Elucidation of the Mechanism Underlying the Anti-Inflammatory Properties of (S)-(+)-Carvone Identifies a Novel Class of Sirtuin-1 Activators in a Murine Macrophage Cell Line
Abstract
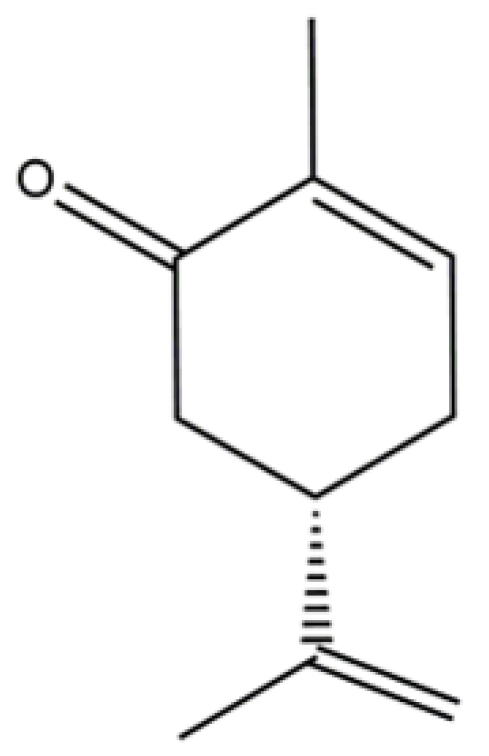
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatments
2.2. Preparation of Cell Extracts
2.3. Western Blotting
2.4. Immunocytochemistry
2.5. SIRT1 Activity Assay
2.6. Statistical Analysis
3. Results
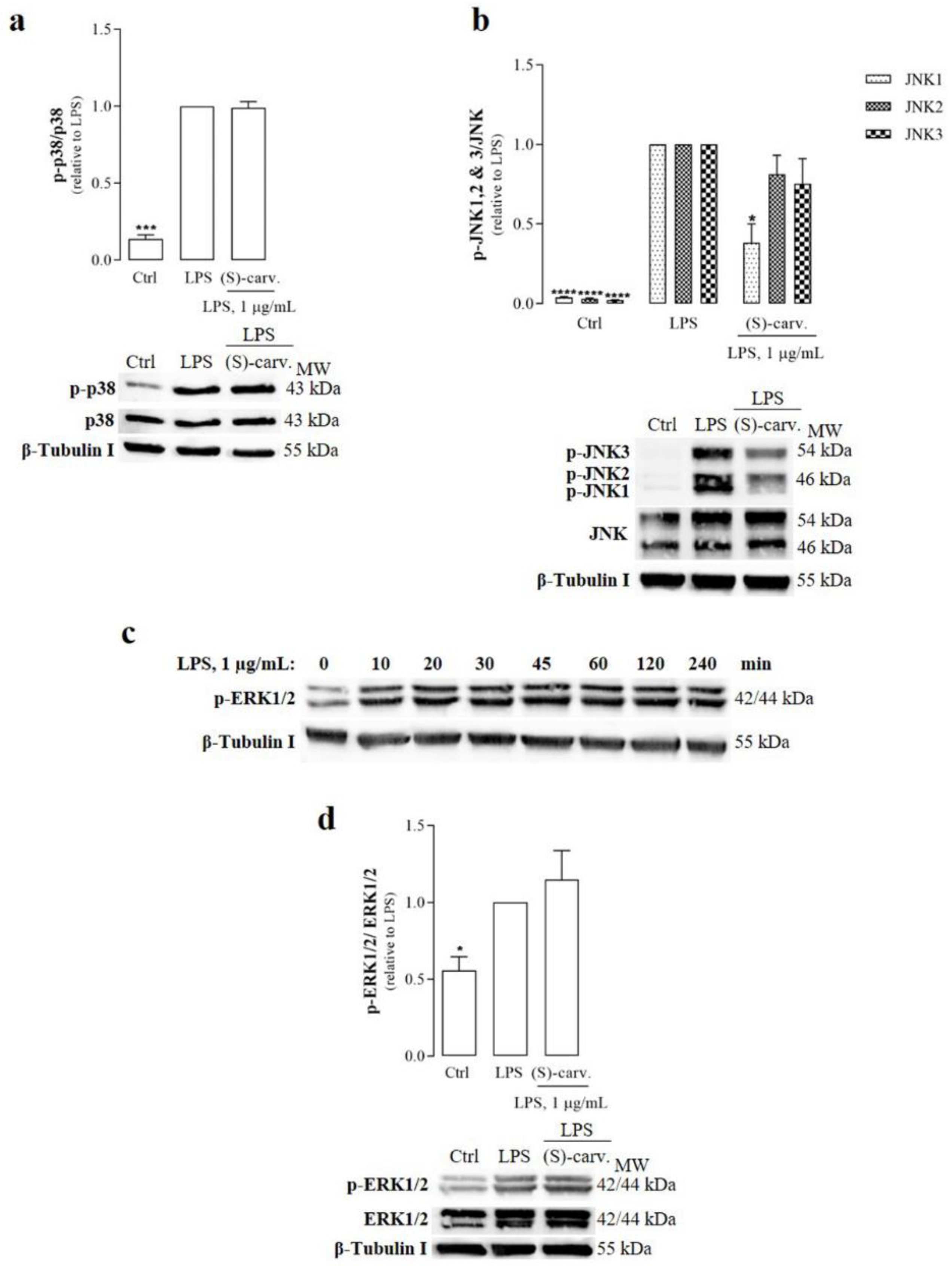
3.1. (S)-(+)-Carvone Decreases JNK1 Phosphorylation Induced by LPS in Macrophages
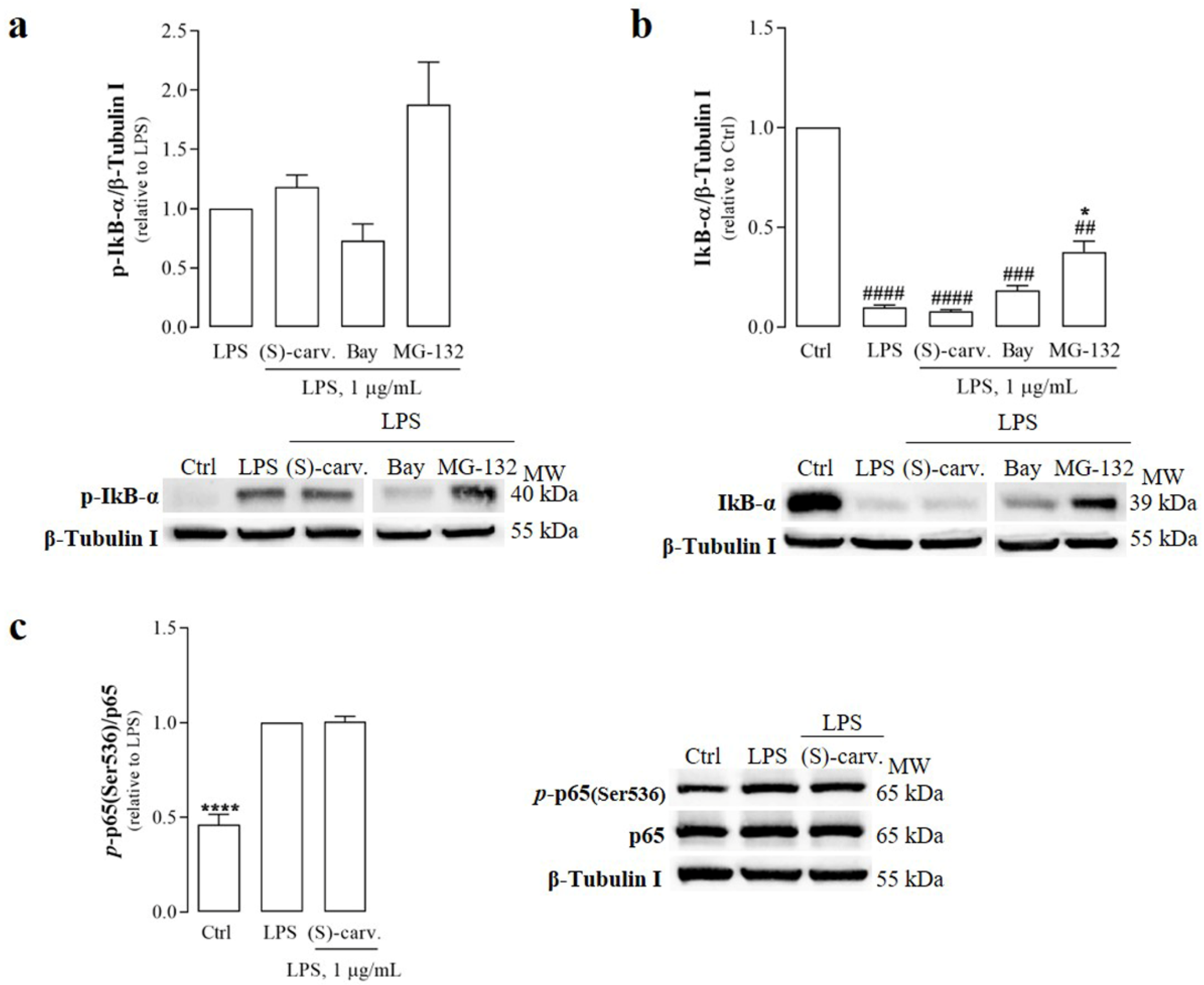
3.2. (S)-(+)-Carvone Does Not Interfere with the Canonical NF-κB Activation Pathway
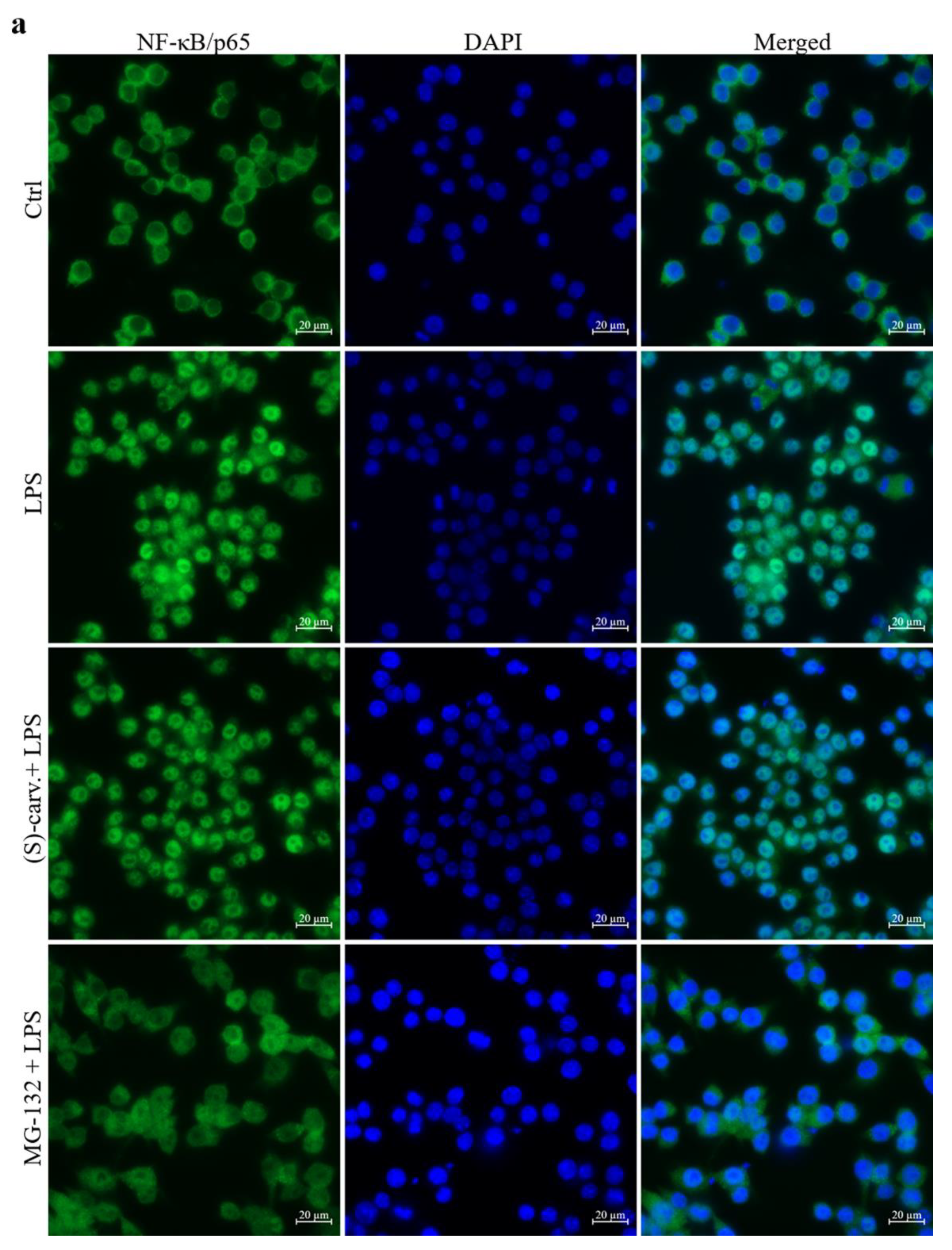
3.3. NF-κB/p65 Nuclear Translocation Is Not Affected by (S)-(+)-Carvone
3.4. NF-κB Transcriptional Activity Is Inhibited by (S)-(+)-Carvone
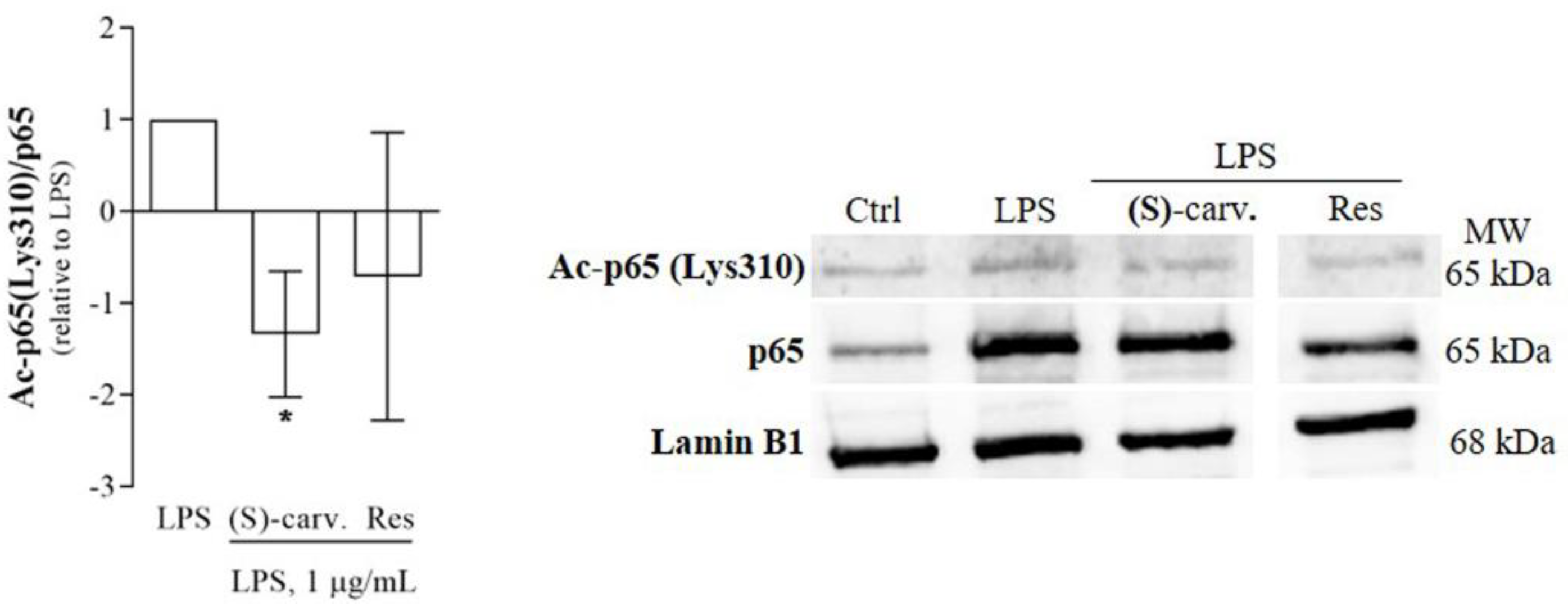
3.5. (S)-(+)-Carvone Promotes NF-κB/p65 Deacetylation at Lys310
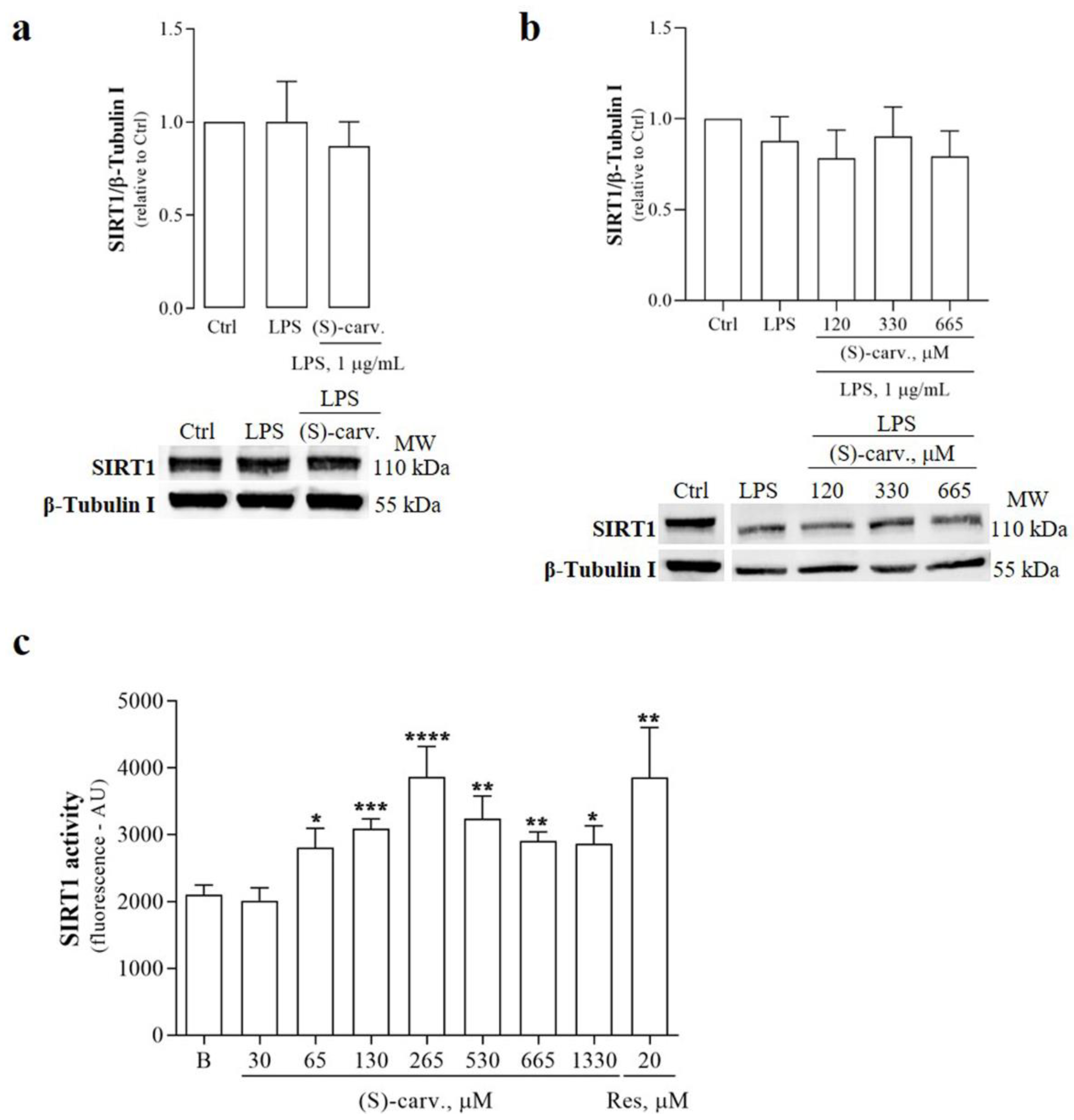
3.6. (S)-(+)-Carvone Activates SIRT1 without Affecting Its Protein Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [Green Version]
- Malaquin, N.; Martinez, A.; Rodier, F. Keeping the senescence secretome under control: Molecular reins on the senescence-associated secretory phenotype. Exp. Gerontol. 2016, 82, 39–49. [Google Scholar] [CrossRef]
- Maniaci, A.; Iannella, G.; Cocuzza, S.; Vicini, C.; Magliulo, G.; Ferlito, S.; Cammaroto, G.; Meccariello, G.; De Vito, A.; Nicolai, A.; et al. Oxidative Stress and Inflammation Biomarker Expression in Obstructive Sleep Apnea Patients. J. Clin. Med. 2021, 10, 277. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.C.; Sundaram, C.; Reuter, S.; Aggarwal, B.B. Inhibiting NF-kappaB activation by small molecules as a therapeutic strategy. Biochim. Biophys. Acta 2010, 1799, 775–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaminska, B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy--from molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta 2005, 1754, 253–262. [Google Scholar] [CrossRef]
- Sousa, C.; Leitão, A.J.; Neves, B.M.; Judas, F.; Cavaleiro, C.; Mendes, A.F. Standardised comparison of limonene-derived monoterpenes identifies structural determinants of anti-inflammatory activity. Sci. Rep. 2020, 10, 7199. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda-Arias, J.C.; Veloza, L.A.; Escobar, L.M.; Orozco, L.M.; Lopera, I.A. Anti-inflammatory effects of the main constituents and epoxides derived from essential oils obtained from Tagetes lucida, Cymbopogon citratus, Lipia alba and Eucalyptus citriodora. J. Essent. Oil Res. 2013, 25, 186–193. [Google Scholar] [CrossRef]
- Sabir, S.M.; Singh, D.; Rocha, J.B.T. In Vitro Antioxidant Activity of S-Carvone Isolated from Zanthoxylum alatum. Pharm. Chem. J. 2015, 49, 187–191. [Google Scholar] [CrossRef]
- Muruganathan, U.; Srinivasan, S. Beneficial effect of carvone, a dietary monoterpene ameliorates hyperglycemia by regulating the key enzymes activities of carbohydrate metabolism in streptozotocin-induced diabetic rats. Biomed. Pharmacother. 2016, 84, 1558–1567. [Google Scholar] [CrossRef]
- Alsanea, S.; Liu, D. BITC and S-Carvone Restrain High-Fat Diet-Induced Obesity and Ameliorate Hepatic Steatosis and Insulin Resistance. Pharm. Res. 2017, 34, 2241–2249. [Google Scholar] [CrossRef]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa, C.; Ribeiro, M.; Rufino, A.T.; Leitao, A.J.; Mendes, A.F. Assessment of cell line competence for studies of pharmacological GPR30 modulation. J. Recept. Signal Transduct. Res. 2017, 37, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Srivastava, M.; Saqib, U.; Liu, D.; Faisal, S.M.; Sugathan, S.; Bishnoi, S.; Baig, M.S. Potential therapeutic targets for inflammation in toll-like receptor 4 (TLR4)-mediated signaling pathways. Int. Immunopharmacol. 2016, 40, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, H.; Chiba, H.; Miyoshi, H.; Sugita, T.; Toriumi, W. IkappaB kinases phosphorylate NF-kappaB p65 subunit on serine 536 in the transactivation domain. J. Biol. Chem. 1999, 274, 30353–30356. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Tang, E.; Guan, K.; Wang, C.Y. IKK beta plays an essential role in the phosphorylation of RelA/p65 on serine 536 induced by lipopolysaccharide. J. Immunol. 2003, 170, 5630–5635. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.D.; Ryseck, R.P.; Attar, R.M.; Dambach, D.; Bravo, R. Functional redundancy of the nuclear factor kappa B inhibitors I kappa B alpha and I kappa B beta. J. Exp. Med. 1998, 188, 1055–1062. [Google Scholar] [CrossRef]
- Huang, B.; Yang, X.D.; Lamb, A.; Chen, L.F. Posttranslational modifications of NF-kappaB: Another layer of regulation for NF-kappaB signaling pathway. Cell. Signal. 2010, 22, 1282–1290. [Google Scholar] [CrossRef] [Green Version]
- Holmes-McNary, M.; Baldwin, A.S., Jr. Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IkappaB kinase. Cancer Res. 2000, 60, 3477–3483. [Google Scholar]
- Manna, S.K.; Mukhopadhyay, A.; Aggarwal, B.B. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: Potential role of reactive oxygen intermediates and lipid peroxidation. J. Immunol. 2000, 164, 6509–6519. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.C.; Kim, Y.H.; Son, S.W.; Moon, E.Y.; Pyo, S.; Um, S.H. Fisetin induces Sirt1 expression while inhibiting early adipogenesis in 3T3-L1 cells. Biochem. Biophys. Res. Commun. 2015, 467, 638–644. [Google Scholar] [CrossRef]
- Miao, Y.; Zhao, S.; Gao, Y.; Wang, R.; Wu, Q.; Wu, H.; Luo, T. Curcumin pretreatment attenuates inflammation and mitochondrial dysfunction in experimental stroke: The possible role of Sirt1 signaling. Brain Res. Bull. 2016, 121, 9–15. [Google Scholar] [CrossRef]
- Dai, H.; Sinclair, D.A.; Ellis, J.L.; Steegborn, C. Sirtuin activators and inhibitors: Promises, achievements, and challenges. Pharmacol. Ther. 2018. [Google Scholar] [CrossRef]
- Lin, G.D.; Li, R.W. Chapter 10—Natural Products Targeting Inflammation Processes and Multiple Mediators. In Natural Products and Drug Discovery; Mandal, S.C., Mandal, V., Konishi, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 277–308. [Google Scholar]
- Azab, A.; Nassar, A.; Azab, A.N. Anti-Inflammatory Activity of Natural Products. Molecules 2016, 21, 1321. [Google Scholar] [CrossRef] [PubMed]
- de Sá Coutinho, D.; Pacheco, M.T.; Frozza, R.L.; Bernardi, A. Anti-Inflammatory Effects of Resveratrol: Mechanistic Insights. Int. J. Mol. Sci. 2018, 19, 1812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rufino, A.T.; Ribeiro, M.; Sousa, C.; Judas, F.; Salgueiro, L.; Cavaleiro, C.; Mendes, A.F. Evaluation of the anti-inflammatory, anti-catabolic and pro-anabolic effects of E-caryophyllene, myrcene and limonene in a cell model of osteoarthritis. Eur. J. Pharmacol. 2015, 750, 141–150. [Google Scholar] [CrossRef]
- Rufino, A.T.; Ribeiro, M.; Judas, F.; Salgueiro, L.; Lopes, M.C.; Cavaleiro, C.; Mendes, A.F. Anti-inflammatory and chondroprotective activity of (+)-alpha-pinene: Structural and enantiomeric selectivity. J. Nat. Prod. 2014, 77, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Wolter, S.; Doerrie, A.; Weber, A.; Schneider, H.; Hoffmann, E.; von der Ohe, J.; Bakiri, L.; Wagner, E.F.; Resch, K.; Kracht, M. c-Jun controls histone modifications, NF-kappaB recruitment, and RNA polymerase II function to activate the ccl2 gene. Mol. Cell. Biol. 2008, 28, 4407–4423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hambleton, J.; Weinstein, S.L.; Lem, L.; DeFranco, A.L. Activation of c-Jun N-terminal kinase in bacterial lipopolysaccharide-stimulated macrophages. Proc. Natl. Acad. Sci. USA 1996, 93, 2774–2778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Q.; Xia, Y. c-Jun, at the crossroad of the signaling network. Protein Cell 2011, 2, 889–898. [Google Scholar] [CrossRef] [Green Version]
- Spitsin, S.V.; Koprowski, H.; Michaels, F.H. Characterization and functional analysis of the human inducible nitric oxide synthase gene promoter. Mol. Med. 1996, 2, 226–235. [Google Scholar] [CrossRef] [Green Version]
- Martin, M.U.; Wesche, H. Summary and comparison of the signaling mechanisms of the Toll/interleukin-1 receptor family. Biochim. Et Biophys. Acta 2002, 1592, 265–280. [Google Scholar] [CrossRef] [Green Version]
- Catterall, J.B.; Carrère, S.; Koshy, P.J.; Degnan, B.A.; Shingleton, W.D.; Brinckerhoff, C.E.; Rutter, J.; Cawston, T.E.; Rowan, A.D. Synergistic induction of matrix metalloproteinase 1 by interleukin-1alpha and oncostatin M in human chondrocytes involves signal transducer and activator of transcription and activator protein 1 transcription factors via a novel mechanism. Arthritis Rheum. 2001, 44, 2296–2310. [Google Scholar] [CrossRef]
- Mengshol, J.A.; Vincenti, M.P.; Brinckerhoff, C.E. IL-1 induces collagenase-3 (MMP-13) promoter activity in stably transfected chondrocytic cells: Requirement for Runx-2 and activation by p38 MAPK and JNK pathways. Nucleic Acids Res. 2001, 29, 4361–4372. [Google Scholar] [CrossRef] [Green Version]
- Wattenberg, L.W.; Sparnins, V.L.; Barany, G. Inhibition of N-nitrosodiethylamine carcinogenesis in mice by naturally occurring organosulfur compounds and monoterpenes. Cancer Res. 1989, 49, 2689–2692. [Google Scholar]
- Haigis, M.C.; Sinclair, D.A. Mammalian sirtuins: Biological insights and disease relevance. Annu. Rev. Pathol. 2010, 5, 253–295. [Google Scholar] [CrossRef] [Green Version]
- Gomes, P.; Leal, H.; Mendes, A.F.; Reis, F.; Cavadas, C. Dichotomous Sirtuins: Implications for Drug Discovery in Neurodegenerative and Cardiometabolic Diseases. Trends Pharmacol. Sci. 2019, 40, 1021–1039. [Google Scholar] [CrossRef] [PubMed]
- Kauppinen, A.; Suuronen, T.; Ojala, J.; Kaarniranta, K.; Salminen, A. Antagonistic crosstalk between NF-kappaB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell. Signal. 2013, 25, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, S.; Xu, L.; Brunet, A. Turning back time with emerging rejuvenation strategies. Nat. Cell Biol. 2019, 21, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, L.S.; Álvaro, A.R.; Carmo-Silva, S.; Mendes, A.F.; Relógio, A.; Cavadas, C. The importance of determining circadian parameters in pharmacological studies. Br. J. Pharmacol. 2019, 176, 2827–2847. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Cardioprotective Effects of Sirtuin-1 and Its Downstream Effectors: Potential Role in Mediating the Heart Failure Benefits of SGLT2 (Sodium-Glucose Cotransporter 2) Inhibitors. Circulation. Heart Fail. 2020, 13, e007197. [Google Scholar] [CrossRef] [PubMed]


| Protein | Source | Clonality | Dilution | Supplier | Catalogue/Lot Number |
|---|---|---|---|---|---|
| IκB-α | rabbit | polyclonal | 1:1000 | Cell Signaling Technology, Inc., Danvers, MA, USA | #9242/9 |
| phospho-p44/42 MAPK (ERK1/2) (Thr202/Tyr204) | rabbit | polyclonal | 1:1000 | Cell Signaling Technology, Inc. | #9101/27 |
| p44/42 MAPK (ERK1/2) | rabbit | polyclonal | 1:1000 | Cell Signaling Technology, Inc. | #9102/26 |
| phospho-p38 MAPK (Thr180/Tyr182) | rabbit | polyclonal | 1:1000 | Cell Signaling Technology, Inc. | #9211/21 |
| p38 MAPK | rabbit | polyclonal | 1:1000 | Cell Signaling Technology, Inc. | #9212/17 |
| SAPK/JNK | rabbit | polyclonal | 1:1000 | Cell Signaling Technology, Inc. | #9252/17 |
| acetyl-NF-κB p65 (Lys310) | rabbit | polyclonal | 1:750 | Cell Signaling Technology, Inc. | #3045/2 |
| Sirtuin-1 | rabbit | polyclonal | 1:1000 | Sigma-Aldrich Co. | 07-131/2736563 |
| Lamin B1 | rabbit | polyclonal | 1:1000 | Abcam, Cambridge, UK | ab16048/GR48958-1 |
| phospho-SAPK/JNK (Thr183/Tyr185) | rabbit | monoclonal | 1:1000 | Cell Signaling Technology, Inc. | #4668/11 |
| NF-κB p65 (D14E12) XP® | rabbit | monoclonal | 1:1000 | Cell Signaling Technology, Inc. | #8242/4 |
| phospho- NF-κB p65 (Ser536) | rabbit | monoclonal | 1:1000 | Cell Signaling Technology, Inc. | #3033/14 |
| phospho-IκB-α (Ser32/36) | mouse | monoclonal | 1:1000 | Cell Signaling Technology, Inc. | #9246/14 |
| β-Tubulin I | mouse | monoclonal | 1:20,000 | Sigma-Aldrich Co. | T7816/052M4835 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, C.; Neves, B.M.; Leitão, A.J.; Mendes, A.F. Elucidation of the Mechanism Underlying the Anti-Inflammatory Properties of (S)-(+)-Carvone Identifies a Novel Class of Sirtuin-1 Activators in a Murine Macrophage Cell Line. Biomedicines 2021, 9, 777. https://doi.org/10.3390/biomedicines9070777
Sousa C, Neves BM, Leitão AJ, Mendes AF. Elucidation of the Mechanism Underlying the Anti-Inflammatory Properties of (S)-(+)-Carvone Identifies a Novel Class of Sirtuin-1 Activators in a Murine Macrophage Cell Line. Biomedicines. 2021; 9(7):777. https://doi.org/10.3390/biomedicines9070777
Chicago/Turabian StyleSousa, Cátia, Bruno Miguel Neves, Alcino Jorge Leitão, and Alexandrina Ferreira Mendes. 2021. "Elucidation of the Mechanism Underlying the Anti-Inflammatory Properties of (S)-(+)-Carvone Identifies a Novel Class of Sirtuin-1 Activators in a Murine Macrophage Cell Line" Biomedicines 9, no. 7: 777. https://doi.org/10.3390/biomedicines9070777
APA StyleSousa, C., Neves, B. M., Leitão, A. J., & Mendes, A. F. (2021). Elucidation of the Mechanism Underlying the Anti-Inflammatory Properties of (S)-(+)-Carvone Identifies a Novel Class of Sirtuin-1 Activators in a Murine Macrophage Cell Line. Biomedicines, 9(7), 777. https://doi.org/10.3390/biomedicines9070777









