Recent Progress in Antimicrobial Strategies for Resin-Based Restoratives
Abstract
1. Introduction
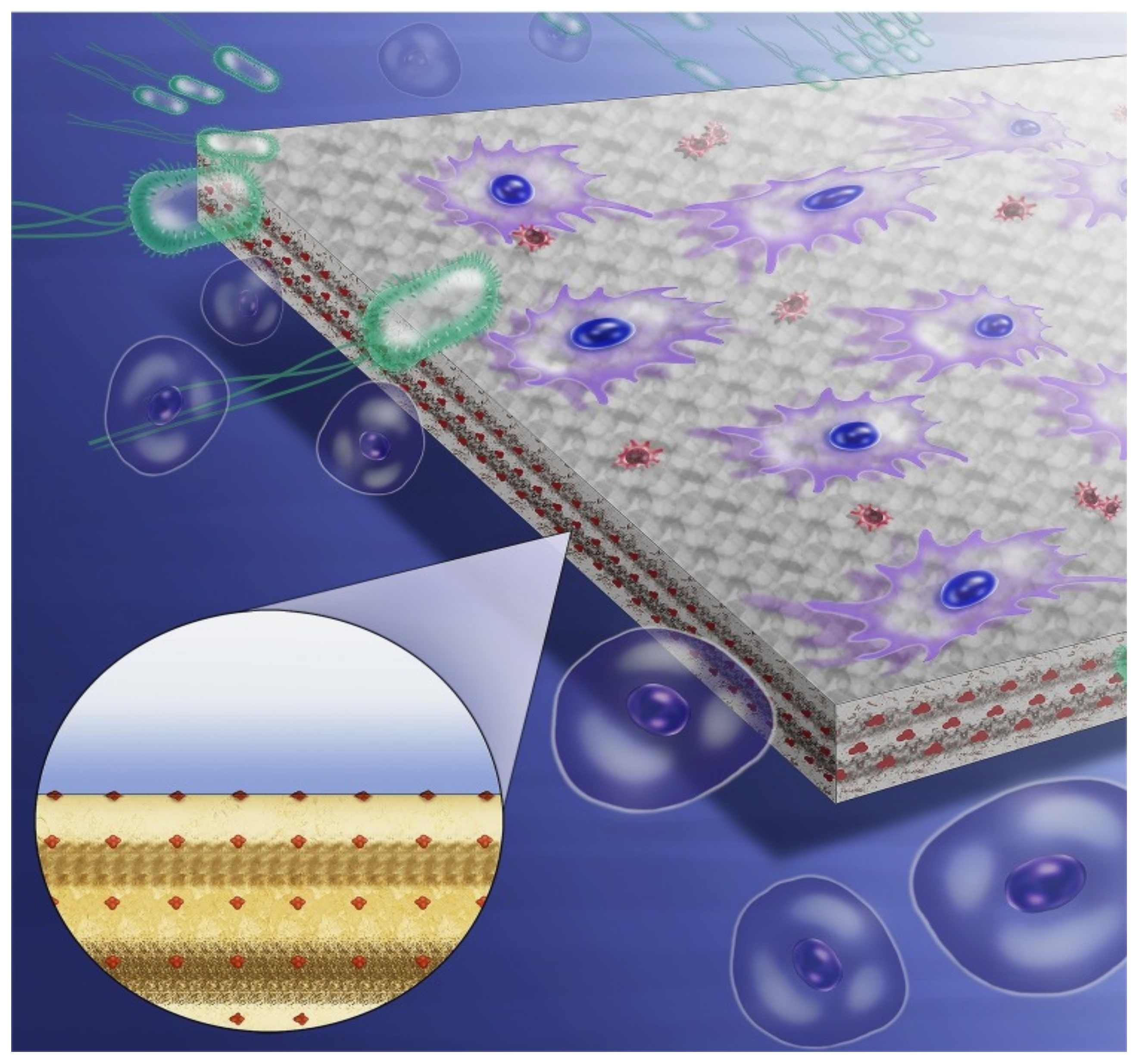
2. Current Antimicrobial Strategies of Resin Composites
2.1. Antimicrobial Agent Release
2.1.1. Applications of Nanotechnology
2.1.2. New Release Systems for Antimicrobial Agents
2.1.3. New Types of Antimicrobial Agents
2.2. Contact-Dependent Strategies
2.2.1. Development of QAC-Based Antimicrobial Systems
2.2.2. Introduction of 2-Methacryloyloxyethyl Phosphorylcholine (MPC)
2.2.3. Development of Other Immobilized Antimicrobial Agents
2.3. Multi-Functional Strategies
3. Challenges Faced by Antimicrobial Dental Restorations
3.1. Biocompatibility
3.2. Drug Resistance
3.3. Controlled Release of Antimicrobial Agents
3.4. Methods of Assessing Antimicrobial Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Habib, E.; Wang, R.; Wang, Y.; Zhu, M.; Zhu, X.X. Inorganic Fillers for Dental Resin Composites: Present and Future. ACS Biomater. Sci. Eng. 2016, 2, 1–11. [Google Scholar] [CrossRef]
- Ferracane, J.L. Resin composite-State of the art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Nedeljkovic, I.; Teughels, W.; De Munck, J.; Van Meerbeek, B.; Van Landuyt, K.L. Is secondary caries with composites a material-based problem? Dent. Mater. 2015, 31, e247–e277. [Google Scholar] [CrossRef]
- Bowen, R.L. Properties of a silica-reinforced polymer for dental restorations. J. Am. Dent. Assoc. 1963, 66, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Xing, A.; Sun, Q.N.; Meng, Y.; Zhang, Y.Y.; Li, X.Y.; Han, B. A hydroxyl-containing hyperbranched polymer as a multi-purpose modifier for a dental epoxy. React. Funct. Polym. 2020, 149. [Google Scholar] [CrossRef]
- Kumar, N.; Zafar, M.S.; Dahri, W.M.; Khan, M.A.; Khurshid, Z.; Najeeb, S. Effects of deformation rate variation on biaxial flexural properties of dental resin composites. J. Taibah Univ. Med. Sci. 2018, 13, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Ghani, F.; Fareed, M.A.; Riaz, S.; Sultan, Z.; Zafar, M.S. Bi-axial flexural strength of resin based dental composites—Influence and reliability of the testing method configuration. Mater. Technol. 2021, 16, 1–7. [Google Scholar] [CrossRef]
- Kumar, N.; Shortall, A. Performance of the experimental resins and dental nanocomposites at varying deformation rates. J. Investig. Clin. Dent. 2013, 5, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Fugolin, A.P.P.; Pfeifer, C.S. New Resins for Dental Composites. J. Dent. Res. 2017, 96, 1085–1091. [Google Scholar] [CrossRef]
- Demarco, F.F.; Correa, M.B.; Cenci, M.S.; Moraes, R.R.; Opdam, N.J. Longevity of posterior composite restorations: Not only a matter of materials. Dent. Mater. 2012, 28, 87–101. [Google Scholar] [CrossRef]
- Melo, M.A.; Guedes, S.F.; Xu, H.H.; Rodrigues, L.K. Nanotechnology-based restorative materials for dental caries management. Trends Biotechnol. 2013, 31, 459–467. [Google Scholar] [CrossRef]
- Opdam, N.J.; van de Sande, F.H.; Bronkhorst, E.; Cenci, M.S.; Bottenberg, P.; Pallesen, U.; Gaengler, P.; Lindberg, A.; Huysmans, M.C.; van Dijken, J.W. Longevity of posterior composite restorations: A systematic review and meta-analysis. J. Dent. Res. 2014, 93, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Kermanshahi, S.; Santerre, J.P.; Cvitkovitch, D.G.; Finer, Y. Biodegradation of resin-dentin interfaces increases bacterial microleakage. J. Dent. Res. 2010, 89, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Imazato, S.; Torii, M.; Tsuchitani, Y.; McCabe, J.F.; Russell, R.R. Incorporation of bacterial inhibitor into resin composite. J. Dent. Res. 1994, 73, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Colton, M.B.; Ehrlich, E. Bactericidal effect obtained by addition of antibiotics to dental cements and direct filling resins. J. Am. Dent. Assoc. 1953, 47, 524–531. [Google Scholar] [CrossRef]
- Boaro, L.C.C.; Campos, L.M.; Varca, G.H.C.; dos Santos, T.M.R.; Marques, P.A.; Sugii, M.M.; Saldanha, N.R.; Cogo-Muller, K.; Brandt, W.C.; Braga, R.R.; et al. Antibacterial resin-based composite containing chlorhexidine for dental applications. Dent. Mater. 2019, 35, 909–918. [Google Scholar] [CrossRef]
- Ali, S.; Sangi, L.; Kumar, N.; Kumar, B.; Khurshid, Z.; Zafar, M.S. Evaluating antibacterial and surface mechanical properties of chitosan modified dental resin composites. Technol. Health Care 2020, 28, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, M.; Zhu, X.X. Functional fillers for dental resin composites. Acta Biomater. 2021, 122, 50–65. [Google Scholar] [CrossRef] [PubMed]
- Ferrando-Magraner, E.; Bellot-Arcis, C.; Paredes-Gallardo, V.; Almerich-Silla, J.M.; Garcia-Sanz, V.; Fernandez-Alonso, M.; Montiel-Company, J.M. Antibacterial Properties of Nanoparticles in Dental Restorative Materials. A Systematic Review and Meta-Analysis. Medicina 2020, 56, 55. [Google Scholar] [CrossRef] [PubMed]
- Kreth, J.; Merritt, J.; Pfeifer, C.S.; Khajotia, S.; Ferracane, J.L. Interaction between the Oral Microbiome and Dental Composite Biomaterials: Where We Are and Where We Should Go. J. Dent. Res. 2020, 99, 1140–1149. [Google Scholar] [CrossRef]
- Ganewatta, M.S.; Miller, K.P.; Singleton, S.P.; Mehrpouya-Bahrami, P.; Chen, Y.P.; Yan, Y.; Nagarkatti, M.; Nagarkatti, P.; Decho, A.W.; Tang, C. Antibacterial and Biofilm-Disrupting Coatings from Resin Acid-Derived Materials. Biomacromolecules 2015, 16, 3336–3344. [Google Scholar] [CrossRef]
- Thongthai, P.; Kitagawa, H.; Kitagawa, R.; Hirose, N.; Noree, S.; Iwasaki, Y.; Imazato, S. Development of novel surface coating composed of MDPB and MPC with dual functionality of antibacterial activity and protein repellency. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 3241–3249. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Tay, F.R.; Niu, L.N.; Chen, J.H. Advancing antimicrobial strategies for managing oral biofilm infections. Int. J. Oral Sci. 2019, 11, 28. [Google Scholar] [CrossRef]
- Cheng, L.; Weir, M.D.; Xu, H.H.; Kraigsley, A.M.; Lin, N.J.; Lin-Gibson, S.; Zhou, X. Antibacterial and physical properties of calcium-phosphate and calcium-fluoride nanocomposites with chlorhexidine. Dent. Mater. 2012, 28, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hua, H.; Li, W.; Wang, R.; Jiang, X.; Zhu, M. Strong antibacterial dental resin composites containing cellulose nanocrystal/zinc oxide nanohybrids. J. Dent. 2019, 80, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Xu, H.; Xie, W.; Wang, M.; Wang, C.; Gao, C.; Gu, F.; Liu, J.; Fu, J. Study on a novel antibacterial light-cured resin composite containing nano-MgO. Colloids Surf. B Biointerfaces 2020, 188, 110774. [Google Scholar] [CrossRef]
- Garoushi, S.; Vallittu, P.K.; Lassila, L. Characterization of fluoride releasing restorative dental materials. Dent. Mater. J. 2018, 37, 293–300. [Google Scholar] [CrossRef]
- Hoshika, T.; Nishitani, Y.; Yoshiyama, M.; Key, W.O., 3rd; Brantley, W.; Agee, K.A.; Breschi, L.; Cadenaro, M.; Tay, F.R.; Rueggeberg, F.; et al. Effects of quaternary ammonium-methacrylates on the mechanical properties of unfilled resins. Dent. Mater. 2014, 30, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.F.; Wu, R.; Fan, Y.; Liao, S.; Wang, Y.; Wen, Z.T.; Xu, X. Antibacterial Dental Composites with Chlorhexidine and Mesoporous Silica. J. Dent. Res. 2014, 93, 1283–1289. [Google Scholar] [CrossRef]
- Pasha, M.; Muhammad, N.; Nayyer, M.; Bokhari, J.H.; Ashraf, H.; Safi, S.Z.; Kaleem, M. Synthesis of an anti-cariogenic experimental dental composite containing novel drug-decorated copper particles. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 114, 111040. [Google Scholar] [CrossRef]
- Barot, T.; Rawtani, D.; Kulkarni, P. Development of Chlorhexidine Loaded Halloysite Nanotube Based Experimental Resin Composite with Enhanced Physico-Mechanical and Biological Properties for Dental Applications. J. Compos. Sci. 2020, 4, 81. [Google Scholar] [CrossRef]
- Barot, T.; Rawtani, D.; Kulkarni, P. Physicochemical and biological assessment of silver nanoparticles immobilized Halloysite nanotubes-based resin composite for dental applications. Heliyon 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Gorain, B.; Low, S.Y.; Tan, S.A.; Ling, E.C.S.; Lim, Y.K.; Chin, C.M.; Lee, P.Y.; Lee, C.M.; Ooi, C.H.; et al. Nanotechnology based approaches for anti-diabetic drugs delivery. Diabetes Res. Clin. Pract. 2018, 136, 52–77. [Google Scholar] [CrossRef]
- Wang, L.L.; Hu, C.; Shao, L.Q. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Padovani, G.C.; Feitosa, V.P.; Sauro, S.; Tay, F.R.; Duran, G.; Paula, A.J.; Duran, N. Advances in Dental Materials through Nanotechnology: Facts, Perspectives anc Toxicological Aspects. Trends Biotechnol. 2015, 33, 621–636. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, M.; Barik, S.K.; MubarakAli, D.; Prakash, P.; Pugazhendhi, A. Synthesis of silver nanoparticles from Bacillus brevis (NCIM 2533) and their antibacterial activity against pathogenic bacteria. Microb. Pathog. 2018, 116, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Noronha, V.T.; Paula, A.J.; Duran, G.; Galembeck, A.; Cogo-Muller, K.; Franz-Montan, M.; Duran, N. Silver nanoparticles in dentistry. Dent. Mater. 2017, 33, 1110–1126. [Google Scholar] [CrossRef]
- Barszczewska-Rybarek, I.; Chladek, G. Studies on the Curing Efficiency and Mechanical Properties of Bis-GMA and TEGDMA Nanocomposites Containing Silver Nanoparticles. Int. J. Mol. Sci. 2018, 19, 3937. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, K.; Weir, M.D.; Melo, M.A.S.; Zhou, X.D.; Xu, H.H.K. Nanotechnology strategies for antibacterial and remineralizing composites and adhesives to tackle dental caries. Nanomedicine 2015, 10, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Dell’Erba, I.E.; Martinez, F.D.; Hoppe, C.E.; Elicabe, G.E.; Ceolin, M.; Zucchi, I.A.; Schroeder, W.F. Mechanism of Particle Formation in Silver/Epoxy Nanocomposites Obtained through a Visible-Light-Assisted in Situ Synthesis. Langmuir 2017, 33, 10248–10258. [Google Scholar] [CrossRef]
- Ren, L.; Pan, Y.; Liang, Q.; He, S.; Liu, Y.; Fan, Y.; Meng, X.; Chen, M. In Situ Synthesis of Dental Resin Matrix Containing Silver Nanoparticles. J. Nanosci. Nanotechnol. 2019, 19, 5774–5782. [Google Scholar] [CrossRef]
- El-Wassefy, N.A.; El-Mahdy, R.H.; El-Kholany, N.R. The impact of silver nanoparticles integration on biofilm formation and mechanical properties of glass ionomer cement. J. Esthet. Restor. Dent. 2018, 30, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.C.; Rolim, W.R.; Viana, M.M.; Souza, T.R.; Goncalves, F.; Tanaka, C.J.; Bueno-Silva, B.; Seabra, A.B. Biogenic synthesis and antimicrobial activity of silica-coated silver nanoparticles for esthetic dental applications. J. Dent. 2020, 96, 103327. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Umar, A.; Kumar, G.; Nalwa, H.S. Antimicrobial properties of ZnO nanomaterials: A review. Ceram. Int. 2017, 43, 3940–3961. [Google Scholar] [CrossRef]
- Mahamuni-Badiger, P.P.; Patil, P.M.; Badiger, M.V.; Patel, P.R.; Thorat-Gadgil, B.S.; Pandit, A.; Bohara, R.A. Biofilm formation to inhibition: Role of zinc oxide-based nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110319. [Google Scholar] [CrossRef] [PubMed]
- Abebe, B.; Zereffa, E.A.; Tadesse, A.; Murthy, H.C.A. A Review on Enhancing the Antibacterial Activity of ZnO: Mechanisms and Microscopic Investigation. Nanoscale Res. Lett. 2020, 15, 190. [Google Scholar] [CrossRef]
- Villegas, N.A.; Compagnucci, M.J.S.; Aja, M.S.; Rocca, D.M.; Becerra, M.C.; Molina, G.F.; Palma, S.D. Novel Antibacterial Resin-Based Filling Material Containing Nanoparticles for the Potential One-Step Treatment of Caries. J. Healthc. Eng. 2019, 2019. [Google Scholar] [CrossRef]
- Stewart, C.A.; Finer, Y. Biostable, antidegradative and antimicrobial restorative systems based on host-biomaterials and microbial interactions. Dent. Mater. 2019, 35, 36–52. [Google Scholar] [CrossRef]
- Yamamoto, E.; Kuroda, K. Colloidal Mesoporous Silica Nanoparticles. B Chem. Soc. Jpn. 2016, 89, 501–539. [Google Scholar] [CrossRef]
- Bai, X.X.; Lin, C.C.; Wang, Y.Y.; Ma, J.; Wang, X.; Yao, X.H.; Tang, B. Preparation of Zn doped mesoporous silica nanoparticles (Zn-MSNs) for the improvement of mechanical and antibacterial properties of dental resin composites. Dent. Mater. 2020, 36, 794–807. [Google Scholar] [CrossRef]
- Chen, H.; Wang, R.; Zhang, J.; Hua, H.; Zhu, M. Synthesis of core-shell structured ZnO@m-SiO2 with excellent reinforcing effect and antimicrobial activity for dental resin composites. Dent. Mater. 2018, 34, 1846–1855. [Google Scholar] [CrossRef]
- Han, N.; Zhao, Q.; Wan, L.; Wang, Y.; Gao, Y.; Wang, P.; Wang, Z.; Zhang, J.; Jiang, T.; Wang, S. Hybrid lipid-capped mesoporous silica for stimuli-responsive drug release and overcoming multidrug resistance. ACS. Appl. Mater. Interfaces 2015, 7, 3342–3351. [Google Scholar] [CrossRef]
- Stewart, C.A.; Finer, Y.; Hatton, B.D. Drug self-assembly for synthesis of highly-loaded antimicrobial drug-silica particles. Sci. Rep. 2018, 8, 895. [Google Scholar] [CrossRef]
- Delaviz, Y.; Nascimento, M.A.; Laschuk, M.W.; Liu, T.W.; Yang, M.; Santerre, J.P. Synthesis and characterization of Ciprofloxacin-containing divinyl oligomers and assessment of their biodegradation in simulated salivary esterase. Dent. Mater. 2018, 34, 711–725. [Google Scholar] [CrossRef]
- Zhang, R.S.; Jones, M.M.; Moussa, H.; Keskar, M.; Huo, N.B.; Zhang, Z.Q.; Visser, M.B.; Sabatini, C.; Swihart, M.T.; Cheng, C. Polymer-antibiotic conjugates as antibacterial additives in dental resins. Biomater. Sci. Uk. 2019, 7, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Su, L.W.; Lin, D.J.; Uan, J.Y. Novel dental resin composites containing LiAl-F layered double hydroxide (LDH) filler: Fluoride release/recharge, mechanical properties, color change, and cytotoxicity. Dent. Mater. 2019, 35, 663–672. [Google Scholar] [CrossRef]
- Hoxha, A.; Gillam, D.G.; Agha, A.; Karpukhina, N.; Bushby, A.J.; Patel, M.P. Novel fluoride rechargeable dental composites containing MgAl and CaAl layered double hydroxide (LDH). Dent. Mater. 2020, 36, 973–986. [Google Scholar] [CrossRef]
- Fullriede, H.; Abendroth, P.; Ehlert, N.; Doll, K.; Schäske, J.; Winkel, A.; Stumpp, S.N.; Stiesch, M.; Behrens, P. pH-responsive release of chlorhexidine from modified nanoporous silica nanoparticles for dental applications. BioNanoMaterials 2016, 17, 59–72. [Google Scholar] [CrossRef]
- Luo, D.; Hasan, M.S.; Shahid, S.; Khlebtsov, B.N.; Cattell, M.J.; Sukhorukov, G.B. Gold Nanorod Mediated Chlorhexidine Microparticle Formation and Near-Infrared Light Induced Release. Langmuir 2017, 33, 7982–7993. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Shahid, S.; Sukhorukov, G.B.; Cattell, M.J. Synthesis of novel chlorhexidine spheres with controlled release from a UDMA-HEMA resin using ultrasound. Dent. Mater. 2017, 33, 713–722. [Google Scholar] [CrossRef]
- Luo, D.; Shahid, S.; Hasan, S.M.; Whiley, R.; Sukhorukov, G.B.; Cattell, M.J. Controlled release of chlorhexidine from a HEMA-UDMA resin using a magnetic field. Dent. Mater. 2018, 34, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Stencel, R.; Kasperski, J.; Pakiela, W.; Mertas, A.; Bobela, E.; Barszczewska-Rybarek, I.; Chladek, G. Properties of Experimental Dental Composites Containing Antibacterial Silver-Releasing Filler. Materials 2018, 11, 1031. [Google Scholar] [CrossRef]
- Miki, S.; Kitagawa, H.; Kitagawa, R.; Kiba, W.; Hayashi, M.; Imazato, S. Antibacterial activity of resin composites containing surface pre-reacted glass-ionomer (S-PRG) filler. Dent. Mater. 2016, 32, 1095–1102. [Google Scholar] [CrossRef]
- Kitagawa, H.; Miki-Oka, S.; Mayanagi, G.; Abiko, Y.; Takahashi, N.; Imazato, S. Inhibitory effect of resin composite containing S-PRG filler on Streptococcus mutans glucose metabolism. J. Dent. 2018, 70, 92–96. [Google Scholar] [CrossRef]
- Szram, A.; Sokolowski, J.; Nowak, J.; Domarecka, M.; Lukomska-Szymanska, M. Mechanical properties of composite material modified with essential oil. Inż. Mater. 2017, 1, 49–53. [Google Scholar] [CrossRef]
- Lapinska, B.; Szram, A.; Zarzycka, B.; Grzegorczyk, J.; Hardan, L.; Sokolowski, J.; Lukomska-Szymanska, M. An In Vitro Study on the Antimicrobial Properties of Essential Oil Modified Resin Composite against Oral Pathogens. Materials 2020, 13, 4383. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, K.; Zhang, N.; Melo, M.A.S.; Weir, M.D.; Zhou, X.D.; Bai, Y.X.; Reynolds, M.A.; Xu, H.H.K. Developing a New Generation of Antimicrobial and Bioactive Dental Resins. J. Dent. Res. 2017, 96, 855–863. [Google Scholar] [CrossRef]
- Jiao, Y.; Niu, L.N.; Ma, S.; Li, J.; Tay, F.R.; Chen, J.H. Quaternary ammonium-based biomedical materials: State-of-the-art, toxicological aspects and antimicrobial resistance. Prog. Polym. Sci. 2017, 71, 53–90. [Google Scholar] [CrossRef]
- Makvandi, P.; Jamaledin, R.; Jabbari, M.; Nikfarjam, N.; Borzacchiello, A. Antibacterial quaternary ammonium compounds in dental materials: A systematic review. Dent. Mater. 2018, 34, 851–867. [Google Scholar] [CrossRef]
- Tanaka, C.B.; Lopes, D.P.; Kikuchi, L.N.T.; Moreira, M.S.; Catalani, L.H.; Braga, R.R.; Kruzic, J.J.; Goncalves, F. Development of novel dental restorative composites with dibasic calcium phosphate loaded chitosan fillers. Dent. Mater. 2020, 36, 551–559. [Google Scholar] [CrossRef]
- Hwang, G.; Koltisko, B.; Jin, X.; Koo, H. Nonleachable Imidazolium-Incorporated Composite for Disruption of Bacterial Clustering, Exopolysaccharide-Matrix Assembly, and Enhanced Biofilm Removal. ACS Appl. Mater. Interfaces 2017, 9, 38270–38280. [Google Scholar] [CrossRef] [PubMed]
- Belkhir, K.; Lacroix, M.; Jamshidian, M.; Salmieri, S.; Jegat, C.; Taha, M. Evaluation of antibacterial activity of branched quaternary ammonium grafted green polymers. Food Packag. Shelf 2017, 12, 28–41. [Google Scholar] [CrossRef]
- Shulman, L.; Pei, L.; Bahnasy, M.F.; Lucy, C.A. High pH instability of quaternary ammonium surfactant coatings in capillary electrophoresis. Analyst 2017, 142, 2145–2151. [Google Scholar] [CrossRef]
- Jiang, Y.L.; Qiu, W.; Zhou, X.D.; Li, H.; Lu, J.Z.; Xu, H.H.K.; Peng, X.A.; Li, M.Y.; Feng, M.Y.; Cheng, L.; et al. Quaternary ammonium-induced multidrug tolerant Streptococcus mutans persisters elevate cariogenic virulence in vitro. Int. J. Oral Sci. 2017, 9. [Google Scholar] [CrossRef]
- Ioannou, C.J.; Hanlon, G.W.; Denyer, S.P. Action of disinfectant quaternary ammonium compounds against Staphylococcus aureus. Antimicrob. Agents Chemother. 2007, 51, 296–306. [Google Scholar] [CrossRef]
- Imazato, S.; Russell, R.R.; McCabe, J.F. Antibacterial activity of MDPB polymer incorporated in dental resin. J. Dent. 1995, 23, 177–181. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, L.; Weir, M.D.; Bai, Y.X.; Xu, H.H. Effects of quaternary ammonium chain length on the antibacterial and remineralizing effects of a calcium phosphate nanocomposite. Int. J. Oral Sci. 2016, 8, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Cherchali, F.Z.; Attik, N.; Mouzali, M.; Tommasino, J.B.; Abouelleil, H.; Decoret, D.; Seux, D.; Grosgogeat, B. Structural stability of DHMAI antibacterial dental composite following in vitro biological aging. Dent. Mater. 2020, 36, 1161–1169. [Google Scholar] [CrossRef]
- He, J.W.; Soderling, E.; Lassila, L.V.; Vallittu, P.K. Preparation of antibacterial and radio-opaque dental resin with new polymerizable quaternary ammonium monomer. Dent. Mater. 2015, 31, 575–582. [Google Scholar] [CrossRef]
- Wang, W.; Wu, F.; Zhang, G.; Zhu, S.; Ban, J.; Wang, L. Preparation of a highly crosslinked biosafe dental nanocomposite resin with a tetrafunctional methacrylate quaternary ammonium salt monomer. RSC Adv. 2019, 9, 41616–41627. [Google Scholar] [CrossRef]
- Cherchali, F.Z.; Mouzali, M.; Tommasino, J.B.; Decoret, D.; Attik, N.; Aboulleil, H.; Seux, D.; Grosgogeat, B. Effectiveness of the DHMAI monomer in the development of an antibacterial dental composite. Dent. Mater. 2017, 33, 1381–1391. [Google Scholar] [CrossRef]
- Makvandi, P.; Ghaemy, M.; Mohseni, M. Synthesis and characterization of photo-curable bis-quaternary ammonium dimethacrylate with antimicrobial activity for dental restoration materials. Eur. Polym. J. 2016, 74, 81–90. [Google Scholar] [CrossRef]
- Jaymand, M.; Lotfi, M.; Lotfi, R. Functional dendritic compounds: Potential prospective candidates for dental restorative materials and in situ re-mineralization of human tooth enamel. RSC Adv. 2016, 6, 43127–43146. [Google Scholar] [CrossRef]
- Jaymand, M.; Lotfi, M.; Barar, J.; Kimyai, S. Synthesis and characterization of potential multifunctional methacrylate-based dental monomers. Res. Chem. Intermediat. 2017, 43, 5707–5722. [Google Scholar] [CrossRef]
- Jaymand, M.; Lotfi, M.; Barar, J.; Eskandani, M.; Maleki, H. Novel dental nanocomposites: Fabrication and investigation of their physicochemical, mechanical and biological properties. B Mater. Sci. 2018, 41. [Google Scholar] [CrossRef]
- Jaymand, M.; Lotfi, M.; Abbasian, M. Fabrication of novel dental nanocomposites and investigation their physicochemical and biological properties. Mater. Res. Express. 2018, 5. [Google Scholar] [CrossRef]
- Zaltsman, N.; Ionescu, A.C.; Weiss, E.I.; Brambilla, E.; Beyth, S.; Beyth, N. Surface-modified nanoparticles as anti-biofilm filler for dental polymers. PLoS ONE 2017, 12, e0189397. [Google Scholar] [CrossRef]
- Zaltsman, N.; Kesler-Shvero, D.; Weiss, E.I.; Beyth, N. Synthesis variants of quaternary ammonium polyethyleneimine nanoparticles and their antibacterial efficacy in dental materials. J. Appl. Biomater. Func. 2016, 14. [Google Scholar] [CrossRef] [PubMed]
- Chroszcz, M.; Barszczewska-Rybarek, I. Nanoparticles of Quaternary Ammonium Polyethylenimine Derivatives for Application in Dental Materials. Polymers 2020, 12, 2551. [Google Scholar] [CrossRef]
- Pietrokovski, Y.; Nisimov, I.; Kesler-Shvero, D.; Zaltsman, N.; Beyth, N. Antibacterial effect of composite resin foundation material incorporating quaternary ammonium polyethyleneimine nanoparticles. J. Prosthet. Dent. 2016, 116, 603–609. [Google Scholar] [CrossRef]
- Muller, R.; Eidt, A.; Hiller, K.A.; Katzur, V.; Subat, M.; Schweikl, H.; Imazato, S.; Ruhl, S.; Schmalz, G. Influences of protein films on antibacterial or bacteria-repellent surface coatings in a model system using silicon wafers. Biomaterials 2009, 30, 4921–4929. [Google Scholar] [CrossRef]
- Koyama, J.; Fukazawa, K.; Ishihara, K.; Mori, Y. In situ surface modification on dental composite resin using 2-methacryloyloxyethyl phosphorylcholine polymer for controlling plaque formation. Mat. Sci. Eng. C-Mater. 2019, 104. [Google Scholar] [CrossRef]
- Ishihara, K.; Nomura, H.; Mihara, T.; Kurita, K.; Iwasaki, Y.; Nakabayashi, N. Why do phospholipid polymers reduce protein adsorption? J. Biomed. Mater. Res. 1998, 39, 323–330. [Google Scholar] [CrossRef]
- Fujiwara, N.; Yumoto, H.; Miyamoto, K.; Hirota, K.; Nakae, H.; Tanaka, S.; Murakami, K.; Kudo, Y.; Ozaki, K.; Miyake, Y. 2-Methacryloyloxyethyl phosphorylcholine (MPC)-polymer suppresses an increase of oral bacteria: A single-blind, crossover clinical trial. Clin. Oral Investig. 2019, 23, 739–746. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, K.; Weir, M.D.; Xu, D.J.; Reynolds, M.A.; Bai, Y.; Xu, H.H.K. Effects of water-aging for 6 months on the durability of a novel antimicrobial and protein-repellent dental bonding agent. Int. J. Oral. Sci. 2018, 10, s41368-s018. [Google Scholar] [CrossRef]
- Lee, M.J.; Kwon, J.S.; Kim, J.Y.; Ryu, J.H.; Seo, J.Y.; Jang, S.; Kim, K.M.; Hwang, C.J.; Choi, S.H. Bioactive resin-based composite with surface pre-reacted glass-ionomer filler and zwitterionic material to prevent the formation of multi-species biofilm. Dent. Mater. 2019, 35, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Schnaider, L.; Ghosh, M.; Bychenko, D.; Grigoriants, I.; Ya’ari, S.; Shalev Antsel, T.; Matalon, S.; Sarig, R.; Brosh, T.; Pilo, R.; et al. Enhanced Nanoassembly-Incorporated Antibacterial Composite Materials. ACS. Appl. Mater. Interfaces 2019, 11, 21334–21342. [Google Scholar] [CrossRef]
- de Souza Araujo, I.J.; de Paula, A.B.; Bruschi Alonso, R.C.; Taparelli, J.R.; Innocentini Mei, L.H.; Stipp, R.N.; Puppin-Rontani, R.M. A novel Triclosan Methacrylate-based composite reduces the virulence of Streptococcus mutans biofilm. PLoS ONE 2018, 13, e0195244. [Google Scholar] [CrossRef]
- Stenhagen, I.S.R.; Rukke, H.V.; Dragland, I.S.; Kopperud, H.M. Effect of methacrylated chitosan incorporated in experimental composite and adhesive on mechanical properties and biofilm formation. Eur. J. Oral Sci. 2019, 127, 81–88. [Google Scholar] [CrossRef]
- Sivakumar, I.; Arunachalam, K.S.; Sajjan, S.; Ramaraju, A.V.; Rao, B.; Kamaraj, B. Incorporation of antimicrobial macromolecules in acrylic denture base resins: A research composition and update. J. Prosthodont. 2014, 23, 284–290. [Google Scholar] [CrossRef]
- Srivastava, R.; Sun, Y. Silver sulfadiazine immobilized glass as antimicrobial fillers for dental restorative materials. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.L.; Modak, S.M. Mechanism of silver sulfadiazine action on burn wound infections. Antimicrob. Agents Chemother. 1974, 5, 582–588. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, K.; Melo, M.A.; Weir, M.D.; Xu, D.J.; Bai, Y.; Xu, H.H. Effects of Long-Term Water-Aging on Novel Anti-Biofilm and Protein-Repellent Dental Composite. Int. J. Mol. Sci. 2017, 18, 186. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, K.; Zhou, C.C.; Weir, M.D.; Zhou, X.D.; Xu, H.H. One-year water-ageing of calcium phosphate composite containing nano-silver and quaternary ammonium to inhibit biofilms. Int. J. Oral Sci. 2016, 8, 172–181. [Google Scholar] [CrossRef]
- Bhadila, G.; Filemban, H.; Wang, X.; Melo, M.A.S.; Arola, D.D.; Tay, F.R.; Oates, T.W.; Weir, M.D.; Sun, J.; Xu, H.H.K. Bioactive low-shrinkage-stress nanocomposite suppresses S. mutans biofilm and preserves tooth dentin hardness. Acta Biomater. 2020, 114, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Balhaddad, A.A.; Ibrahim, M.S.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. Concentration dependence of quaternary ammonium monomer on the design of high-performance bioactive composite for root caries restorations. Dent. Mater. 2020, 36, e266–e278. [Google Scholar] [CrossRef]
- Al-Dulaijan, Y.A.; Cheng, L.; Weir, M.D.; Melo, M.A.S.; Liu, H.; Oates, T.W.; Wang, L.; Xu, H.H.K. Novel rechargeable calcium phosphate nanocomposite with antibacterial activity to suppress biofilm acids and dental caries. J. Dent. 2018, 72, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Wang, L.; Xing, D.; Arola, D.D.; Weir, M.D.; Bai, Y.; Xu, H.H. Protein-repellent and antibacterial functions of a calcium phosphate rechargeable nanocomposite. J. Dent. 2016, 52, 15–22. [Google Scholar] [CrossRef]
- Bhadila, G.; Baras, B.H.; Weir, M.D.; Wang, H.; Melo, M.A.S.; Hack, G.D.; Bai, Y.; Xu, H.H.K. Novel antibacterial calcium phosphate nanocomposite with long-term ion recharge and re-release to inhibit caries. Dent. Mater. J. 2020, 39, 678–689. [Google Scholar] [CrossRef]
- Zhang, N.; Ma, J.F.; Melo, M.A.S.; Weir, M.D.; Bai, Y.X.; Xu, H.H.K. Protein-repellent and antibacterial dental composite to inhibit biofilms and caries. J. Dent. 2015, 43, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.H.; Moreau, J.L.; Sun, L.; Chow, L.C. Nanocomposite containing amorphous calcium phosphate nanoparticles for caries inhibition. Dent. Mater. 2011, 27, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Weir, M.D.; Chow, L.C.; Xu, H.H. Remineralization of demineralized enamel via calcium phosphate nanocomposite. J. Dent. Res. 2012, 91, 979–984. [Google Scholar] [CrossRef]
- Zhang, L.; Weir, M.D.; Chow, L.C.; Antonucci, J.M.; Chen, J.; Xu, H.H. Novel rechargeable calcium phosphate dental nanocomposite. Dent. Mater. 2016, 32, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Yu, Q.; Chen, H. Responsive and Synergistic Antibacterial Coatings: Fighting against Bacteria in a Smart and Effective Way. Adv. Healthc. Mater. 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Shahi, S.; Ozcan, M.; Dizaj, S.M.; Sharifi, S.; Husain, N.A.H.; Eftekhari, A.; Ahmadian, E. A review on potential toxicity of dental material and screening their biocompatibility. Toxicol. Mech. Method. 2019, 29, 368–377. [Google Scholar] [CrossRef]
- Duran, N.; Duran, M.; de Jesus, M.B.; Seabra, A.B.; Favaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomedicine 2016, 12, 789–799. [Google Scholar] [CrossRef]
- Tortella, G.R.; Rubilar, O.; Duran, N.; Diez, M.C.; Martinez, M.; Parada, J.; Seabra, A.B. Silver nanoparticles: Toxicity in model organisms as an overview of its hazard for human health and the environment. J. Hazard. Mater. 2020, 390. [Google Scholar] [CrossRef]
- Sudhakaran, S.; Athira, S.S.; Babu, S.S.; Varma, H.K.; Mohanan, P.V. Determination of the bioavailability of zinc oxide nanoparticles using ICP-AES and associated toxicity. Colloids Surf. B 2020, 188. [Google Scholar] [CrossRef]
- Wang, S.; Wang, H.; Ren, B.; Li, X.; Wang, L.; Zhou, H.; Weir, M.D.; Zhou, X.; Masri, R.M.; Oates, T.W.; et al. Drug resistance of oral bacteria to new antibacterial dental monomer dimethylaminohexadecyl methacrylate. Sci. Rep. 2018, 8, 5509. [Google Scholar] [CrossRef]
- Kitagawa, H.; Izutani, N.; Kitagawa, R.; Maezono, H.; Yamaguchi, M.; Imazato, S. Evolution of resistance to cationic biocides in Streptococcus mutans and Enterococcus faecalis. J. Dent. 2016, 47, 18–22. [Google Scholar] [CrossRef]
- Wang, S.P.; Wang, H.H.; Ren, B.; Li, H.; Weir, M.D.; Zhou, X.D.; Oates, T.W.; Cheng, L.; Xu, H.H.K. Do quaternary ammonium monomers induce drug resistance in cariogenic, endodontic and periodontal bacterial species? Dent. Mater. 2017, 33, 1127–1138. [Google Scholar] [CrossRef]
- Orazi, G.; O’Toole, G.A. “It Takes a Village”: Mechanisms Underlying Antimicrobial Recalcitrance of Polymicrobial Biofilms. J. Bacteriol. 2020, 202. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.S.; Garcia, I.M.; Kensara, A.; Balhaddad, A.A.; Collares, F.M.; Williams, M.A.; Ibrahim, A.S.; Lin, N.J.; Weir, M.D.; Xu, H.H.K.; et al. How we are assessing the developing antibacterial resin-based dental materials? A scoping review. J. Dent. 2020, 99. [Google Scholar] [CrossRef] [PubMed]
- Kreth, J.; Ferracane, J.L.; Pfeifer, C.S.; Khajotia, S.; Merritt, J. At the Interface of Materials and Microbiology: A Call for the Development of Standardized Approaches to Assay Biomaterial-Biofilm Interactions. J. Dent. Res. 2019, 98, 850–852. [Google Scholar] [CrossRef] [PubMed]



| Name | Advantages | Disadvantages |
|---|---|---|
| Antimicrobial agent release | High local doses of antimicrobial agents at specific sites, less systemic toxicity | Short-acting and compromised mechanical properties |
| Contact-dependent strategy | No adverse effects on the physical and mechanical properties of the loaded materials, improved and prolonged antibacterial activity | Relatively weak antimicrobial activity and surface biofouling |
| Multi-functional strategy | Synergistic antibacterial activity | Selection of more effective combinations |
| Speciation | Types of Dental Composite | Microorganisms Tested | Test Method for Antimicrobial Activity | Results | Reference |
|---|---|---|---|---|---|
| MPC, DMAHDM | BisGMA, TEGDMA | Human saliva | CFU counts; live/dead assay; MTT assay; BCA approach | Strongly deterred protein adhesion and diminished biofilm viability | [104] |
| NACP, QADM, NAg | BisGMA, TEGDMA | Human saliva | Live/dead staining; MTT assay; lactate analysis; CFU counts | Significantly stronger antibacterial capability than using QADM or NAg alone | [105] |
| NACP, DMAHDM | UDMA, TEG-DVBE | Human saliva | Live/dead staining assay; CFU counts; lactate dehydrogenase enzymatic method; CV staining | Demonstrated long-term antibacterial activity. | [106] |
| DMAHDM, NACP | BisGMA, TEGDMA | Human saliva | Live/dead staining; MTT assay; enzymatic method; CFU counts | All the microbiological assays were substantially reduced in the presence of 5%DMAHDM | [107] |
| NACP, DMAHDM | EBPADMA, PMGDM | Human saliva | Live/dead staining; MTT assay; enzymatic method; CFU counts | NACP-DMAHM inhibited biofilms’ metabolic activity and lactic acid, and reduced biofilm colony-forming units (CFU) by 3–4 log | [108] |
| NACP, DMAHDM, MPC | EBPADMA, PMGDM | Human saliva | BCA method; live/dead staining; MTT assay; CFU counts | 3% MPC+3% DMAHDM inhibited biofilm growth and viability, reducing biofilm CFU by 3 log | [109] |
| NACP, DMAHDM | EBPADMA, PMGDM | Streptococcus mutans | Live/dead staining assay; CFU counts; lactate dehydrogenase approach | NACP-DMAHM composite reduced biofilm acid, and reduced CFU by 4 log | [110] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Q.; Zhang, L.; Bai, R.; Zhuang, Z.; Zhang, Y.; Yu, T.; Peng, L.; Xin, T.; Chen, S.; Han, B. Recent Progress in Antimicrobial Strategies for Resin-Based Restoratives. Polymers 2021, 13, 1590. https://doi.org/10.3390/polym13101590
Sun Q, Zhang L, Bai R, Zhuang Z, Zhang Y, Yu T, Peng L, Xin T, Chen S, Han B. Recent Progress in Antimicrobial Strategies for Resin-Based Restoratives. Polymers. 2021; 13(10):1590. https://doi.org/10.3390/polym13101590
Chicago/Turabian StyleSun, Qiannan, Lingyun Zhang, Rushui Bai, Zimeng Zhuang, Yunfan Zhang, Tingting Yu, Liying Peng, Tianyi Xin, Si Chen, and Bing Han. 2021. "Recent Progress in Antimicrobial Strategies for Resin-Based Restoratives" Polymers 13, no. 10: 1590. https://doi.org/10.3390/polym13101590
APA StyleSun, Q., Zhang, L., Bai, R., Zhuang, Z., Zhang, Y., Yu, T., Peng, L., Xin, T., Chen, S., & Han, B. (2021). Recent Progress in Antimicrobial Strategies for Resin-Based Restoratives. Polymers, 13(10), 1590. https://doi.org/10.3390/polym13101590






