 100% oxygen at 2.45 ATA (hereafter: 2.5 ATA) for 90 min (3 periods of 25 min each, separated by air breaks of 5 min each; inclusive of compression and decompression times).
2.3. Measurements and Data Collection
2.3.1. Oxidative Stress and Oxidative Damage
2.3.2. Isoprostane
2.3.3. Nitrite and Nitrate Levels (NOx)
2.3.4. Inducible Nitric Oxide Synthase (iNOS)
2.3.5. Inflammatory Status
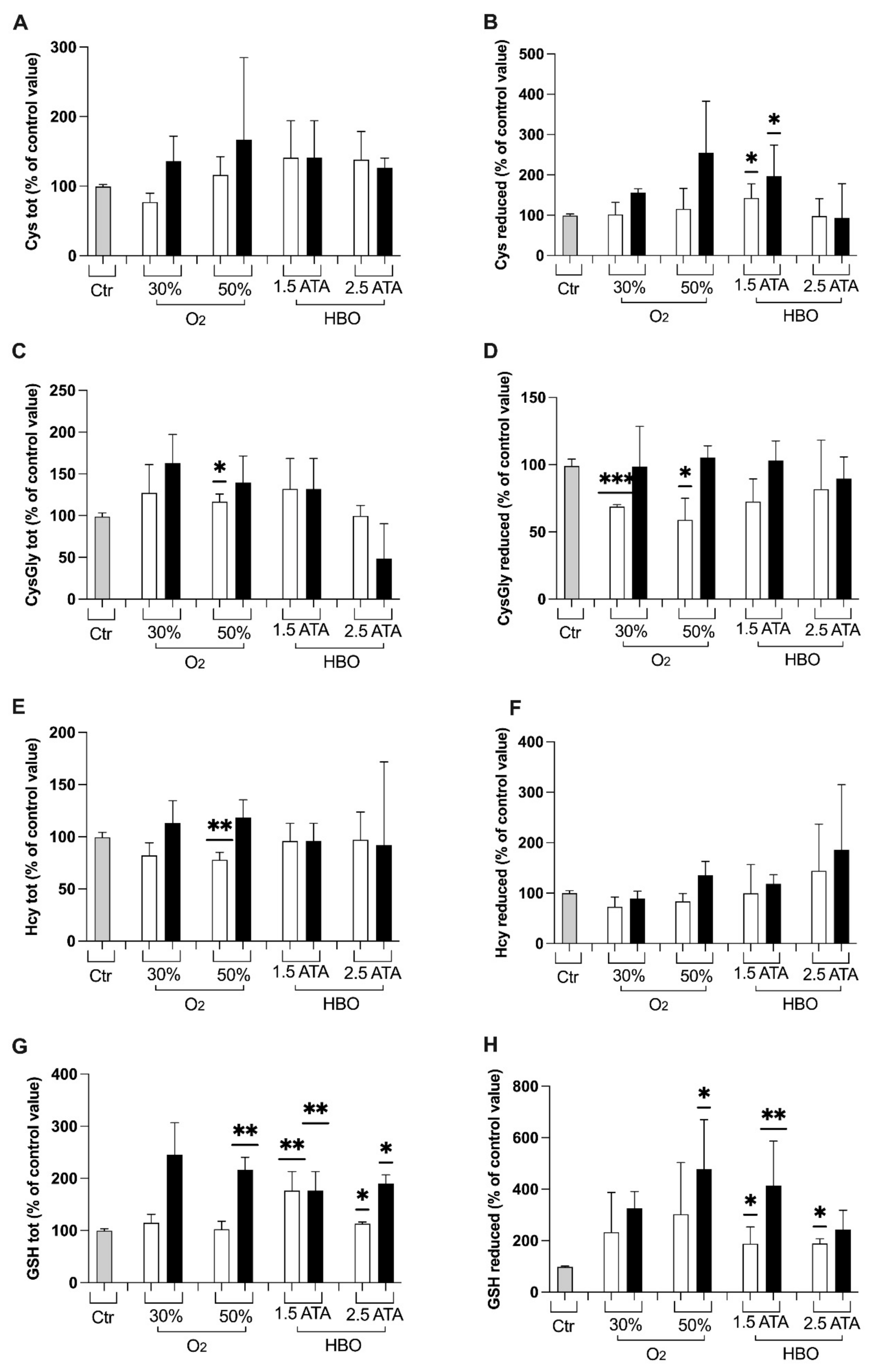
2.3.6. Thiols
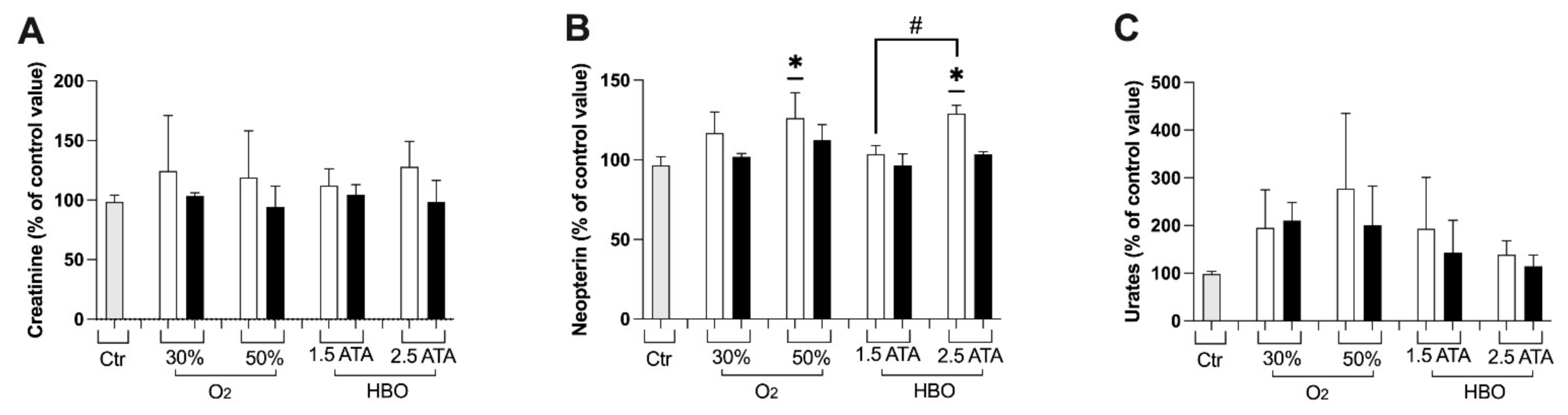
2.3.7. Creatinine, Neopterin and Uric Acid Concentration
2.3.8. Total RNA and miRNA Isolation
2.3.9. Hemoglobin
2.4. Modifications to the Protocol
2.5. Statistical Analysis
3. Results
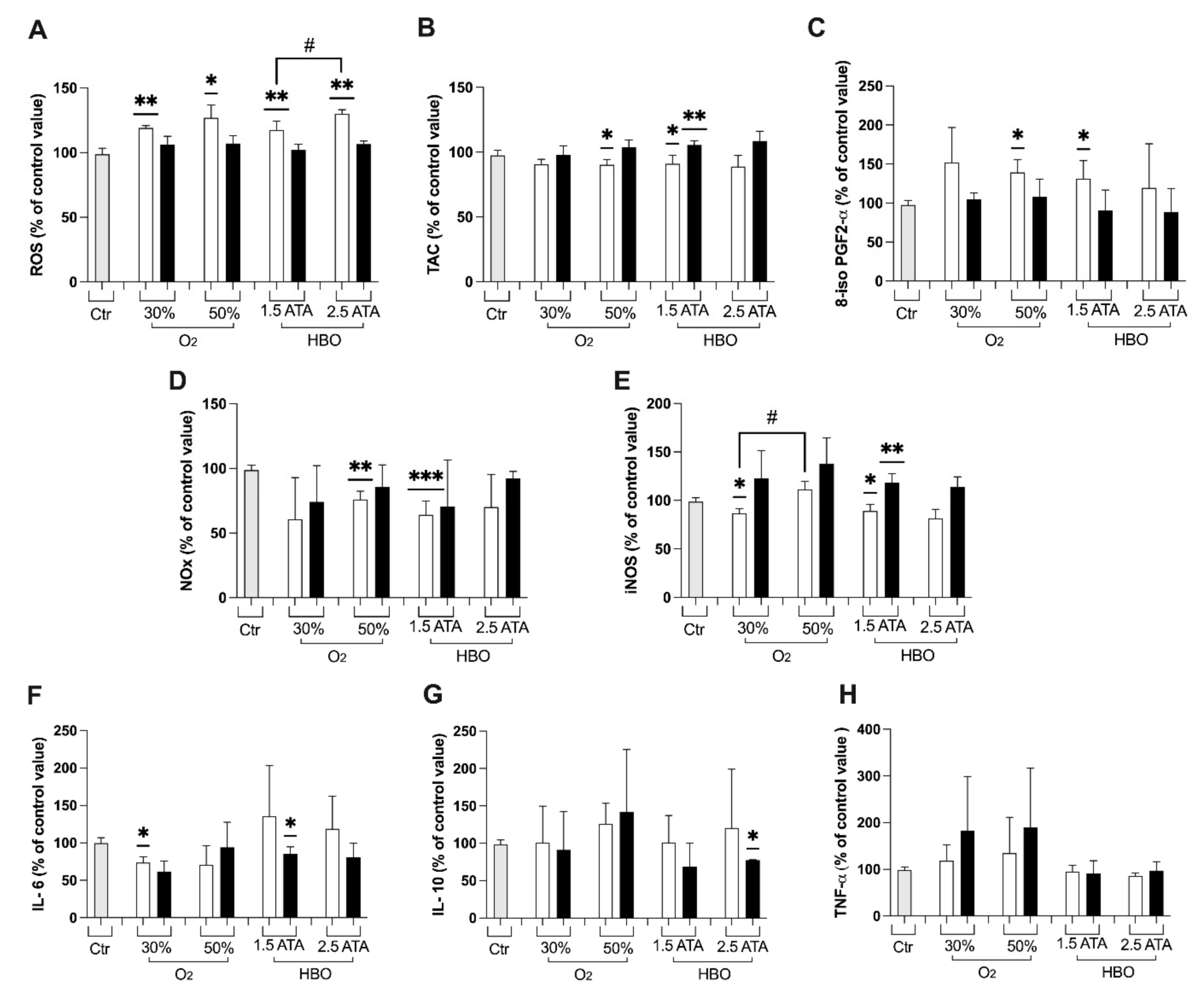
3.1. Oxidative Stress, Nitric Oxide, and Inflammation Status
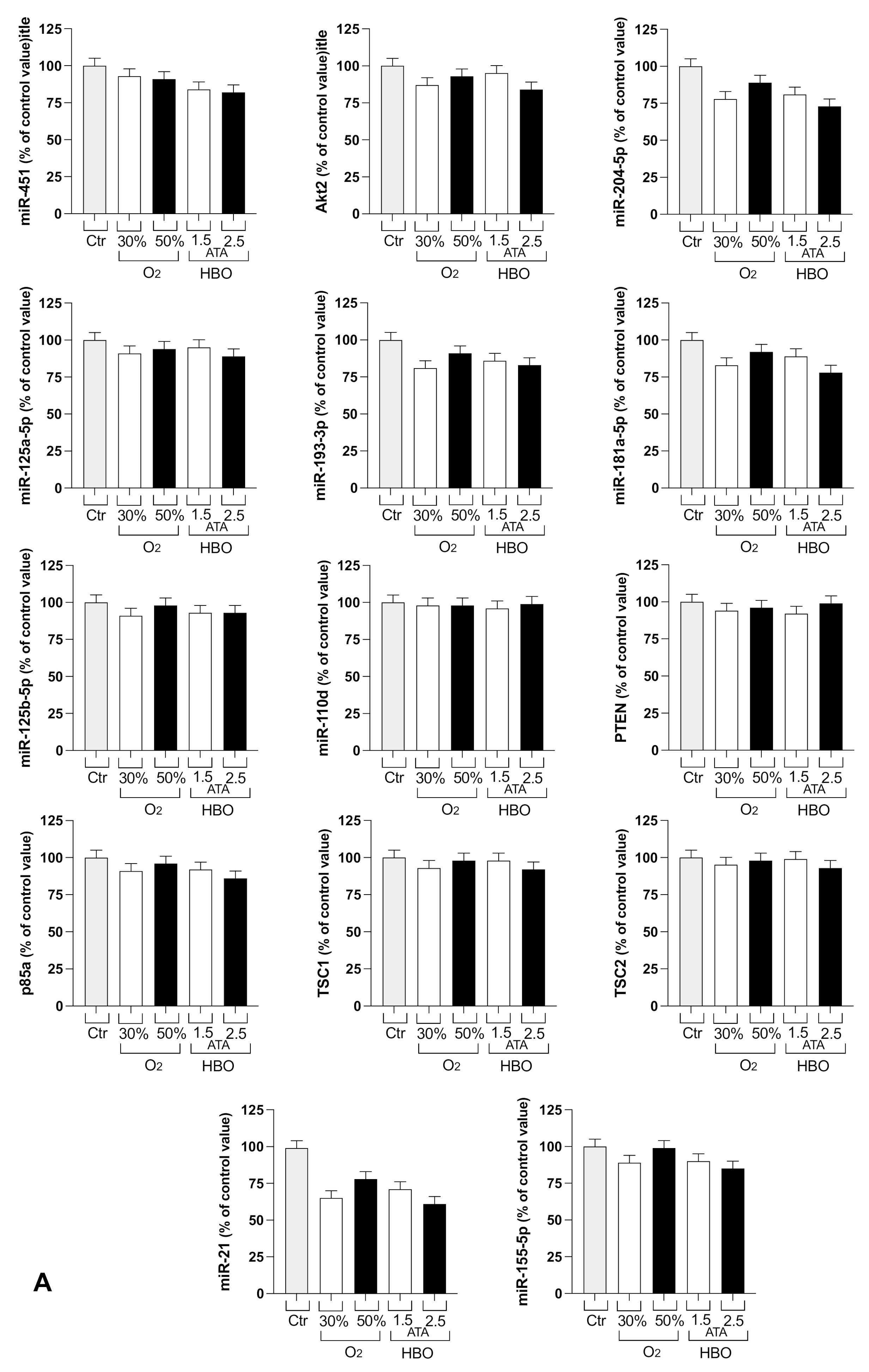
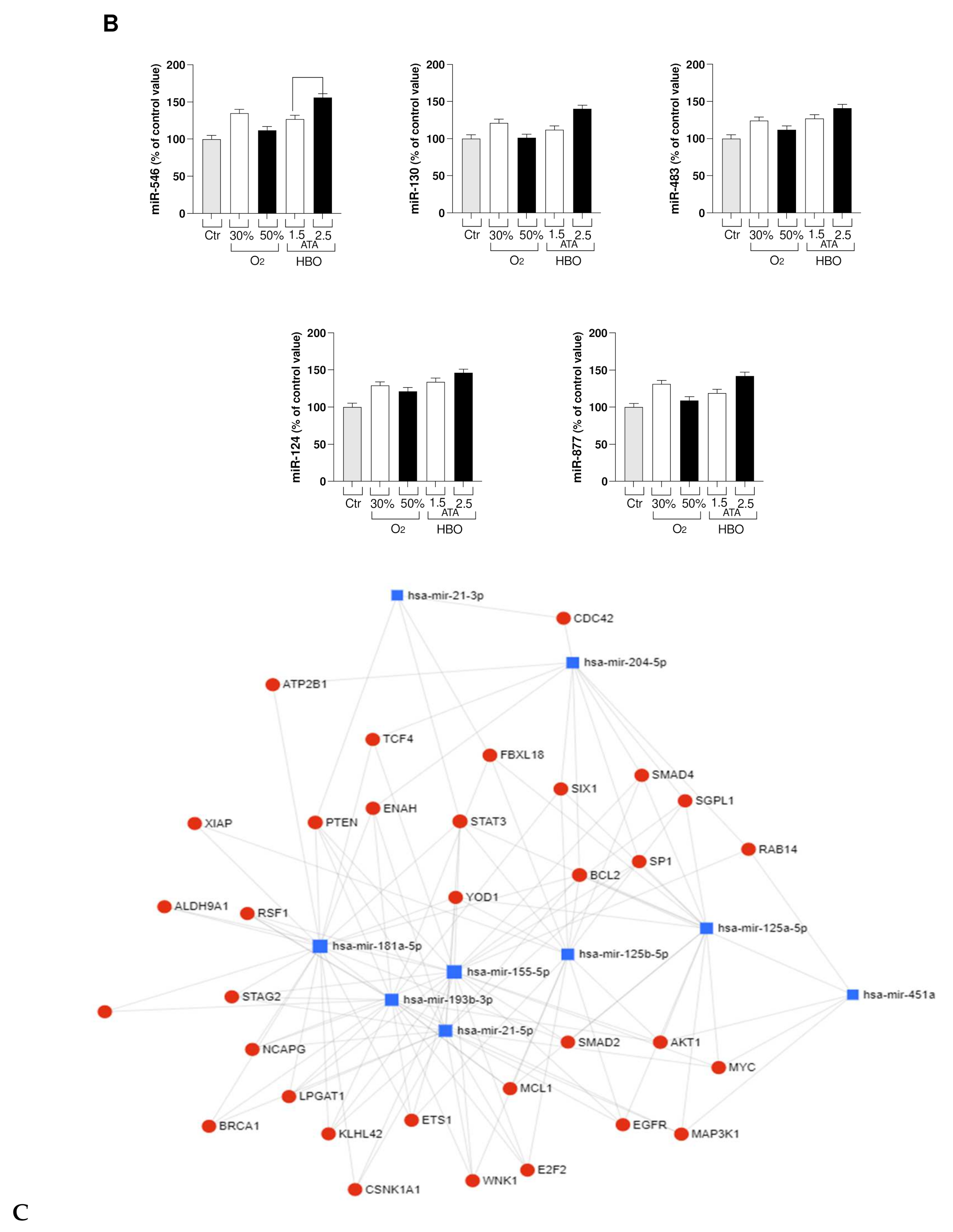
3.2. Circulating miRNA
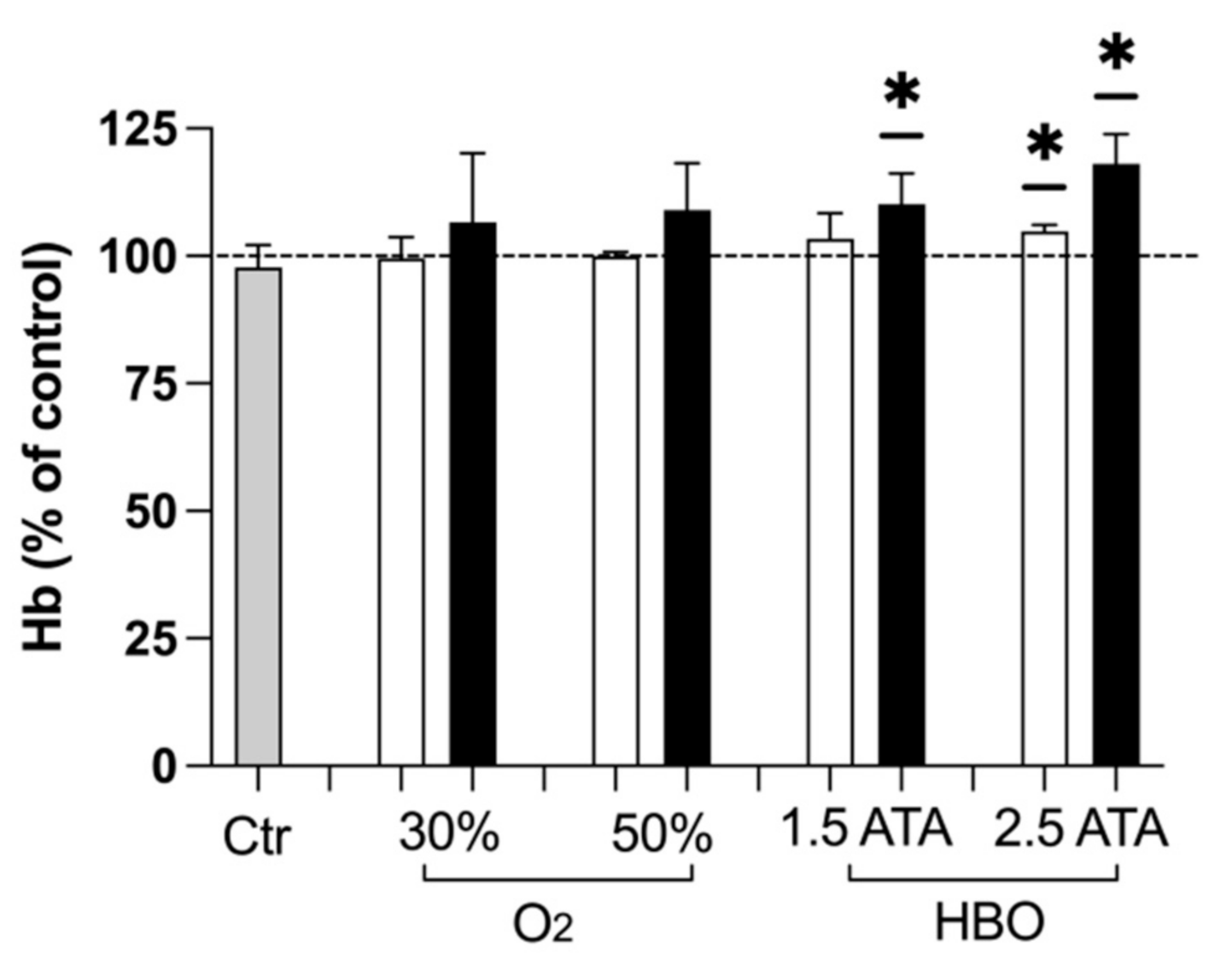
3.3. Hemoglobin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef]
- Sakellariou, G.K.; Jackson, M.J.; Vasilaki, A. Redefining the major contributors to superoxide production in contracting skeletal muscle. The role of NAD(P)H oxidases. Free Radic. Res. 2014, 48, 12–29. [Google Scholar] [CrossRef]
- Kuwahara, H.; Horie, T.; Ishikawa, S.; Tsuda, C.; Kawakami, S.; Noda, Y.; Kaneko, T.; Tahara, S.; Tachibana, T.; Okabe, M.; et al. Oxidative stress in skeletal muscle causes severe disturbance of exercise activity without muscle atrophy. Free Radic. Biol. Med. 2010, 48, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Peternelj, T.T.; Coombes, J.S. Antioxidant supplementation during exercise training: Beneficial or detrimental? Sports Med. 2011, 41, 1043–1069. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Li, J.; Liu, Z.; Chuang, C.-C.; Yang, W.; Zuo, L. Redox Mechanism of Reactive Oxygen Species in Exercise. Front. Physiol. 2016, 7. [Google Scholar] [CrossRef]
- Chevion, S.; Moran, D.S.; Heled, Y.; Shani, Y.; Regev, G.; Abbou, B.; Berenshtein, E.; Stadtman, E.R.; Epstein, Y. Plasma Antioxidant Status and Cell Injury after Severe Physical Exercise. Proc. Natl. Acad. Sci. USA 2003, 100, 5119–5123. [Google Scholar] [CrossRef] [PubMed]
- Goto, C.; Higashi, Y.; Kimura, M.; Noma, K.; Hara, K.; Nakagawa, K.; Kawamura, M.; Chayama, K.; Yoshizumi, M.; Nara, I. Effect of Different Intensities of Exercise on Endothelium-Dependent Vasodilation in Humans: Role of Endothelium-Dependent Nitric Oxide and Oxidative Stress. Circulation 2003, 108, 530–535. [Google Scholar] [CrossRef]
- Castell, L.M.; Poortmans, J.R.; Leclercq, R.; Brasseur, M.; Duchateau, J.; Newsholme, E.A. Some Aspects of the Acute Phase Response after a Marathon Race, and the Effects of Glutamine Supplementation. Eur. J. Appl. Physiol. 1996, 75, 47–53. [Google Scholar] [CrossRef]
- Hellsten, Y.; Frandsen, U.; Orthenblad, N.; Sjødin, B.; Richter, E.A. Xanthine Oxidase in Human Skeletal Muscle Following Eccentric Exercise: A Role in Inflammation. J. Physiol. 1997, 498, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Dyakova, E.Y.; Kapilevich, L.V.; Shylko, V.G.; Popov, S.V.; Anfinogenova, Y. Physical Exercise Associated with NO Production: Signaling Pathways and Significance in Health and Disease. Front. Cell Dev. Biol. 2015, 3. [Google Scholar] [CrossRef]
- Farney, T.M.; Mccarthy, C.G.; Canale, R.E.; Schilling, B.K.; Whitehead, P.N.; Bloomer, R.J. Absence of Blood Oxidative Stress in Trained Men after Strenuous Exercise. Med. Sci. Sports Exerc. 2012, 44, 1855–1863. [Google Scholar] [CrossRef]
- Radák, Z.; Sasvári, M.; Nyakas, C.; Pucsok, J.; Nakamoto, H.; Goto, S. Exercise Preconditioning against Hydrogen Peroxide-Induced Oxidative Damage in Proteins of Rat Myocardium. Arch. Biochem. Biophys. 2000, 376, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Mrakic-Sposta, S.; Vezzoli, A.; Rizzato, A.; Della Noce, C.; Malacrida, S.; Montorsi, M.; Paganini, M.; Cancellara, P.; Bosco, G. Oxidative Stress Assessment in Breath-Hold Diving. Eur. J. Appl. Physiol. 2019, 119, 2449–2456. [Google Scholar] [CrossRef]
- Bosco, G.; Rizzato, A.; Quartesan, S.; Camporesi, E.; Mrakic-Sposta, S.; Moretti, S.; Balestra, C.; Rubini, A. Spirometry and Oxidative Stress after Rebreather Diving in Warm Water. Undersea Hyperb. Med. 2018, 45, 191–198. [Google Scholar] [CrossRef]
- Joulia, F.; Steinberg, J.G.; Faucher, M.; Jamin, T.; Ulmer, C.; Kipson, N.; Jammes, Y. Breath-Hold Training of Humans Reduces Oxidative Stress and Blood Acidosis after Static and Dynamic Apnea. Respir. Physiol. Neurobiol. 2003, 137, 19–27. [Google Scholar] [CrossRef]
- Morabito, C.; Bosco, G.; Pilla, R.; Corona, C.; Mancinelli, R.; Yang, Z.; Camporesi, E.M.; Fanò, G.; Mariggiò, M.A. Effect of Pre-Breathing Oxygen at Different Depth on Oxidative Status and Calcium Concentration in Lymphocytes of Scuba Divers: Pre-Breathing O2 and Lymphocyte Status. Acta. Physiol. 2011, 202, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Ferrer, M.D.; Batle, J.M.; Tauler, P.; Tur, J.A.; Pons, A. Scuba Diving Increases Erythrocyte and Plasma Antioxidant Defenses and Spares NO without Oxidative Damage. Med. Sci. Sports Exerc. 2009, 41, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Bulmer, A.C.; Coombes, J.S.; Sharman, J.E.; Stewart, I.B. Effects of Maximal Static Apnea on Antioxidant Defenses in Trained Free Divers. Med. Sci. Sports Exerc. 2008, 40, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.D.; Sureda, A.; Batle, J.M.; Tauler, P.; Tur, J.A.; Pons, A. Scuba Diving Enhances Endogenous Antioxidant Defenses in Lymphocytes and Neutrophils. Free Radic. Res. 2007, 41, 274–281. [Google Scholar] [CrossRef]
- Perović, A.; Sobočanec, S.; Dabelić, S.; Balog, T.; Dumić, J. Effect of Scuba Diving on the Oxidant/Antioxidant Status, SIRT1 and SIRT3 Expression in Recreational Divers after a Winter Nondive Period. Free Radic. Res. 2018, 52, 188–197. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, X.; Luo, Q.; Zhang, X. MicroRNAs Involved in Oxidative Stress Processes Regulating Physiological and Pathological Responses. MicroRNA 2021, 10. [Google Scholar] [CrossRef]
- Ciesielska, S.; Slezak-Prochazka, I.; Bil, P.; Rzeszowska-Wolny, J. Micro RNAs in Regulation of Cellular Redox Homeostasis. Int. J. Mol. Sci. 2021, 22, 6022. [Google Scholar] [CrossRef]
- Burgos, C.; Henríquez-Olguín, C.; Andrade, D.C.; Ramírez-Campillo, R.; Araneda, O.F.; White, A.; Cerda-Kohler, H. Effects of Exercise Training under Hyperbaric Oxygen on Oxidative Stress Markers and Endurance Performance in Young Soccer Players: A Pilot Study. J. Nutr. Metab. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Woo, J.; Min, J.-H.; Lee, Y.-H.; Roh, H.-T. Effects of Hyperbaric Oxygen Therapy on Inflammation, Oxidative/Antioxidant Balance, and Muscle Damage after Acute Exercise in Normobaric, Normoxic and Hypobaric, Hypoxic Environments: A Pilot Study. Int. J. Environ. Res. Public Health 2020, 17, 7377. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J. Endurance Performance Is Enhanced by Intermittent Hyperbaric Exposure via Up-Regulation of Proteins Involved in Mitochondrial Biogenesis in Mice. Physiol. Rep. 2017, 5, e13349. [Google Scholar] [CrossRef]
- Suzuki, J. Effects of Intermittent Hyperbaric Exposure on Endurance and Interval Exercise Performance in Well-trained Mice. Exp. Physiol. 2018, EP087360. [Google Scholar] [CrossRef] [PubMed]
- Linossier, M.T.; Dormois, D.; Arsac, L.; Denis, C.; Gay, J.P.; Geyssant, A.; Lacour, J.R. Effect of Hyperoxia on Aerobic and Anaerobic Performances and Muscle Metabolism during Maximal Cycling Exercise: Effects of Hyperoxia on Performances and Muscle Metabolism. Acta Physiol. Scand. 2000, 168, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Chou, W.-Y.; Ko, J.-Y.; Lee, M.S.; Wu, R.-W. Early Recovery of Exercise-Related Muscular Injury by HBOT. Biomed. Res. Int. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Sperlich, B.; Zinner, C.; Hauser, A.; Holmberg, H.-C.; Wegrzyk, J. The Impact of Hyperoxia on Human Performance and Recovery. Sports Med. 2017, 47, 429–438. [Google Scholar] [CrossRef]
- Fratantonio, D.; Virgili, F.; Zucchi, A.; Lambrechts, K.; Latronico, T.; Lafère, P.; Germonpré, P.; Balestra, C. Increasing Oxygen Partial Pressures Induce a Distinct Transcriptional Response in Human PBMC: A Pilot Study on the “Normobaric Oxygen Paradox”. Int. J. Mol. Sci. 2021, 22, 458. [Google Scholar] [CrossRef]
- Nes, B.M.; Janszky, I.; Wisløff, U.; Støylen, A.; Karlsen, T. Age-Predicted Maximal Heart Rate in Healthy Subjects: The HUNT Fitness Study: Maximal Heart Rate in a Population. Scand. J. Med. Sci. Sports 2013, 23, 697–704. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Vezzoli, A.; D’Alessandro, F.; Paganini, M.; Dellanoce, C.; Cialoni, D.; Bosco, G. Change in Oxidative Stress Biomarkers During 30 Days in Saturation Dive: A Pilot Study. Int. J. Environ. Res. Public Health 2020, 17, 7118. [Google Scholar] [CrossRef] [PubMed]
- Mrakic-Sposta, S.; Gussoni, M.; Montorsi, M.; Porcelli, S.; Vezzoli, A. Assessment of a Standardized ROS Production Profile in Humans by Electron Paramagnetic Resonance. Oxidative Med. Cell. Longev. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mrakic-Sposta, S.; Gussoni, M.; Montorsi, M.; Porcelli, S.; Vezzoli, A. A Quantitative Method to Monitor Reactive Oxygen Species Production by Electron Paramagnetic Resonance in Physiological and Pathological Conditions. Oxidative Med. Cell. Longev. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bosco, G.; Vezzani, G.; Mrakic Sposta, S.; Rizzato, A.; Enten, G.; Abou-samra, A.; Malacrida, S.; Quartesan, S.; Vezzoli, A.; Camporesi, E. Hyperbaric Oxygen Therapy Ameliorates Osteonecrosis in Patients by Modulating Inflammation and Oxidative Stress. J. Enzym. Inhib. Med. Chem. 2018, 33, 1501–1505. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of Nitrate, Nitrite, and [15N]Nitrate in Biological Fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Vezzoli, A.; Dellanoce, C.; Mrakic-Sposta, S.; Montorsi, M.; Moretti, S.; Tonini, A.; Pratali, L.; Accinni, R. Oxidative Stress Assessment in Response to Ultraendurance Exercise: Thiols Redox Status and ROS Production According to Duration of a Competitive Race. Oxidative Med. Cell. Longev. 2016, 2016, 1–13. [Google Scholar] [CrossRef]
- Dellanoce, C.; Cozzi, L.; Zuddas, S.; Pratali, L.; Accinni, R. Determination of Different Forms of Aminothiols in Red Blood Cells without Washing Erythrocytes: Determination of Different Forms of Aminothiols in RBC. Biomed. Chromatogr. 2014, 28, 327–331. [Google Scholar] [CrossRef]
- Gardin, C.; Ferroni, L.; Piattelli, A.; SIvolella, S.; Zavan, B.; Mijiritsky, E. Non-Washed Resorbable Blasting Media (NWRBM) on Titanium Surfaces Could Enhance Osteogenic Properties of MSCs through Increase of MiRNA-196a And VCAM1. Stem Cell Rev. Rep. 2016, 12, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Calvaresi, E.C.; La’ulu, S.L.; Snow, T.M.; Allison, T.R.; Genzen, J.R. Plasma Hemoglobin: A Method Comparison of Six Assays for Hemoglobin and Hemolysis Index Measurement. Int. J. Lab. Hematol. 2021, ijlh.13457. [Google Scholar] [CrossRef] [PubMed]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative Stress, Prooxidants, and Antioxidants: The Interplay. Biomed. Res. Int. 2014, 2014, 1–19. [Google Scholar] [CrossRef]
- Yamada, M.; Suzuki, K.; Kudo, S.; Totsuka, M.; Simoyama, T.; Nakaji, S.; Sugawara, K. Effect of Exhaustive Exercise on Human Neutrophils in Athletes. Luminescence 2000, 15, 15–20. [Google Scholar] [CrossRef]
- Silveira, L.S.; Antunes, B.; Minari, A.L.A.; dos Santos, R.V.T.; Neto, J.C.R.; Lira, F.S. Macrophage Polarization: Implications on Metabolic Diseases and the Role of Exercise. Crit. Rev. Eukaryot. Gene Expr. 2016, 26, 115–132. [Google Scholar] [CrossRef]
- Nunes-Silva, A.; Bernardes, P.T.T.; Rezende, B.M.; Lopes, F.; Gomes, E.C.; Marques, P.E.; Lima, P.M.A.; Coimbra, C.C.; Menezes, G.B.; Teixeira, M.M.; et al. Treadmill Exercise Induces Neutrophil Recruitment into Muscle Tissue in a Reactive Oxygen Species-Dependent Manner. An Intravital Microscopy Study. PLoS ONE 2014, 9, e96464. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.J.; Steiner, L.A.; Gupta, A.K.; Menon, D.K. Cerebral Oxygen Vasoreactivity and Cerebral Tissue Oxygen Reactivity. Br. J. Anaesth. 2003, 90, 774–786. [Google Scholar] [CrossRef]
- Nocella, C.; Cammisotto, V.; Pigozzi, F.; Borrione, P.; Fossati, C.; D’Amico, A.; Cangemi, R.; Peruzzi, M.; Gobbi, G.; Ettorre, E.; et al. Impairment between Oxidant and Antioxidant Systems: Short- and Long-Term Implications for Athletes’ Health. Nutrients 2019, 11, 1353. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Ulrich, K.; Jakob, U. The Role of Thiols in Antioxidant Systems. Free Radic. Biol. Med. 2019, 140, 14–27. [Google Scholar] [CrossRef]
- Benson, R.M.; Minter, L.M.; Osborne, B.A.; Granowitz, E.V. Hyperbaric Oxygen Inhibits Stimulus-Induced Proinflammatory Cytokine Synthesis by Human Blood-Derived Monocyte-Macrophages: HBO Inhibits Cytokine Synthesis. Clin. Exp. Immunol. 2003, 134, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Peltonen, J.E.; Tikkanen, H.O.; Rusko, H.K. Cardiorespiratory Responses to Exercise in Acute Hypoxia, Hyperoxia and Normoxia. Eur. J. Appl. Physiol. 2001, 85, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.G.R.; Reid, J.; Perry, W.; Wilson, B.A. Effects of Hyperoxic Training on Performance and Cardiorespiratory Response to Exercise. Med. Sci. Sports Exerc. 2005, 37, 1175–1179. [Google Scholar] [CrossRef]
- Perry, C.G.R.; Talanian, J.L.; Heigenhauser, G.J.F.; Spriet, L.L. The Effects of Training in Hyperoxia vs. Normoxia on Skeletal Muscle Enzyme Activities and Exercise Performance. J. Appl. Physiol. 2007, 102, 1022–1027. [Google Scholar] [CrossRef][Green Version]
- Ploutz-Snyder, L.L.; Simoneau, J.-A.; Gilders, R.M.; Staron, R.S.; Hagerman, F.C. Cardiorespiratory and Metabolic Adaptations to Hyperoxic Training. Eur. J. Appl. Physiol. 1996, 73, 38–48. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Jiang, B.-H. Interplay Between Reactive Oxygen Species and MicroRNAs in Cancer. Curr. Pharm. Rep. 2016, 2, 82–90. [Google Scholar] [CrossRef]
- La Sala, L.; Mrakic-Sposta, S.; Tagliabue, E.; Prattichizzo, F.; Micheloni, S.; Sangalli, E.; Specchia, C.; Uccellatore, A.C.; Lupini, S.; Spinetti, G.; et al. Circulating MicroRNA-21 Is an Early Predictor of ROS-Mediated Damage in Subjects with High Risk of Developing Diabetes and in Drug-Naïve T2D. Cardiovasc. Diabetol. 2019, 18, 18. [Google Scholar] [CrossRef]
- Natarelli, L.; Parca, L.; Mazza, T.; Weber, C.; Virgili, F.; Fratantonio, D. MicroRNAs and Long Non-Coding RNAs as Potential Candidates to Target Specific Motifs of SARS-CoV-2. Non-Coding RNA 2021, 7, 14. [Google Scholar] [CrossRef]
- Balestra, C.; Germonpré, P.; Poortmans, J.R.; Marroni, A. Serum Erythropoietin Levels in Healthy Humans after a Short Period of Normobaric and Hyperbaric Oxygen Breathing: The “Normobaric Oxygen Paradox”. J. Appl. Physiol. 2006, 100, 512–518. [Google Scholar] [CrossRef]
- Balestra, C.; Germonpré, P.; Lafere, P.; Ciccarella, Y.; Van Der Linden, P. The ‘Normobaric Oxygen Paradox’: A Simple Way to Induce Endogenous Erythropoietin Production and Concomitantly Raise Hemoglobin Levels in Anemic Patients. Transfus. Altern. Transfus. Med. 2010, 11, 39–42. [Google Scholar] [CrossRef]
- De Bels, D.; Theunissen, S.; Devriendt, J.; Germonpré, P.; Lafere, P.; Valsamis, J.; Snoeck, T.; Meeus, P.; Balestra, C. The “Normobaric Oxygen Paradox”: Does It Increase Haemoglobin? Diving Hyperb. Med. 2012, 42, 67–71. [Google Scholar] [PubMed]

| Parameter (Median, IQR) | |
|---|---|
| Age (years) | 37 (33–46) |
| Weight T0 (kg) | 73 (64–79) |
| Height (cm) | 174 (168–180) |
| Leukocytes (109/L) | 6.29 (5.49–8.23) |
| Erythrocytes (1012/L) | 4.98 (4.77–5.39) |
| Hemoglobin (g/dL) | 15.35 (14.00–15.9) |
| Hematocrit (%) | 45.00 (42.78–46.18) |
| Platelets (109/L) | 248.00 (200.50–283.75) |
| Erythrocyte Sedimentation Rate (mm) | 5.50 (3.75–6.50) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosco, G.; Paganini, M.; Giacon, T.A.; Oppio, A.; Vezzoli, A.; Dellanoce, C.; Moro, T.; Paoli, A.; Zanotti, F.; Zavan, B.; et al. Oxidative Stress and Inflammation, MicroRNA, and Hemoglobin Variations after Administration of Oxygen at Different Pressures and Concentrations: A Randomized Trial. Int. J. Environ. Res. Public Health 2021, 18, 9755. https://doi.org/10.3390/ijerph18189755
Bosco G, Paganini M, Giacon TA, Oppio A, Vezzoli A, Dellanoce C, Moro T, Paoli A, Zanotti F, Zavan B, et al. Oxidative Stress and Inflammation, MicroRNA, and Hemoglobin Variations after Administration of Oxygen at Different Pressures and Concentrations: A Randomized Trial. International Journal of Environmental Research and Public Health. 2021; 18(18):9755. https://doi.org/10.3390/ijerph18189755
Chicago/Turabian StyleBosco, Gerardo, Matteo Paganini, Tommaso Antonio Giacon, Alberto Oppio, Alessandra Vezzoli, Cinzia Dellanoce, Tatiana Moro, Antonio Paoli, Federica Zanotti, Barbara Zavan, and et al. 2021. "Oxidative Stress and Inflammation, MicroRNA, and Hemoglobin Variations after Administration of Oxygen at Different Pressures and Concentrations: A Randomized Trial" International Journal of Environmental Research and Public Health 18, no. 18: 9755. https://doi.org/10.3390/ijerph18189755
APA StyleBosco, G., Paganini, M., Giacon, T. A., Oppio, A., Vezzoli, A., Dellanoce, C., Moro, T., Paoli, A., Zanotti, F., Zavan, B., Balestra, C., & Mrakic-Sposta, S. (2021). Oxidative Stress and Inflammation, MicroRNA, and Hemoglobin Variations after Administration of Oxygen at Different Pressures and Concentrations: A Randomized Trial. International Journal of Environmental Research and Public Health, 18(18), 9755. https://doi.org/10.3390/ijerph18189755















