Lasting Effects of Low to Non-Lethal Radiation Exposure during Late Gestation on Offspring’s Cardiac Metabolism and Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care and Experimental Groups
2.2. Tissue Preparation
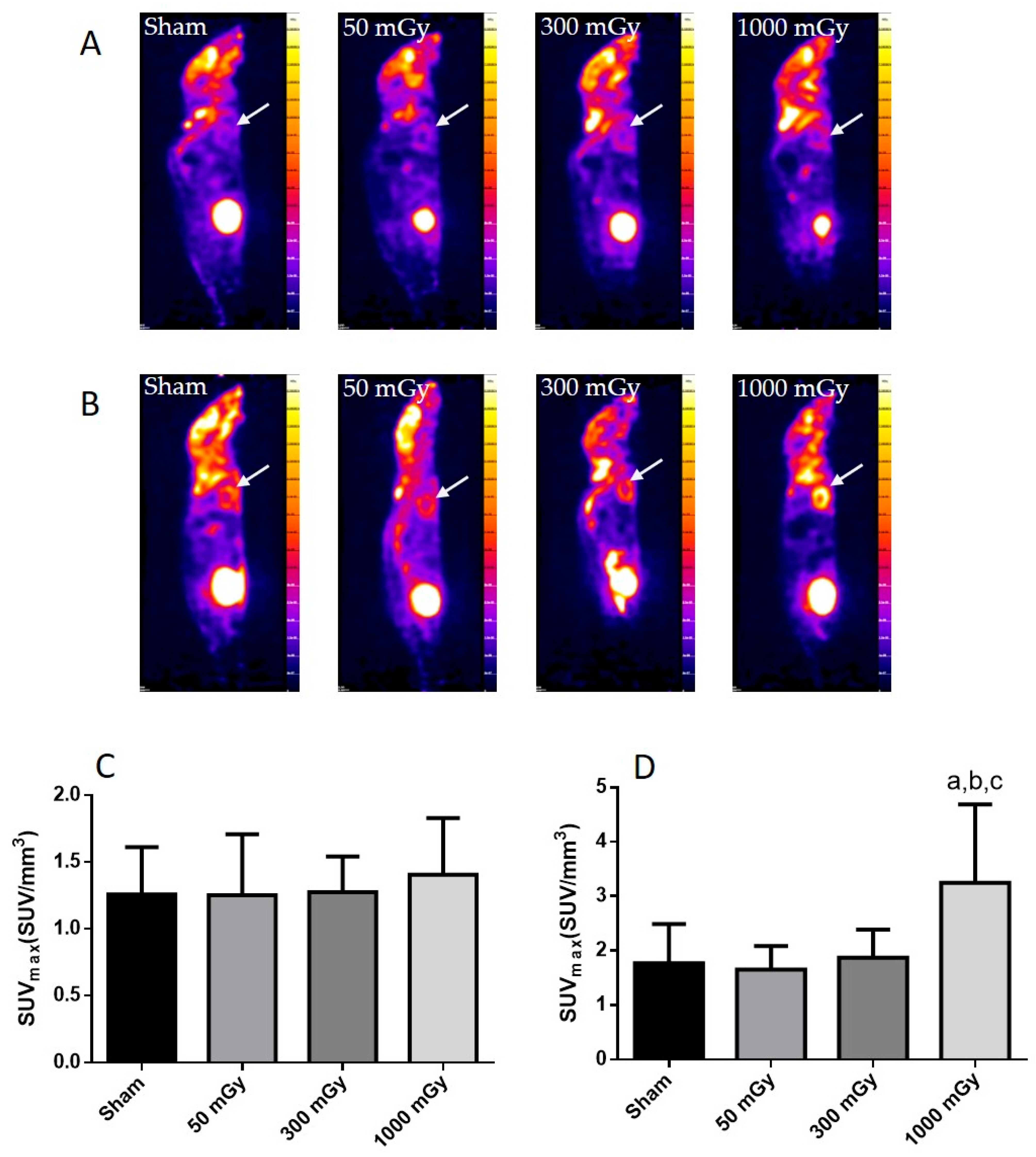
2.3. Positron Emission Tomography
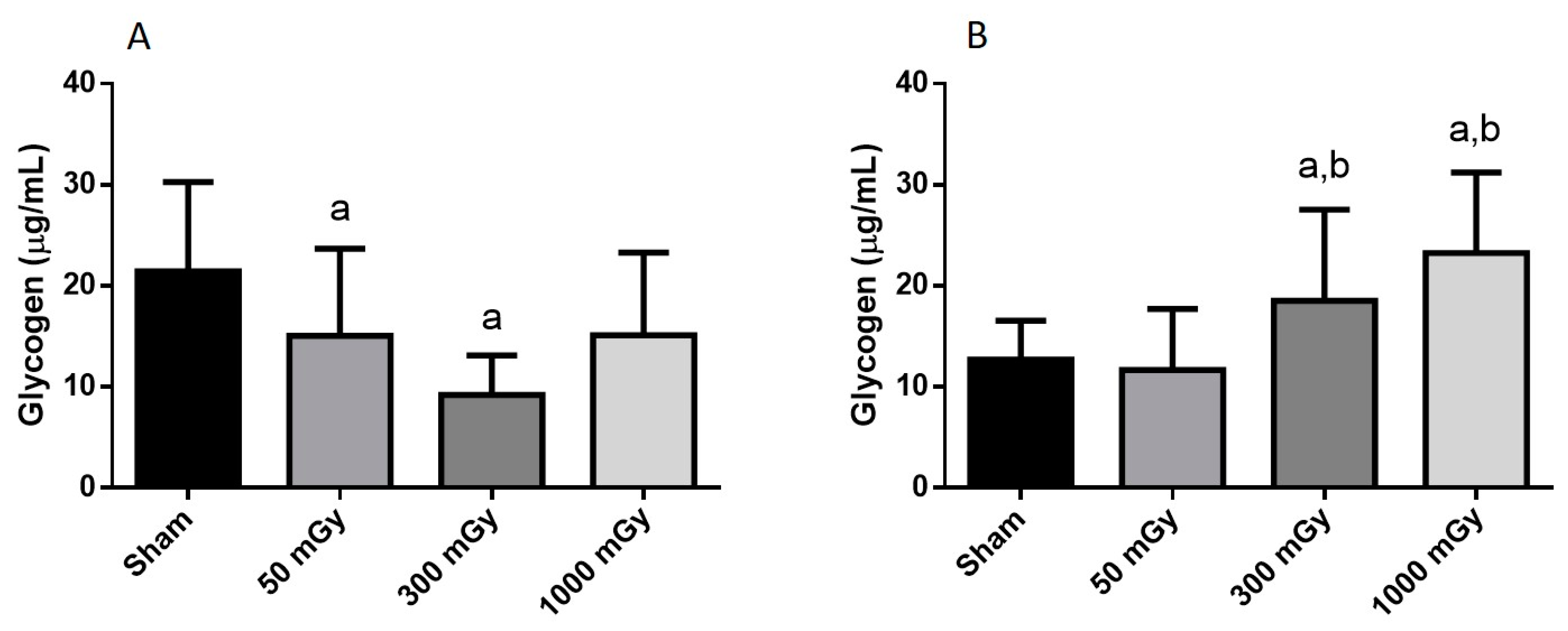
2.4. Glycogen Content
2.5. Antioxidant Enzyme Activity
2.6. NADPH Oxidase Enzyme Activity
2.7. Total ROS Content
2.8. Redox Ratio
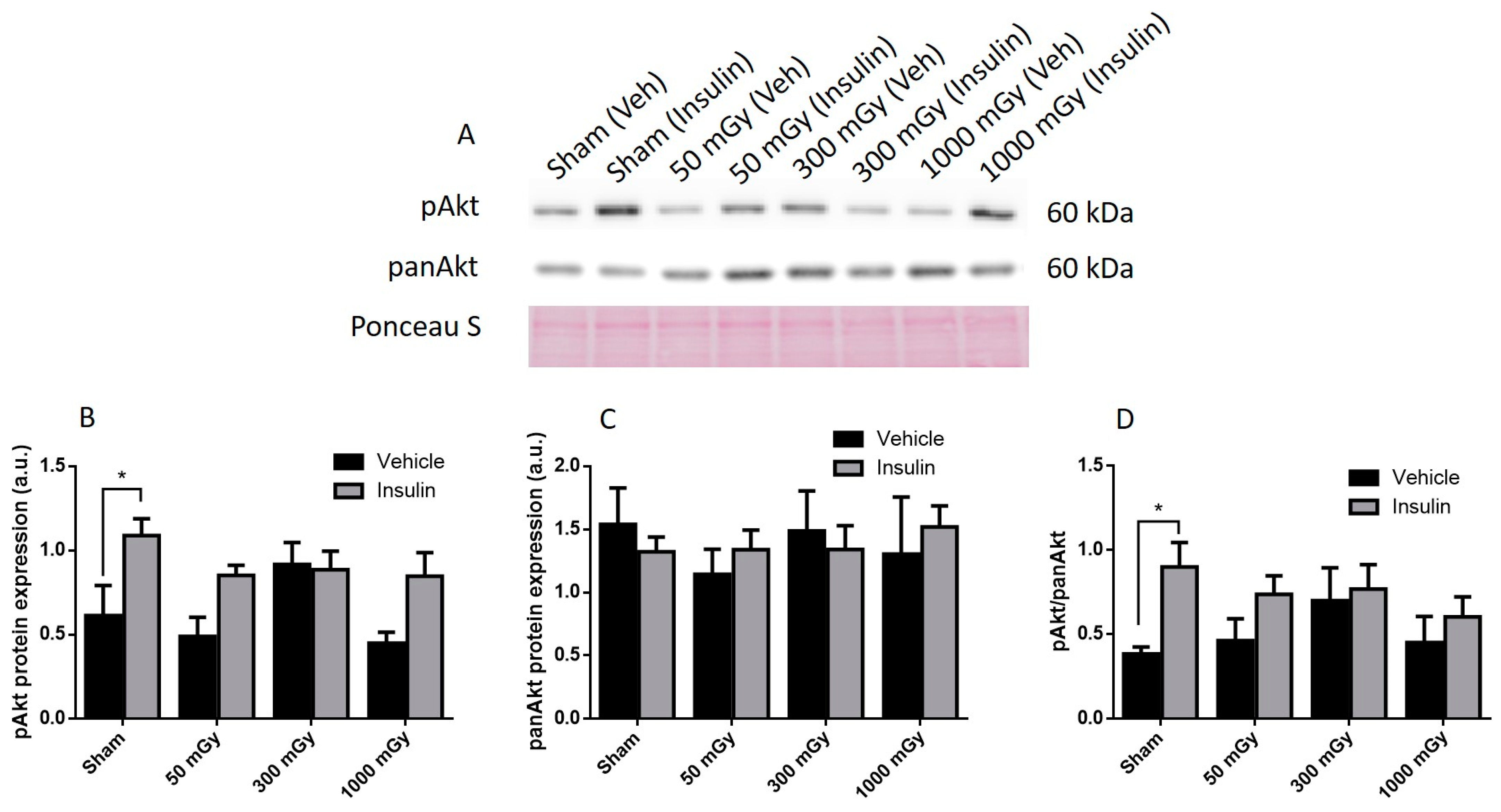
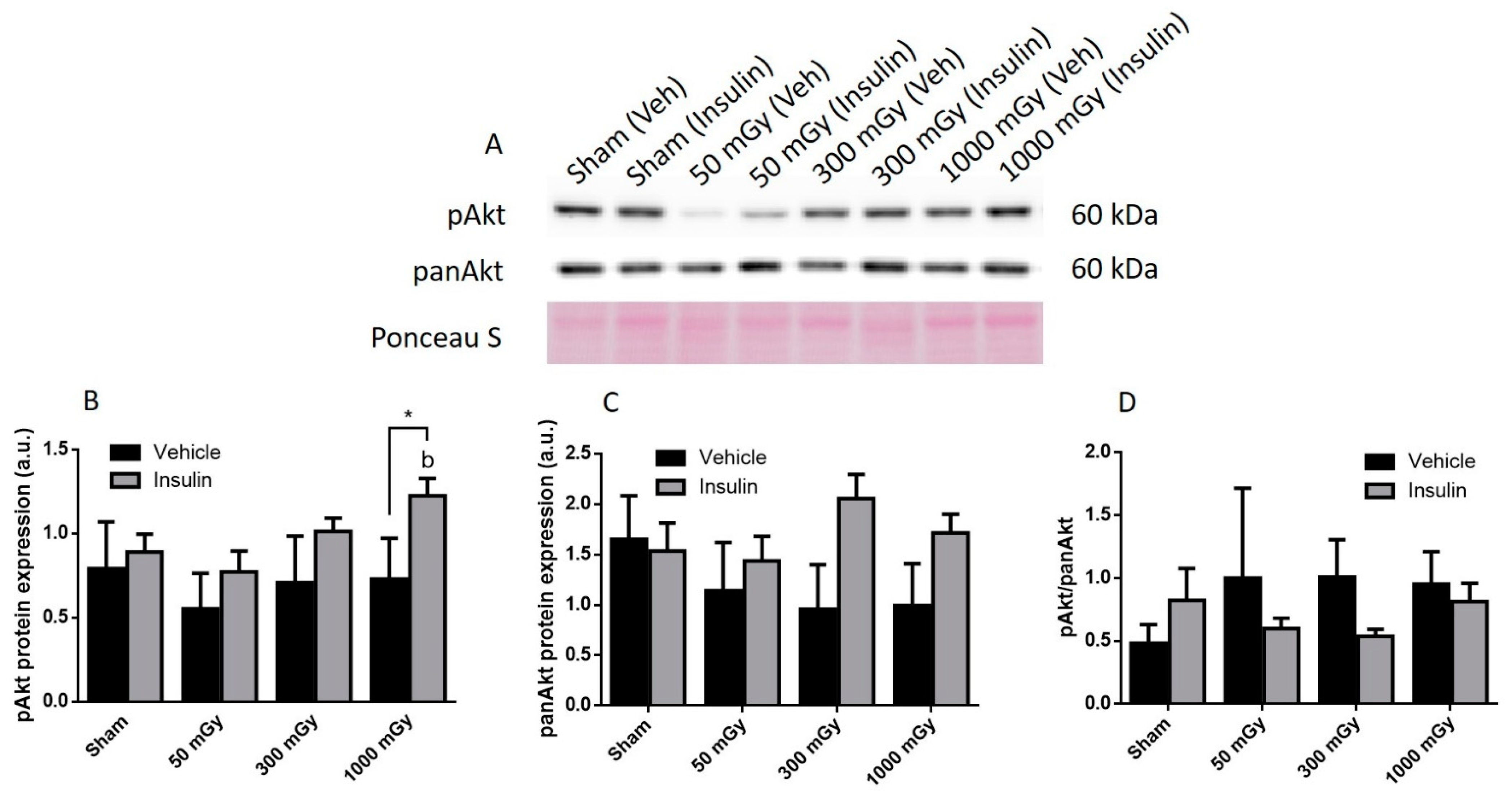
2.9. Western Blot
2.10. Plasma Cytokine Concentration
2.11. Statistical Analysis
3. Results
3.1. Body and Heart Weight
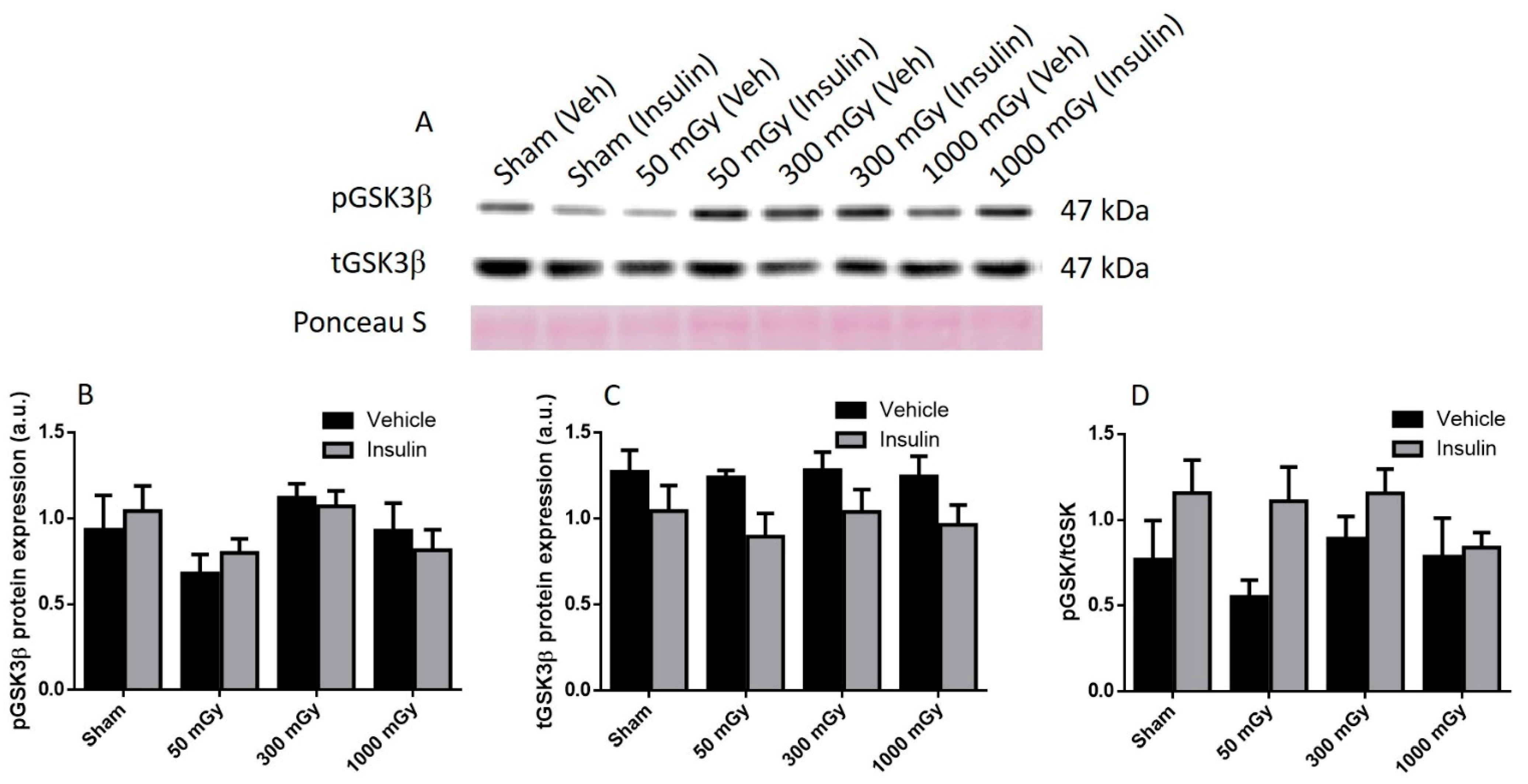
3.2. Cardiac Glucose Uptake, Storage, and Insulin Signaling
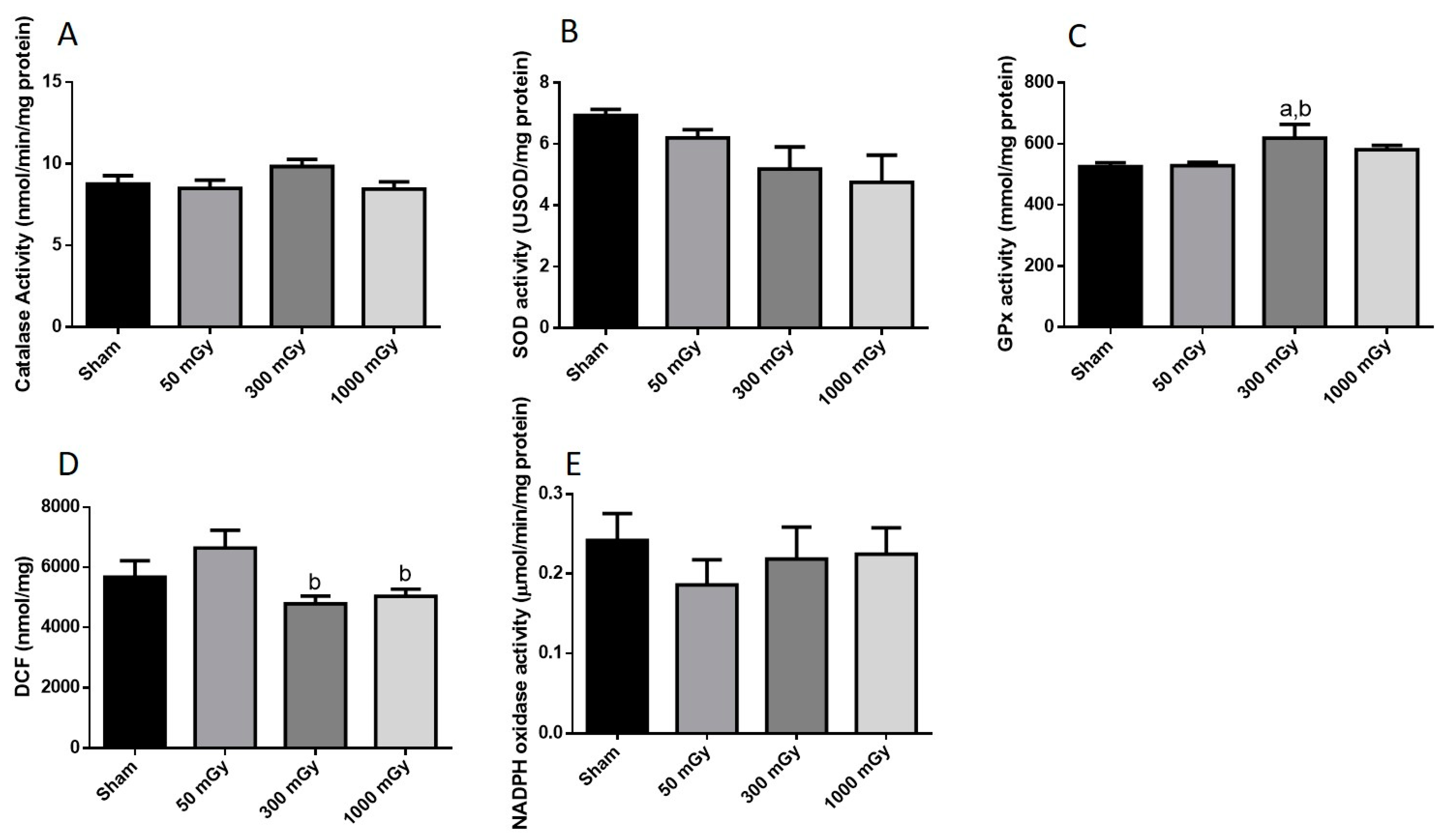
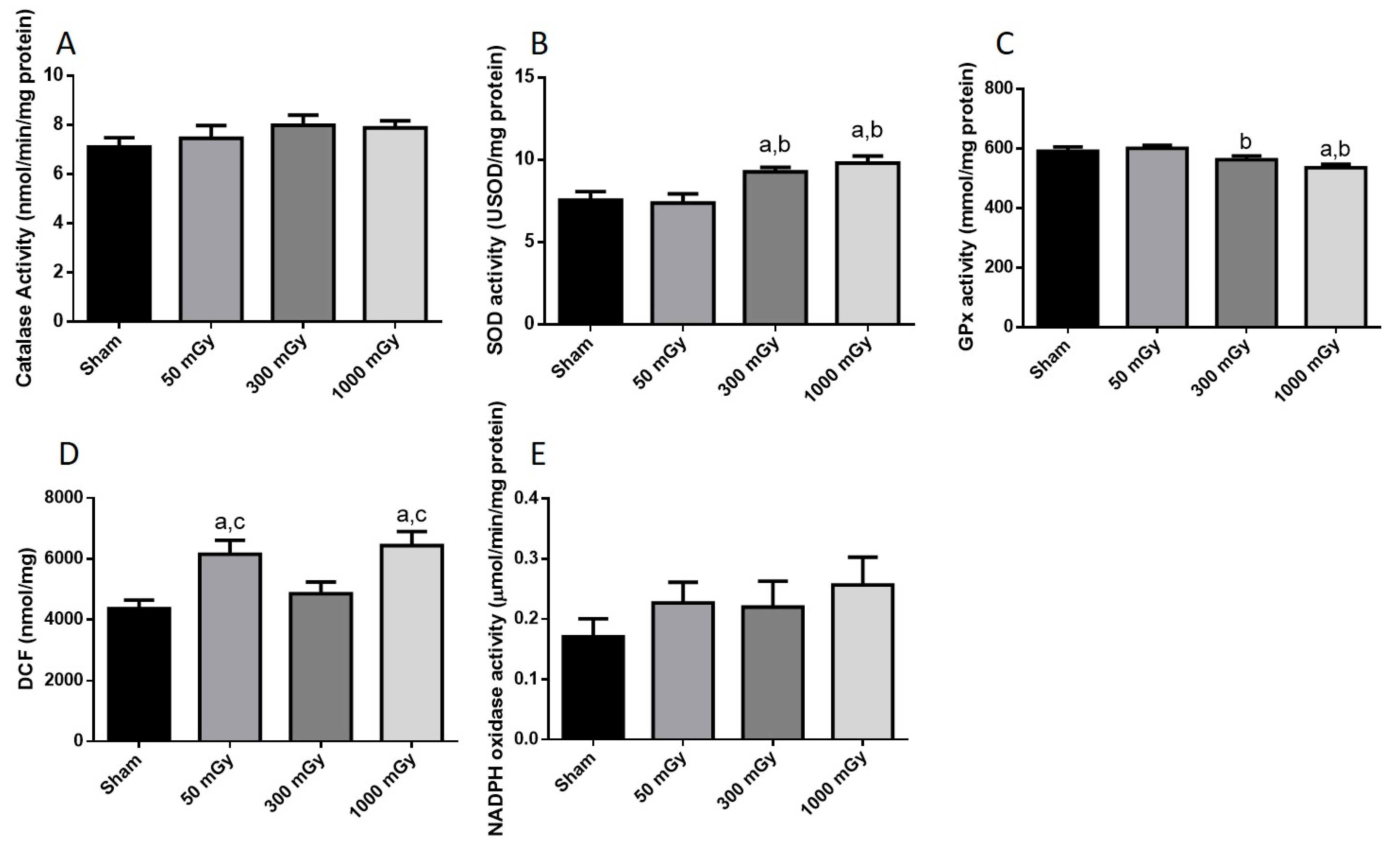
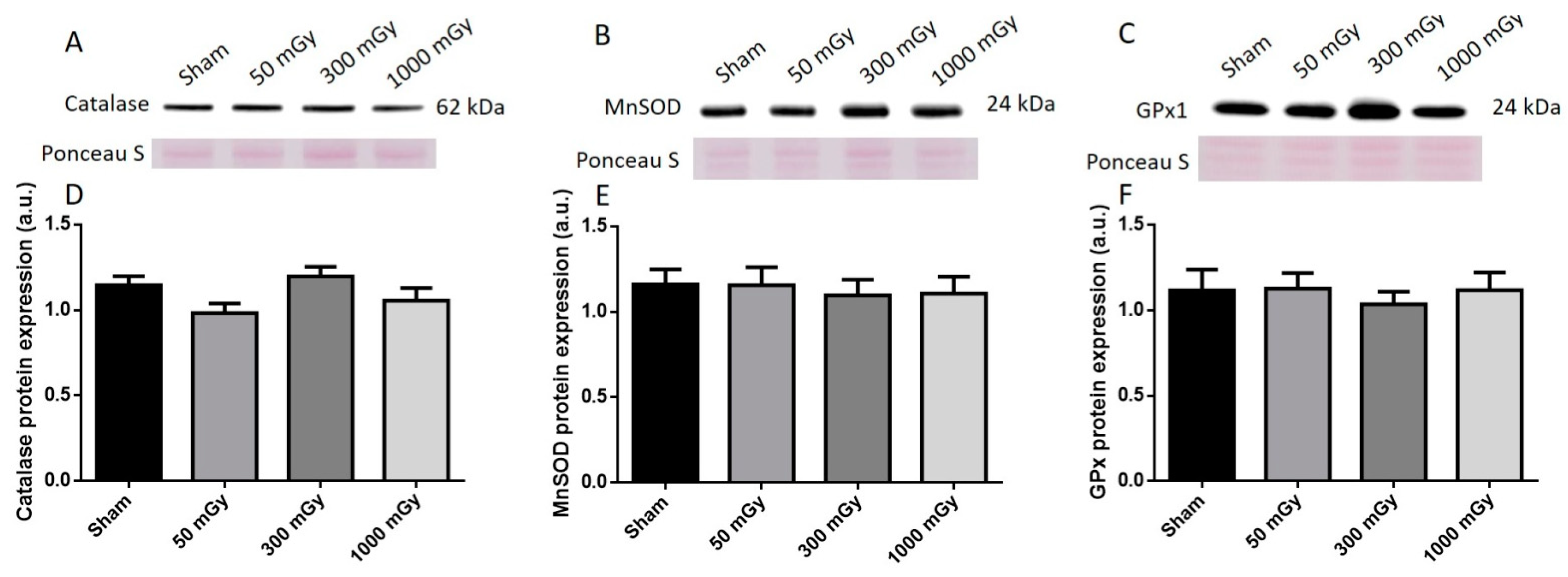
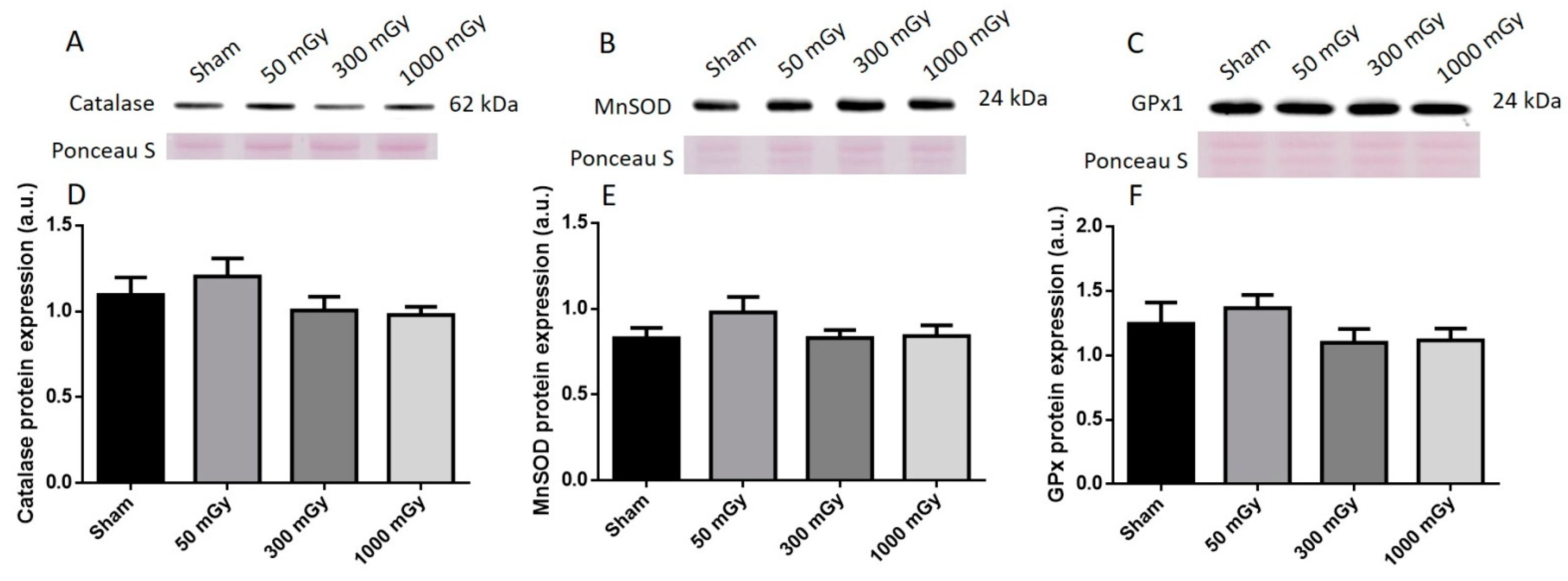
3.3. Antioxidant and Oxidative Stress Status
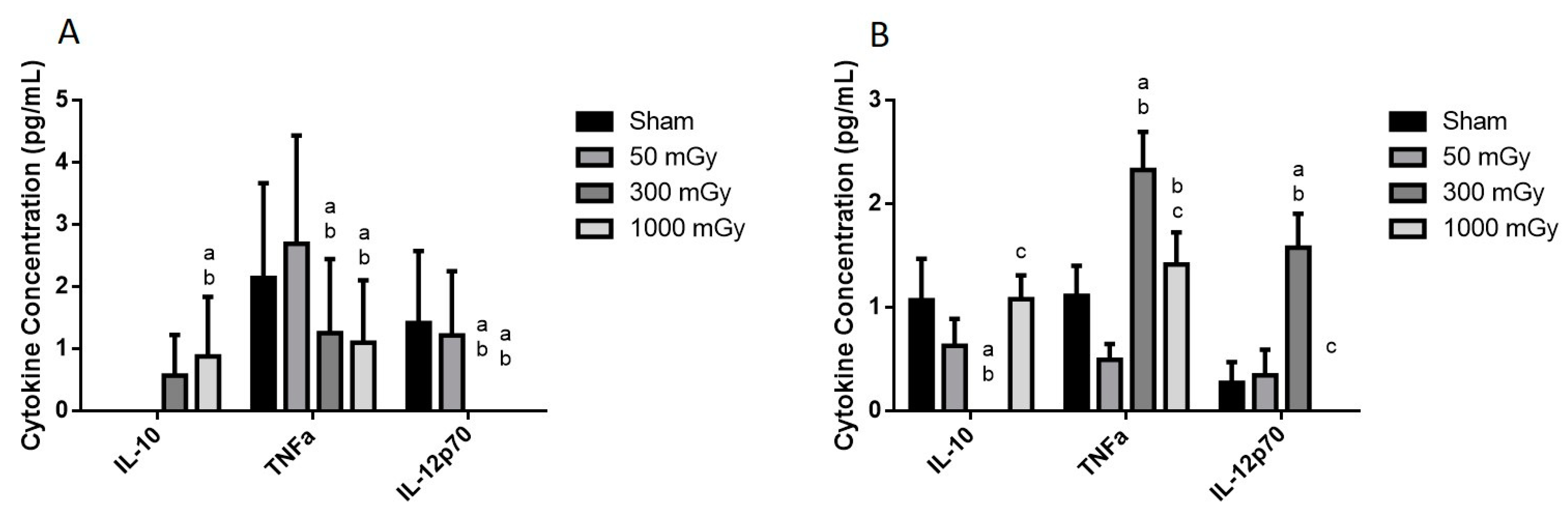
3.4. Circulating Cytokines
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crispi, F.; Miranda, J.; Gratacós, E. Long-Term Cardiovascular Consequences of Fetal Growth Restriction: Biology, Clinical Implications, and Opportunities for Prevention of Adult Disease. Am. J. Obstet. Gynecol. 2018, 218, S869–S879. [Google Scholar] [CrossRef] [PubMed]
- Moisiadis, V.G.; Matthews, S.G. Glucocorticoids and Fetal Programming Part 1: Outcomes. Nat. Rev. Endocrinol. 2014, 10, 391–402. [Google Scholar] [CrossRef]
- Barker, D.J.; Gluckman, P.D.; Godfrey, K.M.; Harding, J.E.; Owens, J.A.; Robinson, J.S. Fetal Nutrition and Cardiovascular Disease in Adult Life. Lancet Lond. Engl. 1993, 341, 938–941. [Google Scholar] [CrossRef]
- Frankel, S.; Elwood, P.; Sweetnam, P.; Yarnell, J.; Smith, G.D. Birthweight, Body-Mass Index in Middle Age, and Incident Coronary Heart Disease. Lancet Lond. Engl. 1996, 348, 1478–1480. [Google Scholar] [CrossRef]
- Syddall, H.E.; Sayer, A.A.; Simmonds, S.J.; Osmond, C.; Cox, V.; Dennison, E.M.; Barker, D.J.P.; Cooper, C. Birth Weight, Infant Weight Gain, and Cause-Specific Mortality: The Hertfordshire Cohort Study. Am. J. Epidemiol. 2005, 161, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Seckl, J.R.; Cleasby, M.; Nyirenda, M.J. Glucocorticoids, 11β-Hydroxysteroid Dehydrogenase, and Fetal Programming. Kidney Int. 2000, 57, 1412–1417. [Google Scholar] [CrossRef]
- Joseph, D.N.; Whirledge, S. Stress and the HPA Axis: Balancing Homeostasis and Fertility. Int. J. Mol. Sci. 2017, 18, 2224. [Google Scholar] [CrossRef]
- Puthanveetil, P.; Rodrigues, B. Glucocorticoid excess induces accumulation of cardiac glycogen and triglyceride: Suggested role for AMPK. Curr. Pharm. Des. 2013, 19, 4818–4830. [Google Scholar] [CrossRef]
- Qi, D.; Rodrigues, B. Glucocorticoids Produce Whole Body Insulin Resistance with Changes in Cardiac Metabolism. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E654–E667. [Google Scholar] [CrossRef]
- Skobowiat, C.; Slominski, A.T. Ultraviolet B (UVB) Activates Hypothalamic-Pituitary-Adrenal (HPA) Axis in C57BL/6 Mice. J. Investig. Dermatol. 2015, 135, 1638–1648. [Google Scholar] [CrossRef]
- Velicković, N.; Djordjević, A.; Matić, G.; Horvat, A. Radiation-Induced Hyposuppression of the Hypothalamic-Pituitary-Adrenal Axis Is Associated with Alterations of Hippocampal Corticosteroid Receptor Expression. Radiat. Res. 2008, 169, 397–407. [Google Scholar] [CrossRef] [PubMed]
- United Nations Scientific Committee on the Effects of Atomic Radiation, N.A. of In This Issue. Proc. Natl. Acad. Sci. USA 2008, 105, 9131–9132. [CrossRef]
- Canandian Nuclear Safety Commission, Radiation Doses. Available online: http://nuclearsafety.gc.ca/eng/resources/radiation/introduction-to-radiation/radiation-doses.cfm (accessed on 12 April 2021).
- Plett, P.A.; Sampson, C.H.; Chua, H.L.; Joshi, M.; Booth, C.; Gough, A.; Johnson, C.S.; Katz, B.P.; Farese, A.M.; Parker, J.; et al. Establishing a Murine Model of the Hematopoietic Syndrome of the Acute Radiation Syndrome. Health Phys. 2012, 103, 343–355. [Google Scholar] [CrossRef]
- Zhang, S.B.; Maguire, D.; Zhang, M.; Tian, Y.; Yang, S.; Zhang, A.; Casey-Sawicki, K.; Han, D.; Ma, J.; Yin, L.; et al. Mitochondrial DNA and Functional Investigations into the Radiosensitivity of Four Mouse Strains. Int. J. Cell Biol. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.J.; Giaccia, A.J. Radiobiology for the Radiologist; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; ISBN 978-0-7817-4151-4. [Google Scholar]
- Lau, C.; Rogers, J.M. Embryonic and Fetal Programming of Physiological Disorders in Adulthood. Birth Defects Res. Part C Embryo Today Rev. 2004, 72, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Tam, P.; Tan, S. The Somitogenetic Potential of Cells in the Primitive Streak and the Tail Bud of the Organogenesis-Stage Mouse Embryo. Development 1992, 115, 703–715. Available online: https://pubmed.ncbi.nlm.nih.gov/1425350/ (accessed on 25 April 2021). [CrossRef]
- Streffer, C.; Molls, M. Cultures of Preimplantation Mouse Embryos: A Model for Radiobiological Studies. In Advances in Radiation Biology; Lett, J.T., Ed.; Academic Press: New York, NY, USA, 1987; Volume 13, pp. 169–213. [Google Scholar]
- Culiat, C.T.; Stubbs, L.; Nicholls, R.D.; Montgomery, C.S.; Russell, L.B.; Johnson, D.K.; Rinchik, E.M. Concordance between Isolated Cleft Palate in Mice and Alterations within a Region Including the Gene Encoding the Beta 3 Subunit of the Type A Gamma-Aminobutyric Acid Receptor. Proc. Natl. Acad. Sci. USA 1993, 90, 5105–5109. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, J.H.; Oh, H.; Kim, S.R.; Lee, C.S.; Jo, S.K.; Kim, T.H.; Lee, Y.S. Dependence of Malformation upon Gestational Age and Exposed Dose of Gamma Radiation. J. Radiat. Res. 2001, 42, 255–264. [Google Scholar] [CrossRef]
- Lindsay, K.L.; Buss, C.; Wadhwa, P.D.; Entringer, S. The interplay between nutrition and stress in pregnancy: Implications for fetal programming of brain development. Biol. Psychiatry 2019, 85, 135–149. [Google Scholar] [CrossRef]
- Spitz, D.R.; Azzam, E.I.; Jian Li, J.; Gius, D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: A unifying concept in stress response biology. Cancer Metastasis Rev. 2004, 23, 311–322. [Google Scholar] [CrossRef]
- Szumiel, I. Ionizing radiation-induced oxidative stress, epigenetic changes and genomic instability: The pivotal role of mitochondria. Int. J. Radiat. Biol. 2015, 91, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Taverne, Y.J.H.J.; Bogers, A.J.J.C.; Duncker, D.J.; Merkus, D. Reactive oxygen species and the cardiovascular system. Oxid. Med. Cell. Longev. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Azzam, E.I.; Jay-Gerin, J.-P.; Pain, D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Singla, N.; Chadha, V.D.; Dhawan, D.K. A concept of radiation hormesis: Stimulation of antioxidant machinery in rats by low dose ionizing radiation. Hell. J. Nucl. Med. 2019, 22, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Shibamoto, Y.; Nakamura, H. Overview of biological, epidemiological, and clinical evidence of radiation hormesis. Int. J. Mol. Sci. 2018, 19, 2387. [Google Scholar] [CrossRef]
- Weiss, J.F.; Landauer, M.R. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology 2003, 189, 1–20. [Google Scholar] [CrossRef]
- Đurović, B.; Spasić-Jokić, V.; Đurović, B. Influence of occupational exposure to low-dose ionizing radiation on the plasma activity of superoxide dismutase and glutathione level. Vojnosanit. Pregl. 2008, 65, 613–618. [Google Scholar] [CrossRef]
- Pathak, C.M.; Avti, P.K.; Kumar, S.; Khanduja, K.L.; Sharma, S.C. Whole body exposure to low-dose gamma radiation promotes kidney antioxidant status in balb/c mice. J. Radiat. Res. 2007, 48, 113–120. [Google Scholar] [CrossRef]
- Cui, J.; Yang, G.; Pan, Z.; Zhao, Y.; Liang, X.; Li, W.; Cai, L. Hormetic response to low-dose radiation: Focus on the immune system and its clinical implications. Int. J. Mol. Sci. 2017, 18, 280. [Google Scholar] [CrossRef]
- Miura, Y. Oxidative stress, radiation-adaptive responses, and aging. J. Radiat. Res. 2004, 45, 357–372. [Google Scholar] [CrossRef]
- Yang, G.; Li, W.; Jiang, H.; Liang, X.; Zhao, Y.; Yu, D.; Zhou, L.; Wang, G.; Tian, H.; Han, F.; et al. Low-dose radiation may be a novel approach to enhance the effectiveness of cancer therapeutics. Int. J. Cancer 2016, 139, 2157–2168. [Google Scholar] [CrossRef] [PubMed]
- Sreetharan, S.; Thome, C.; Tharmalingam, S.; Jones, D.E.; Kulesza, A.V.; Khaper, N.; Lees, S.J.; Wilson, J.Y.; Boreham, D.R.; Tai, T.C. Ionizing radiation exposure during pregnancy: Effects on postnatal development and life. Radiat. Res. 2017, 187, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.; Vitale, C. Metabolic modulation of cardiac metabolism in heart failure. J. Metab. Modul. Card. Metab. Heart Fail. 2018, 67, 177–181. [Google Scholar] [CrossRef]
- Tran, D.H.; Wang, Z.V. Glucose metabolism in cardiac hypertrophy and heart failure. J. Am. Heart Assoc. 2019, 8, e012673. [Google Scholar] [CrossRef] [PubMed]
- Duque-Guimarães, D.E.; Ozanne, S.E. Nutritional programming of insulin resistance: Causes and consequences. Trends Endocrinol. Metab. TEM 2013, 24, 525–535. [Google Scholar] [CrossRef]
- Zhai, P.; Sciarretta, S.; Galeotti, J.; Volpe, M.; Sadoshima, J. Differential roles of GSK-3β during myocardial ischemia and ischemia/reperfusion. Circ. Res. 2011, 109, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Vélez, D.E.; Mestre-Cordero, V.E.; Hermann, R.; Perego, J.; Harriet, S.; Fernandez-Pazos, M.; de las, M.; Mourglia, J.; Marina-Prendes, M.G. Rosuvastatin protects isolated hearts against ischemia-reperfusion injury: Role of Akt-GSK-3β, metabolic environment, and mitochondrial permeability transition pore. J. Physiol. Biochem. 2020, 76, 85–98. [Google Scholar] [CrossRef]
- Yao, H.; Han, X.; Han, X. The Cardioprotection of the Insulin-Mediated PI3K/Akt/mTOR Signaling Pathway. Am. J. Cardiovasc. Drugs 2014, 14, 433–442. [Google Scholar] [CrossRef]
- Davidson, C.Q.; Tharmalingam, S.; Niccoli, S.; Nemec-Bakk, A.; Khurana, S.; Murray, A.; Tai, T.C.; Boreham, D.R.; Khaper, N.; Lees, S.J. Dose threshold for radiation induced fetal programming in a mouse model at 4 months of age: Hepatic expression of genes and proteins involved in glucose metabolism and glucose uptake in brown adipose tissue. PLoS ONE 2020, 15, e0231650. [Google Scholar] [CrossRef]
- Tomášová, L.; Šmajda, B.; Ševc, J. Effects of prenatal irradiation on behaviour and hippocampal neurogenesis in adult rats. Acta Physiol. Hung. 2012, 99, 126–132. [Google Scholar] [CrossRef]
- Sreetharan, S.; Stoa, L.; Cybulski, M.E.; Jones, D.E.; Lee, A.H.; Kulesza, A.V.; Tharmalingam, S.; Boreham, D.R.; Tai, T.C.; Wilson, J.Y. Cardiovascular and growth outcomes of C57Bl/6J mice offspring exposed to maternal stress and ionizing radiation during pregnancy. Int. J. Radiat. Biol. 2019, 95, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase. Methods Enzym. Anal. 1983, 105, 121–126. [Google Scholar]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Sowers, J.; Nistala, R.; Gong, H.; Uptergrove, G.; Clark, S.; Morris, E.; Szary, N.; Manrique, C.; Stump, C. Angiotensin II-induced NADPH oxidase activation impairs insulin signaling in skeletal muscle cells. J. Biol. Chem. 2006, 281, 35137–35146. [Google Scholar] [CrossRef]
- LeBel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the Probe 2′,7′-dichlorofluorescin as an Indicator of Reactive Oxygen Species Formation and Oxidative Stress. Chem. Res. Toxicol. 1992, 5, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Treadway, J.L.; Hargrove, D.M.; Nardone, N.A.; McPherson, R.K.; Russo, J.F.; Milici, A.J.; Stukenbrok, H.A.; Gibbs, E.M.; Stevenson, R.W.; Pessin, J.E. Enhanced peripheral glucose utilization in transgenic mice expressing the human GLUT4 gene. J. Biol. Chem. 1994, 269, 29956–29961. [Google Scholar] [CrossRef]
- Belke, D.D.; Larsen, T.S.; Gibbs, E.M.; Severson, D.L. Glucose Metabolism in Perfused Mouse Hearts Overexpressing Human GLUT-4 Glucose Transporter. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E420–E427. [Google Scholar] [CrossRef] [PubMed]
- Dokken, B.B.; Saengsirisuwan, V.; Kim, J.S.; Teachey, M.K.; Henriksen, E.J. Oxidative stress-induced insulin resistance in rat skeletal muscle: Role of glycogen synthase kinase-3. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E615–E621. [Google Scholar] [CrossRef]
- Williams, P.M.; Fletcher, S. Health effects of prenatal radiation exposure. Am. Fam. Physician 2010, 82, 488–493. [Google Scholar]
- Jackson, A.A. Nutrients, growth, and the development of programmed metabolic function. Adv. Exp. Med. Biol. 2000, 478, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Devi, P.U.; Bisht, K.S. Effect of prenatal gamma irradiation during the late fetal period on the postnatal development of the mouse. Teratology 1999, 59, 133–138. [Google Scholar] [CrossRef]
- Grigore, D.; Ojeda, N.B.; Alexander, B.T. Sex differences in the fetal programming of cardiovascular disease. Gend. Med. 2008, 5, S121–S132. [Google Scholar] [CrossRef] [PubMed]
- O’Regan, D.; Kenyon, C.J.; Seckl, J.R.; Holmes, M.C. Glucocorticoid exposure in late gestation in the rat permanently programs gender-specific differences in adult cardiovascular and metabolic physiology. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E863–E870. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, M.V.; Azimzadeh, O.; Merl-Pham, J.; Verreet, T.; Hauck, S.M.; Benotmane, M.A.; Atkinson, M.J.; Tapio, S. In-utero low-dose irradiation leads to persistent alterations in the mouse heart proteome. PLoS ONE 2016, 11, e0156952. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Allard, C.; Xu, W.; Mauvais-Jarvis, F. The role of androgens in metabolism, obesity and diabetes in males and females. Obesity 2015, 23, 713–719. [Google Scholar] [CrossRef]
- Smith, C.; Ryckman, K.; Barnabei, V.M.; Howard, B.; Isasi, C.R.; Sarto, G.; Tom, S.E.; Van Horn, L.; Wallace, R.; Robinson, J.G. The Impact of birth weight on cardiovascular disease risk in the women’s health initiative. Nutr. Metab. Cardiovasc. Dis. NMCD 2016, 26, 239–245. [Google Scholar] [CrossRef]
- Nielsen, K.M.; Offersen, B.V.; Nielsen, H.M.; Vaage-Nilsen, M.; Yusuf, S.W. Short and long term radiation induced cardiovascular disease in patients with cancer. Clin. Cardiol. 2017, 40, 255–261. [Google Scholar] [CrossRef]
- Yusuf, S.W.; Venkatesulu, B.P.; Mahadevan, L.S.; Krishnan, S. Radiation-induced cardiovascular disease: A clinical perspective. Front. Cardiovasc. Med. 2017, 4. [Google Scholar] [CrossRef]
- Muaku, S.M.; Thissen, J.-P.; Gerard, G.; Ketelslegers, J.-M.; Maiter, D. Postnatal catch-up growth induced by growth hormone and insulin-like growth factor-I in rats with intrauterine growth retardation caused by maternal protein malnutrition. Pediatr. Res. 1997, 42, 370–377. [Google Scholar] [CrossRef]
- Chan, Z.; Chooi, Y.C.; Ding, C.; Choo, J.; Sadananthan, S.A.; Michael, N.; Velan, S.S.; Leow, M.K.; Magkos, F. Sex differences in glucose and fatty acid metabolism in Asians who are nonobese. J. Clin. Endocrinol. Metab. 2019, 104, 127–136. [Google Scholar] [CrossRef]
- Peterson, L.R.; Soto, P.F.; Herrero, P.; Schechtman, K.B.; Dence, C.; Gropler, R.J. Sex differences in myocardial oxygen and glucose metabolism. J. Nucl. Cardiol. 2007, 14, 573–581. [Google Scholar] [CrossRef]
- Foryst-Ludwig, A.; Kreissl, M.C.; Sprang, C.; Thalke, B.; Böhm, C.; Benz, V.; Gürgen, D.; Dragun, D.; Schubert, C.; Mai, K.; et al. Sex differences in physiological cardiac hypertrophy are associated with exercise-mediated changes in energy substrate availability. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H115–H122. [Google Scholar] [CrossRef] [PubMed]
- Alsbeih, G.; Al-Meer, R.S.; Al-Harbi, N.; Bin Judia, S.; Al-Buhairi, M.; Venturina, N.Q.; Moftah, B. Gender Bias in Individual Radiosensitivity and the Association with Genetic Polymorphic Variations. Radiother. Oncol. 2016, 119, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Narendran, N.; Luzhna, L.; Kovalchuk, O. Sex difference of radiation response in occupational and accidental exposure. Front. Genet. 2019, 10. [Google Scholar] [CrossRef]
- Pogribny, I.; Raiche, J.; Slovack, M.; Kovalchuk, O. Dose-dependence, sex- and tissue-specificity, and persistence of radiation-induced genomic DNA methylation changes. Biochem. Biophys. Res. Commun. 2004, 320, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Pereira, R.O.; O’Neill, B.T.; Riehle, C.; Ilkun, O.; Wende, A.R.; Rawlings, T.A.; Zhang, Y.C.; Zhang, Q.; Klip, A.; et al. Cardiac PI3K-Akt Impairs Insulin-Stimulated Glucose Uptake Independent of mTORC1 and GLUT4 Translocation. Mol. Endocrinol. 2013, 27, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Bloch-Damti, A.; Bashan, N. Proposed mechanisms for the induction of insulin resistance by oxidative stress. Antioxid. Redox Signal. 2005, 7, 1553–1567. [Google Scholar] [CrossRef]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef]
- Boerma, M.; Sridharan, V.; Mao, X.-W.; Nelson, G.A.; Cheema, A.K.; Koturbash, I.; Singh, S.P.; Tackett, A.J.; Hauer-Jensen, M. Effects of ionizing radiation on the heart. Mutat. Res. 2016, 770, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15. [Google Scholar] [CrossRef]
- Thompson, L.P.; Al-Hasan, Y. Impact of oxidative stress in fetal programming. J. Pregnancy 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Role of oxidative stress in pathophysiology of nonalcoholic fatty liver disease. Oxid. Med. Cell. Longev. 2018, 2018, 9547613. [Google Scholar] [CrossRef] [PubMed]
- Heney, D.; Whicher, J.T. Factors affecting the measurement of cytokines in biological fluids: Implications for their clinical measurement. Ann. Clin. Biochem. 1995, 32, 358–368. [Google Scholar] [CrossRef]
- Ye, F.; Han, L.; Lu, Q.; Dong, W.; Chen, Z.; Shao, H.; Kaplan, H.J.; Li, Q.; Lu, Q. Retinal Self-Antigen Induces a Predominantly Th1 Effector Response in Axl and Mertk Double-Knockout Mice. J. Immunol. 2011, 187, 4178–4186. [Google Scholar] [CrossRef] [PubMed]
- Lacoste-Collin, L.; Jozan, S.; Cances-Lauwers, V.; Pipy, B.; Gasset, G.; Caratero, C.; Courtade-Saïdi, M. Effect of continuous irradiation with a very low dose of gamma rays on life span and the immune system in SJL mice prone to B-cell lymphoma. Radiat. Res. 2007, 168, 725–732. [Google Scholar] [CrossRef]
- Shin, S.C.; Lee, K.-M.; Kang, Y.M.; Kim, K.; Kim, C.S.; Yang, K.H.; Jin, Y.-W.; Kim, C.S.; Kim, H.S. Alteration of cytokine profiles in mice exposed to chronic low-dose ionizing radiation. Biochem. Biophys. Res. Commun. 2010, 397, 644–649. [Google Scholar] [CrossRef]

| Sham | 50 mGy | 300 mGy | 1000 mGy | |
|---|---|---|---|---|
| Male Body Weight (g) | 30.63 ± 0.83 | 28.11 ± 0.45 a | 30.47 ± 0.82 b | 26.79 ± 0.57 ac |
| Female Body Weight (g) | 22.95 ± 0.63 | 21.26 ± 0.93 a | 21.69 ± 0.34 a | 20.71 ± 0.53 a |
| Male Heart Weight (mg) | 118.3 ± 3.46 | 115.7 ± 2.65 | 123.7 ± 2.95 | 109.3 ± 3.19 ac |
| Female Heart Weight (mg) | 98.82 ± 2.43 | 92.17 ± 1.47 a | 96.23 ± 1.62 | 89.79 ± 1.63 ac |
| Male Heart/Body Weight (mg/g) | 3.87 ± 0.071 | 4.11 ± 0.11 | 4.08 ± 0.084 | 4.08 ± 0.063 |
| Female Heart/Body Weight (mg/g) | 4.31 ± 0.067 | 4.34 ± 0.056 | 4.44 ± 0.063 | 4.35 ± 0.062 |
| Sham | 50 mGy | 300 mGy | 1000 mGy | |
|---|---|---|---|---|
| Male GSH (μM) | 106.2 ± 4.15 | 114.3 ± 18.72 | 100.2 ± 9.62 | 105.3 ± 6.94 |
| Male GSSG (μM) | 60.97 ± 4.82 | 66.75 ± 7.42 | 55.88 ± 4.88 | 60.72 ± 3.76 |
| Male GSH/GSSG | 1.84 ± 0.15 | 1.96 ± 0.33 | 1.955 ± 0.26 | 1.80 ± 0.15 |
| Female GSH (μM) | 96.76 ± 5.57 | 98.32 ± 5.32 | 83.4 ± 4.86 | 86.68 ± 6.94 |
| Female GSSG (μM) | 35.13 ± 3.034 | 42.24 ± 3.79 | 55.57 ± 5.67 a | 86.82 ± 5.62 abc |
| Female GSH/GSSG | 2.95 ± 0.30 | 2.56 ± 0.37 | 1.779 ± 0.21 ab | 1.05 ± 0.10 abc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemec-Bakk, A.S.; Niccoli, S.; Davidson, C.; Roy, D.; Stoa, L.; Sreetharan, S.; Simard, A.; Boreham, D.R.; Wilson, J.Y.; Tai, T.C.; et al. Lasting Effects of Low to Non-Lethal Radiation Exposure during Late Gestation on Offspring’s Cardiac Metabolism and Oxidative Stress. Antioxidants 2021, 10, 816. https://doi.org/10.3390/antiox10050816
Nemec-Bakk AS, Niccoli S, Davidson C, Roy D, Stoa L, Sreetharan S, Simard A, Boreham DR, Wilson JY, Tai TC, et al. Lasting Effects of Low to Non-Lethal Radiation Exposure during Late Gestation on Offspring’s Cardiac Metabolism and Oxidative Stress. Antioxidants. 2021; 10(5):816. https://doi.org/10.3390/antiox10050816
Chicago/Turabian StyleNemec-Bakk, Ashley S., Sarah Niccoli, Caitlund Davidson, Danika Roy, Lisa Stoa, Shayenthiran Sreetharan, Alain Simard, Douglas R. Boreham, Joanna Y. Wilson, T.C. Tai, and et al. 2021. "Lasting Effects of Low to Non-Lethal Radiation Exposure during Late Gestation on Offspring’s Cardiac Metabolism and Oxidative Stress" Antioxidants 10, no. 5: 816. https://doi.org/10.3390/antiox10050816
APA StyleNemec-Bakk, A. S., Niccoli, S., Davidson, C., Roy, D., Stoa, L., Sreetharan, S., Simard, A., Boreham, D. R., Wilson, J. Y., Tai, T. C., Lees, S. J., & Khaper, N. (2021). Lasting Effects of Low to Non-Lethal Radiation Exposure during Late Gestation on Offspring’s Cardiac Metabolism and Oxidative Stress. Antioxidants, 10(5), 816. https://doi.org/10.3390/antiox10050816






