Effect of Rhizophagus irregularis on Growth and Quality of Cannabis sativa Seedlings
Abstract
1. Introduction
2. Results
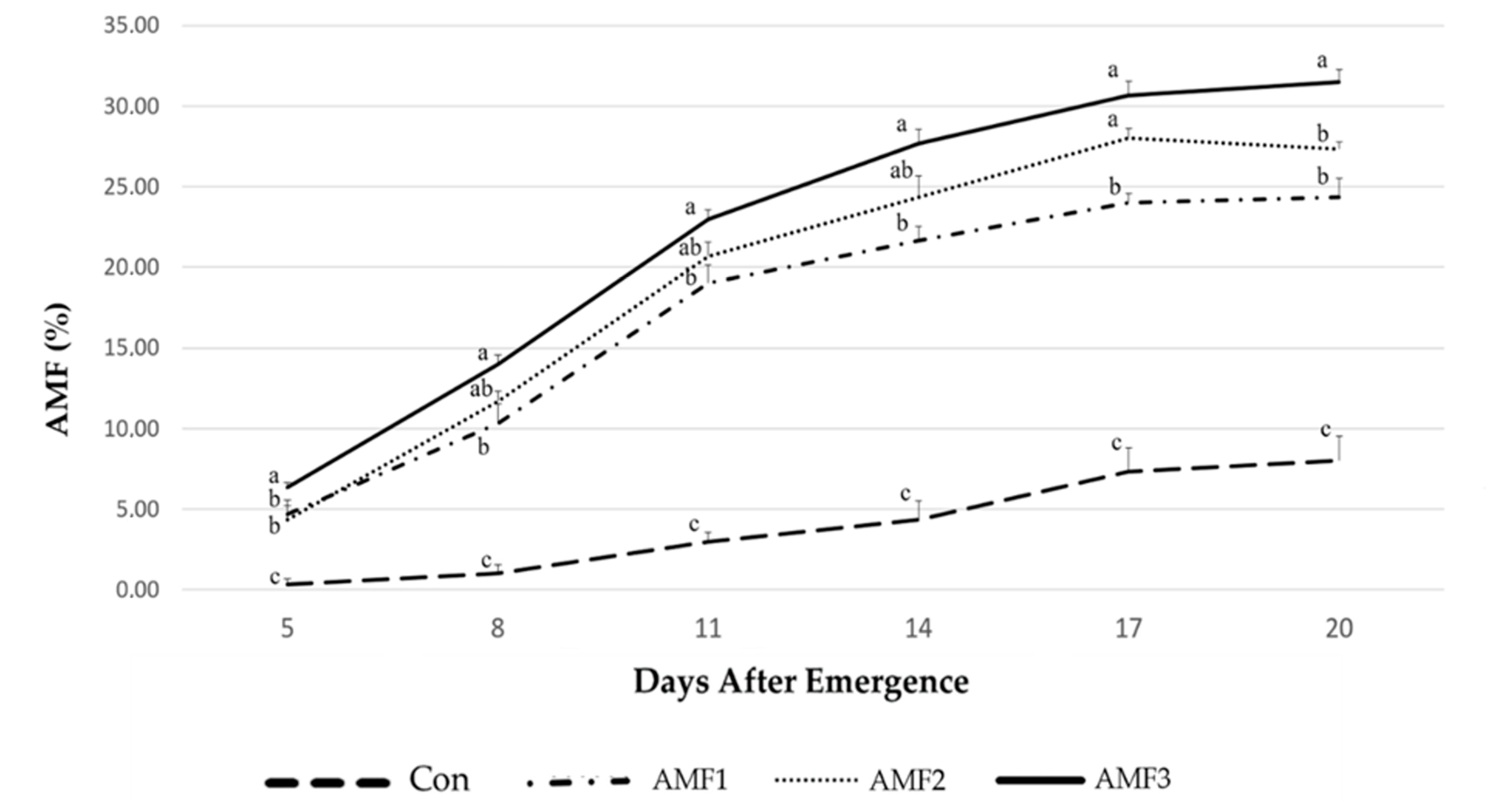
2.1. Mycorrhizal Colonization
2.2. Plant Growth
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Zuardi, A.W. History of cannabis as a medicine: A review. Rev. Bras. Psiquiatr. 2006, 28, 153–157. [Google Scholar] [CrossRef]
- Angelini, L.G.; Tavarini, S.; Cestone, B.; Beni, C. Variation in mineral composition in three different plant organs of five fibre cannabis (Cannabis sativa L.) cultivars. Agrochimica Pisa 2014, 58, 1–18. [Google Scholar]
- Carus, M.; Sarmento, L. The European Hemp Industry: Cultivation, processing and applications for fibres, shivs, seeds and flowers. Eur. Ind. Hemp Assoc. 2016, 2016, 1–9. [Google Scholar]
- Pisanti, S.; Bifulco, M. MedicalCannabis: A plurimillennial history of an evergreen. J. Cell. Physiol. 2019, 234, 8342–8351. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Chanet, G.; Morin-Crini, N. Applications of hemp in textiles, paper industry, insulation and building materials, horticulture, animal nutrition, food and beverages, nutraceuticals, cosmetics and hygiene, medicine, agrochemistry, energy production and environment: A review. Environ. Chem. Lett. 2020, 18, 1–26. [Google Scholar] [CrossRef]
- Gorelick, J.; Bernstein, N. Chemical and Physical Elicitation for Enhanced Cannabinoid Production in Cannabis. In Cannabis sativa L.—Botany and Biotechnology, 1st ed.; Chandra, S., Lata, H., ElSohly, M., Eds.; Springer: Cham, Switzerland, 2017; pp. 439–456. [Google Scholar] [CrossRef]
- Bouloc, P. Cannabis: Industrial Production and Uses; CABI Publishing: Cambridge, MA, USA, 2013; pp. 5–23. [Google Scholar]
- Amaducci, S.; Scordia, D.; Liu, F.; Zhang, Q.; Guo, H.; Testa, G.; Cosentino, S. Key cultivation techniques for hemp in Europe and China. Ind. Crop. Prod. 2015, 68, 2–16. [Google Scholar] [CrossRef]
- Bifulco, M.; Pisanti, S. Medicinal use of cannabis in E urope. EMBO Rep. 2015, 16, 130–132. [Google Scholar] [CrossRef]
- Kousta, A.; Papastylianou, P.; Cheimona, N.; Travlos, I.; Kakabouki, I.; Bilalis, D. Effect of Fertilization and Weed Management on Weed Flora of Hemp Crop. Bull. Univ. Agric. Sci. Veter Med. Cluj-Napoca. Hortic. 2020, 77, 44–51. [Google Scholar] [CrossRef]
- Adesina, I.; Bhowmik, A.; Sharma, H.; Shahbazi, A. A Review on the Current State of Knowledge of Growing Conditions, Agronomic Soil Health Practices and Utilities of Hemp in the United States. Agriculture 2020, 10, 129. [Google Scholar] [CrossRef]
- Johnson, R. Cannabis as an Agricultural Commodity; Congressional Research Service: Washington, DC, USA, 2014. [Google Scholar]
- Jin, D.; Jin, S.; Chen, J. Cannabis Indoor Growing Conditions, Management Practices, and Post-Harvest Treatment: A Review. Am. J. Plant Sci. 2019, 10, 925–946. [Google Scholar] [CrossRef]
- Poulsen, H.; Sutherland, G. The potency of cannabis in New Zealand from 1976 to 1996. Sci. Justice 2000, 40, 171–176. [Google Scholar] [CrossRef]
- Rideout, J.W.; Overstreet, L.F. Phosphorus Rate in Combination with Cultural Practices Reduces Excessive Growth of Tomato Seedlings in the Float System. HortScience 2003, 38, 524–528. [Google Scholar] [CrossRef]
- Leal, R.S. The use of Confidor S in the float, a new tobacco seedling production system in the south of Brazil. Pflanzenschutz-Nachr. Bayer 2001, 54, 337–352. [Google Scholar]
- Zhang, X. Ecology and Management of Pythium Species in Float Greenhouse Tobacco Transplant Production. Ph.D. Thesis, Virginia Tech, Blacksburg, VA, USA, 2021. [Google Scholar]
- Roberto, K. How-to Hydroponics, 4th ed.; The Futuregarden Press: Farmingdale, NY, USA, 2003; p. 23. [Google Scholar]
- Akoumianaki-Ioannidou, A.; Rasouli, M.; Podaropoulou, L.; Bilalis, D. Seedlings production of mentha × piperita (papermint) and mentha spicata (spearmint) in float system with organic and inorganic fertilization. Acta Hortic. 2012, 1307–1311. [Google Scholar] [CrossRef]
- Kanatas, P. Float system and crucial points of the method for seedling production and crop cultivation with or without organic fertilization. Agron. Res. 2020, 18, 137–147. [Google Scholar]
- Gregory, P.J. Plant Roots: Growth, Activity and Interactions with the Soil; Blackwell Publishing: Oxford, UK, 2008; pp. 108–109. [Google Scholar]
- Qin, R.; Stamp, P.; Richner, W. Impact of tillage on maize rooting in a Cambisol and Luvisol in Switzerland. Soil Tillage Res. 2006, 85, 50–61. [Google Scholar] [CrossRef]
- Akoumianaki-Ioannidou, A.; Podaropoulou, L.; Rasouli, M.; Bilalis, D. Seedlings Production of Ocimum Basilicum in two systems (float and seedbed) with organic and unorganic fertilization. Acta Hortic. 2012, 937, 1301–1306. [Google Scholar] [CrossRef]
- Bantis, F.; Dangitsis, C.; Koukounaras, A. Influence of Light Spectra from LEDs and Scion × Rootstock Genotype Combinations on the Quality of Grafted Watermelon Seedlings. Plants 2021, 10, 353. [Google Scholar] [CrossRef] [PubMed]
- Bócsa, I.; Máthé, P.; Hangyel, L. Effect of nitrogen on tetrahydrocannabinol (THC) content in hemp (Cannabis sativa L.) leaves at different positions. JIHA 1997, 4, 78–79. [Google Scholar]
- Taura, F.; Morimoto, S.; Shoyama, Y.; Mechoulam, R. First direct evidence for the mechanism of .DELTA.1-tetrahydrocannabinolic acid biosynthesis. J. Am. Chem. Soc. 1995, 117, 9766–9767. [Google Scholar] [CrossRef]
- Taura, F.; Sirikantaramas, S.; Shoyama, Y.; Yoshikai, K.; Shoyama, Y.; Morimoto, S. Cannabidiolic-acid synthase, the chemotype-determining enzyme in the fiber-typeCannabis sativa. FEBS Lett. 2007, 581, 2929–2934. [Google Scholar] [CrossRef]
- Cockson, P.; Landis, H.; Smith, T.; Hicks, K.; Whipker, B.E. Characterization of Nutrient Disorders of Cannabis sativa. Appl. Sci. 2019, 9, 4432. [Google Scholar] [CrossRef]
- Rainer, G.; Kuhnert, R.; Unterholzer, M.; Dresch, P.; Gruber, A.; Peintner, U. Host-Specialist Dominated Ectomycorrhizal Communities of Pinus cembra are not Affected by Temperature Manipulation. J. Fungi 2015, 1, 55–75. [Google Scholar] [CrossRef] [PubMed]
- Fernández, F.; Vicente-Sánchez, J.; Maestre-Valero, J.F.; Bernabé, A.J.; Nicolás, E.; Pedrero, F.; Alarcon, J.J. Physiological and growth responses of young tomato seedlings to drip-irrigation containing two low doses of the arbuscular mycorrhizal fungus Glomus iranicumvar.tenuihypharumsp.nova. J. Hortic. Sci. Biotechnol. 2014, 89, 679–685. [Google Scholar] [CrossRef]
- Klinsukon, C.; Lumyong, S.; Kuyper, T.W.; Boonlue, S. Colonization by arbuscular mycorrhizal fungi improves salinity tolerance of eucalyptus (Eucalyptus camaldulensis) seedlings. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Chang, W.; Fan, X.; Song, F. Effects of Arbuscular mycorrhizal fungi on photosynthetic and chlorophyll fluorescence characteristics in Elaeagnus angustifolia seedlings under salt stress. Acta Ecol. Sin. 2018, 38, 1337–1347. [Google Scholar] [CrossRef]
- Altomare, C.; Tringovska, I. Beneficial Soil Microorganisms, an Ecological Alternative for Soil Fertility Management. In Ge-netics, Biofuels and Local Farming Systems, 1st ed.; Lichtfouse, E., Ed.; Springer: Dordrecht, The Netherlands, 2011; Volume 7, pp. 161–214. [Google Scholar] [CrossRef]
- Valadares, R.B.S.; Perotto, S.; Lucheta, A.R.; Santos, E.C.; Oliveira, R.M.; Lambais, M.R. Proteomic and Transcriptomic Analyses Indicate Metabolic Changes and Reduced Defense Responses in Mycorrhizal Roots of Oeceoclades maculata (Orchidaceae) Collected in Nature. J. Fungi 2020, 6, 148. [Google Scholar] [CrossRef] [PubMed]
- Kakabouki, I.; Tataridas, A.; Mavroeidis, A.; Kousta, A.; Karydogianni, S.; Zisi, C.; Kouneli, V.; Konstantinou, A.; Folina, A.; Konstantas, A.; et al. Effect of Colonization of Trichoderma harzianum on Growth Development and CBD Content of Hemp (Cannabis sativa L.). Microorganisms 2021, 9, 518. [Google Scholar] [CrossRef] [PubMed]
- Paliwoda, D.; Mikiciuk, G. Use of Rhizosphere Microorganisms in Plant Production—A Review Study. J. Ecol. Eng. 2020, 21, 292–310. [Google Scholar] [CrossRef]
- Chen, S.; Jin, W.; Liu, A.; Zhang, S.; Liu, D.; Wang, F.; Lin, X.; He, C. Arbuscular mycorrhizal fungi (AMF) increase growth and secondary metabolism in cucumber subjected to low temperature stress. Sci. Hortic. 2013, 160, 222–229. [Google Scholar] [CrossRef]
- Dodd, J.C.; Boddington, C.L.; Rodríguez, A.; Gonzalez-Chavez, C.; Mansur, I. Mycelium of Arbuscular Mycorrhizal fungi (AMF) from different genera: Form, function and detection. Plant Soil 2000, 226, 131–151. [Google Scholar] [CrossRef]
- Spagnoletti, F.; Carmona, M.; Gómez, N.E.; Chiocchio, V.; Lavado, R.S. Arbuscular mycorrhiza reduces the negative effects of M. phaseolina on soybean plants in arsenic-contaminated soils. Appl. Soil Ecol. 2017, 121, 41–47. [Google Scholar] [CrossRef]
- Mota, I.; Sánchez-Sánchez, J.; Pedro, L.G.; Sousa, M.J. Composition variation of the essential oil from Ocimum basilicum L. cv. Genovese Gigante in response to Glomus intraradices and mild water stress at different stages of growth. Biochem. Syst. Ecol. 2020, 90, 104021. [Google Scholar] [CrossRef]
- Khalediyan, N.; Weisany, W.; Schenk, P.M. Arbuscular mycorrhizae and rhizobacteria improve growth, nutritional status and essential oil production in Ocimum basilicum and Satureja hortensis. Ind. Crop. Prod. 2021, 160, 113163. [Google Scholar] [CrossRef]
- Latef, A.A.H.A.; Hashem, A.; Rasool, S.; Abd_Allah, E.F.; Alqarawi, A.A.; Egamberdieva, D.; Jan, S.; Anjum, N.A.; Ahmad, P. Arbuscular mycorrhizal symbiosis and abiotic stress in plants: A review. J. Plant Biol. 2016, 59, 407–426. [Google Scholar] [CrossRef]
- He, F.; Sheng, M.; Tang, M. Effects of Rhizophagus irregularis on Photosynthesis and Antioxidative Enzymatic System in Robinia pseudoacacia L. under Drought Stress. Front. Plant Sci. 2017, 8, 183. [Google Scholar] [CrossRef]
- Ortiz, N.; Armada, E.; Duque, E.; Roldán, A.; Azcón, R. Contribution of arbuscular mycorrhizal fungi and/or bacteria to enhancing plant drought tolerance under natural soil conditions: Effectiveness of autochthonous or allochthonous strains. J. Plant Physiol. 2015, 174, 87–96. [Google Scholar] [CrossRef]
- Wang, F.; Li, K.; Shi, Z. Phosphorus fertilization and mycorrhizal colonization change silver nanoparticle impacts on maize. Ecotoxicology 2021, 30, 118–129. [Google Scholar] [CrossRef]
- Nacoon, S.; Ekprasert, J.; Riddech, N.; Mongkolthanaruk, W.; Jogloy, S.; Vorasoot, N.; Cooper, J.; Boonlue, S. Growth enhancement of sunchoke by arbuscular mycorrhizal fungi under drought condition. Rhizosphere 2021, 17, 100308. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Landi, L.; Renella, G. Role of phosphatase enzymes in soil. In Phosphorus in Action; Bünemann, E., Oberson, A., Frossard, E., Eds.; Soil Biology; Springer: Berlin/Heidelberg, Germany, 2011; Volume 26, pp. 215–243. [Google Scholar]
- White, P.J.; Hammond, J.P. Phosphorus nutrition of terrestrial plants. In The Ecophysiology of Plant-Phosphorus Interactions, 1st ed.; White, P.J., Hammond, J.P., Eds.; Springer: Dordrecht, The Netherlands, 2008; Volume 7, pp. 51–81. [Google Scholar] [CrossRef]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Functions of macronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2011; pp. 135–189. ISBN 9780123849052. [Google Scholar]
- Chen, S.; Zhao, H.; Zou, C.; Li, Y.; Chen, Y.; Wang, Z.; Jiang, Y.; Liu, A.; Zhao, P.; Wang, M.; et al. Combined Inoculation with Multiple Arbuscular Mycorrhizal Fungi Improves Growth, Nutrient Uptake and Photosynthesis in Cucumber Seedlings. Front. Microbiol. 2017, 8, 2516. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Campbell, W.F.; Rupp, L.A.; Allen, E.B. Effects of mycorrhizae on sorghum growth, photosynthesis, and stomatal conductance under drought conditions. Arid. Soil Res. Rehabil. 1990, 4, 99–107. [Google Scholar] [CrossRef]
- Caravaca, F.; Díaz, E.; Barea, J.; Azcon, C.; Roldán, A. Photosynthetic and Transpiration Rates of Olea europaea subsp. sylvestris and Rhamnus lycioides as Affected by Water Deficit and Mycorrhiza. Biol. Plant. 2003, 46, 637–639. [Google Scholar] [CrossRef]
- Zuccarini, P.; Okurowska, P. Effects of Mycorrhizal Colonization and Fertilization on Growth and Photosynthesis of Sweet Basil Under Salt Stress. J. Plant Nutr. 2008, 31, 497–513. [Google Scholar] [CrossRef]
- Herold, A.; Walker, D.A. Transport across Chloroplast Envelopes the Role of Phosphate. In Transport Across Single Biological Membranes, 1st ed.; Tosteson, D.C., Ed.; Springer: Berlin, Germany, 1979; Volume 2, pp. 411–439. [Google Scholar] [CrossRef]
- Nemec, S.; Vu, J.C.V. Effects of soil phosphorus and Glomus intraradices on growth, nonstructural carbohydrates, and photosynthetic activity of Citrus aurantium. Plant Soil 1990, 128, 257–263. [Google Scholar] [CrossRef]
- Al-Arjani, A.-B.F.; Hashem, A.; Abd_Allah, E.F. Arbuscular mycorrhizal fungi modulates dynamics tolerance expression to mitigate drought stress in Ephedra foliata Boiss. Saudi J. Biol. Sci. 2020, 27, 380–394. [Google Scholar] [CrossRef]
- Jaitieng, S.; Sinma, K.; Rungcharoenthong, P.; Amkha, S. Arbuscular mycorrhiza fungi applications and rock phosphate fertilizers enhance available phosphorus in soil and promote plant immunity in robusta coffee. Soil Sci. Plant Nutr. 2021, 67, 97–101. [Google Scholar] [CrossRef]
- Vlot, A.C.; Dempsey, D.A.; Klessig, D.F. Salicylic acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef]
- Bolandnazar, S.; Neyshabouri, M.R.; Aliasgharzad, N.; Chaparzadeh, N. Effects of Mycorrhizal Colonization on Growth Parameters of Onion under Different Irrigation and Soil Conditions. Pak. J. Biol. Sci. 2007, 10, 1491–1495. [Google Scholar] [CrossRef]
- Annuum, C.; De Cabra, L.C.; Ccastilluct, C. Effect of Arbucula Myrrhizal Fungi on an Ecological Crop of Chili Peppers (Capsicum annuum L.). Chil. J. Agric. Res. 2009, 69, 79–87. [Google Scholar] [CrossRef]
- Krishna, H.; Singh, S.K.; Minakshi; Patel, V.B.; Khawale, R.N.; Deshmukh, P.S.; Jindal, P.C. Arbuscular-mycorrhizal fungi alleviate transplantation shock in micropropagated grapevine (Vitis vinifera L.). J. Hortic. Sci. Biotechnol. 2006, 81, 259–263. [Google Scholar] [CrossRef]
- Kishor, P.B.K.; Kumari, P.H.; Sunita, M.S.L.; Sreenivasulu, N. Role of proline in cell wall synthesis and plant development and its implications in plant ontogeny. Front. Plant Sci. 2015, 6, 544. [Google Scholar] [CrossRef]
- Montaño-Mata, N.J.; Núñez, J.C. Evaluación del efecto de la edad de transplante sobre el rendimiento en tres selecciones de ají dulce Capsicum chinense Jacq. en Jusepín, estado Monagas. Rev. Fac. Agron. 2003, 20, 144–155. [Google Scholar]
- Balota, E.L.; Machineski, O.; Truber, P.V.; Scherer, A.; De Souza, F.S. Physic nut plants present high mycorrhizal dependency under conditions of low phosphate availability. Braz. J. Plant Physiol. 2011, 23, 33–44. [Google Scholar] [CrossRef]
- Carmo, É.R.D.; Da Silva, C.F.; Freitas, M.S.M.; Lima, K.B.; Martins, M.A. Production of Autralian Cedar Seedlings Iinoculated with Abuscular Mycorrhizal Fungi in different types of containers. Rev. Árvore 2016, 40, 269–278. [Google Scholar] [CrossRef][Green Version]
- Aguilar, E.E.Q.; Montoya-Martínez, A.C.; Rincón-Enriquez, G.; Lobit, P.; López-Pérez, L. Effectiveness of native arbuscular mycorrhizal consortia on the growth of Agave inaequidens. J. Soil Sci. Plant Nutr. 2016, 16, 1052–1064. [Google Scholar] [CrossRef]
- Gomes, J.M.; Couto, L.; Leite, H.G.; Xavier, A.; Garcia, S.L.R. Parâmetros morfológicos na avaliação de qualidade de mudas de Eucalyptus grandis. Rev. Árvore 2002, 26, 655–664. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. AN Evaluation of Techniques for Measuring Vesicular Arbuscular Mycorrhizal Infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
| Total Root Length (cm) | Root Dry Mass (g) | Plant Height (cm) | Fresh Weight (g) | Dry Stem Weight (g) | DSW/RDM | Stem Diameter (cm) | Total DW (g) | |
|---|---|---|---|---|---|---|---|---|
| Control | 207 a | 1.19 ns | 11.54 ns | 4.97 ns | 2.01 a | 1.70 ns | 0.40 a | 3.20 a |
| AMF1 | 245 ab | 1.23 ns | 11.23 ns | 4.74 ns | 2.20 b | 1.80 ns | 0.37 b | 3.43 bc |
| AMF2 | 258.33 bc | 1.26 ns | 11.45 ns | 4.80 ns | 2.30 bc | 1.83 ns | 0.38 b | 3.56 b |
| AMF3 | 277.67 c | 1.29 ns | 11.69 ns | 4.99 ns | 2.44 c | 1.89 ns | 0.40 a | 3.73 c |
| F | 6.13 * | 1.08 ns | 0.39 ns | 1.71 ns | 19.70 *** | 1.37 ns | 3.96 * | 13.48 ** |
| Survival Rate | N Content % | P Content (mg/kg) | Seedling Index (DQI) | |
|---|---|---|---|---|
| Control | 90.83 a | 2.26 ns | 0.37 a | 0.1084 a |
| AMF1 | 92.93 a | 2.45 ns | 0.41 a | 0.1088 ab |
| AMF2 | 93.60 a | 2.46 ns | 0.43 a | 0.1138 bc |
| AMF3 | 95.53 b | 2.58 ns | 0.46 b | 0.1218 c |
| F | 4.20 * | 0.73 ns | 4.15 * | 7.18 * |
| Total Root Length (cm) | Root Dry Mass (g) | Plant Heigh (cm) | Fresh Weight (g) | Dry Stem Weight (g) | DSW/RDM | Stem Diameter (cm) | Total DW | Survival Rate | N Content % | P Content mg/kg | Seedling Index (DQI) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMF%20 DAE | 0.8233 * | 0.4935 ns | −0.0227 ns | 0.1564 ns | 0.9019 *** | 0.5607 ns | −0.1119 ns | 0.8689 *** | 0.7319 ns | 0.6861 ns | 0.7660 * | 0.7584 * |
| Seedling Index | 0.5569 ns | 0.4462 ns | −0.2340 ns | 0.4793 ns | 0.6476 ns | 0.3137 ns | 0.4291 ns | 0.6560 ns | 0.4873 ns | 0.7977 * | 0.4205 ns | - |
| Treatment | MycoPlant® Polvo Grow Dose (g L−1) | Total Applied Fungus Spores |
|---|---|---|
| Control | - | - |
| AMF1 | 0.1 | 2000 |
| AMF2 | 0.2 | 4000 |
| AMF3 | 0.3 | 6000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakabouki, I.; Mavroeidis, A.; Tataridas, A.; Kousta, A.; Efthimiadou, A.; Karydogianni, S.; Katsenios, N.; Roussis, I.; Papastylianou, P. Effect of Rhizophagus irregularis on Growth and Quality of Cannabis sativa Seedlings. Plants 2021, 10, 1333. https://doi.org/10.3390/plants10071333
Kakabouki I, Mavroeidis A, Tataridas A, Kousta A, Efthimiadou A, Karydogianni S, Katsenios N, Roussis I, Papastylianou P. Effect of Rhizophagus irregularis on Growth and Quality of Cannabis sativa Seedlings. Plants. 2021; 10(7):1333. https://doi.org/10.3390/plants10071333
Chicago/Turabian StyleKakabouki, Ioanna, Antonios Mavroeidis, Alexandros Tataridas, Angeliki Kousta, Aspasia Efthimiadou, Stella Karydogianni, Nikolaos Katsenios, Ioannis Roussis, and Panayiota Papastylianou. 2021. "Effect of Rhizophagus irregularis on Growth and Quality of Cannabis sativa Seedlings" Plants 10, no. 7: 1333. https://doi.org/10.3390/plants10071333
APA StyleKakabouki, I., Mavroeidis, A., Tataridas, A., Kousta, A., Efthimiadou, A., Karydogianni, S., Katsenios, N., Roussis, I., & Papastylianou, P. (2021). Effect of Rhizophagus irregularis on Growth and Quality of Cannabis sativa Seedlings. Plants, 10(7), 1333. https://doi.org/10.3390/plants10071333











