Prevention of Postprandial Hyperglycemia by Ophthalmic Nanoparticles Based on Protamine Zinc Insulin in the Rabbit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemicals
2.3. Rabbit Model with Oral Glucose Tolerance Test (OGTT)
2.4. Preparation of INS Ophthalmic Formulations
2.5. Characteristics in INS Ophthalmic Formulations
2.6. Dispersibility in INS Ophthalmic Formulations
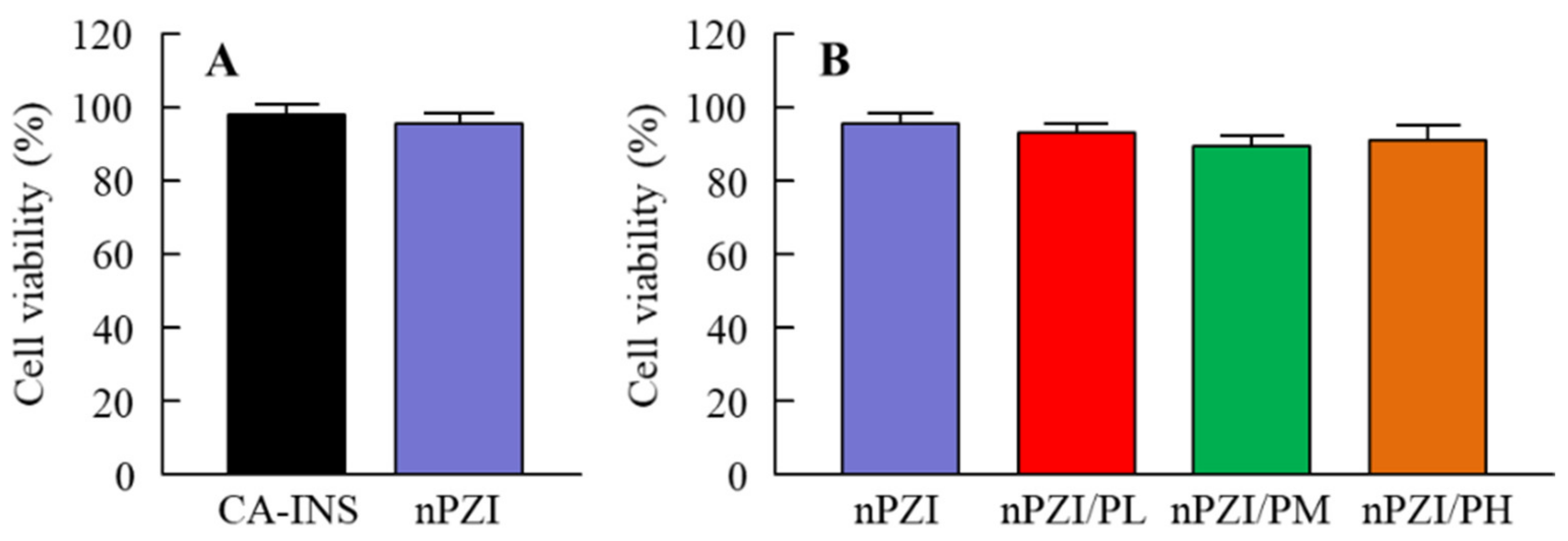
2.7. Corneal Toxicity of INS Ophthalmic Formulations
2.8. Measurement of Plasma INS in Rabbits
2.9. Measurement of PG in Rabbits
2.10. Statistical Analysis
3. Results
3.1. Development of the INS Ophthalmic Formulations
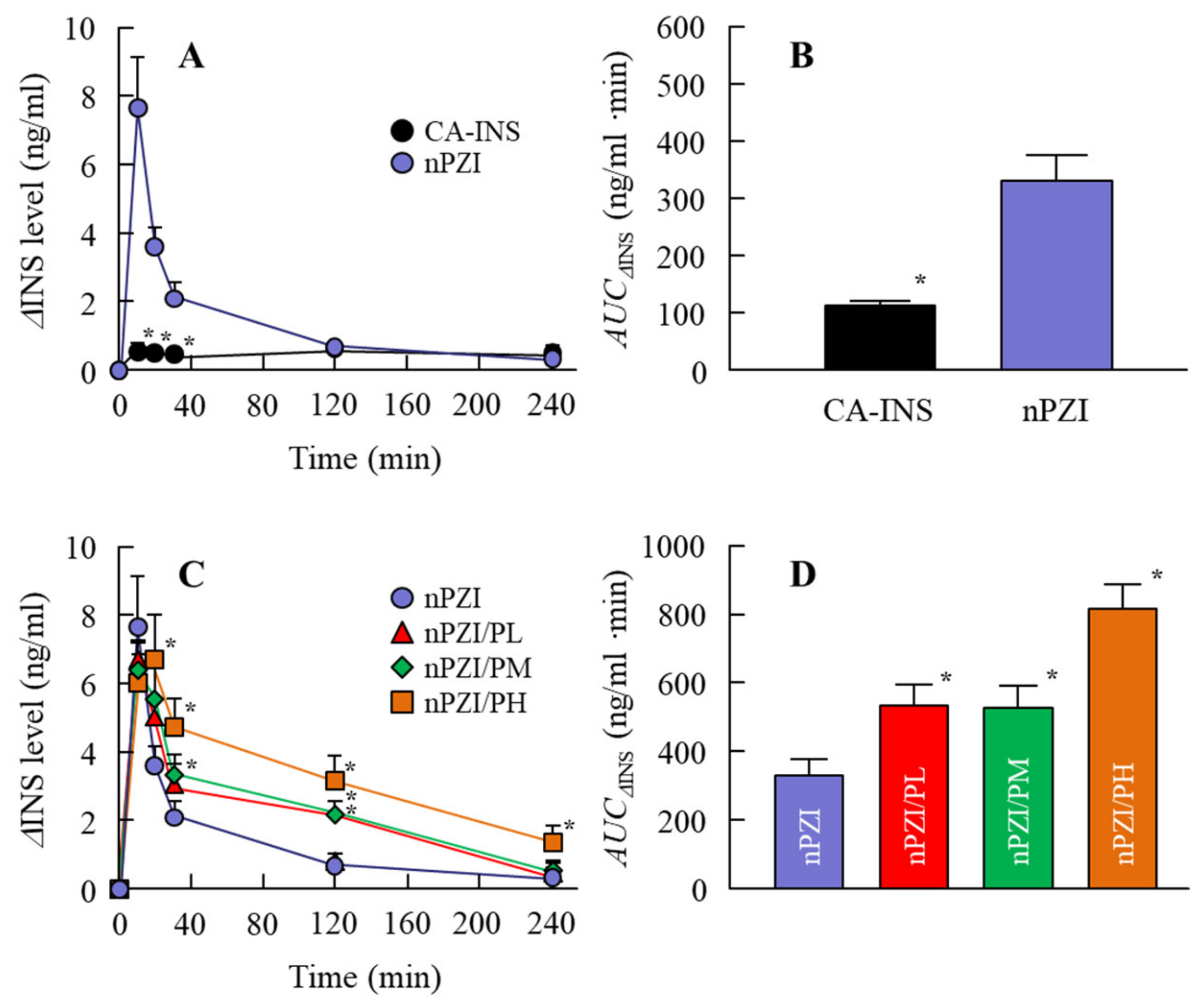
3.2. Changes in Plasma INS and PG Levels in Normal Rabbits Instilled with INS Ophthalmic Formulations
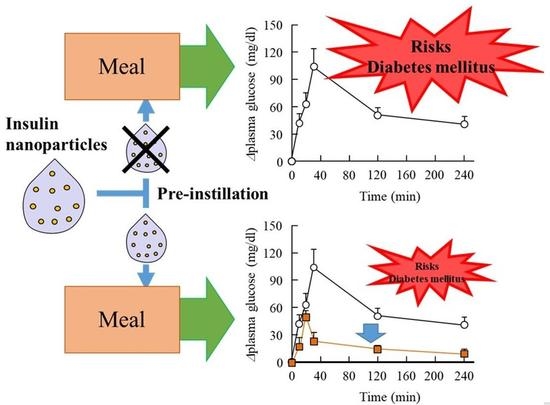
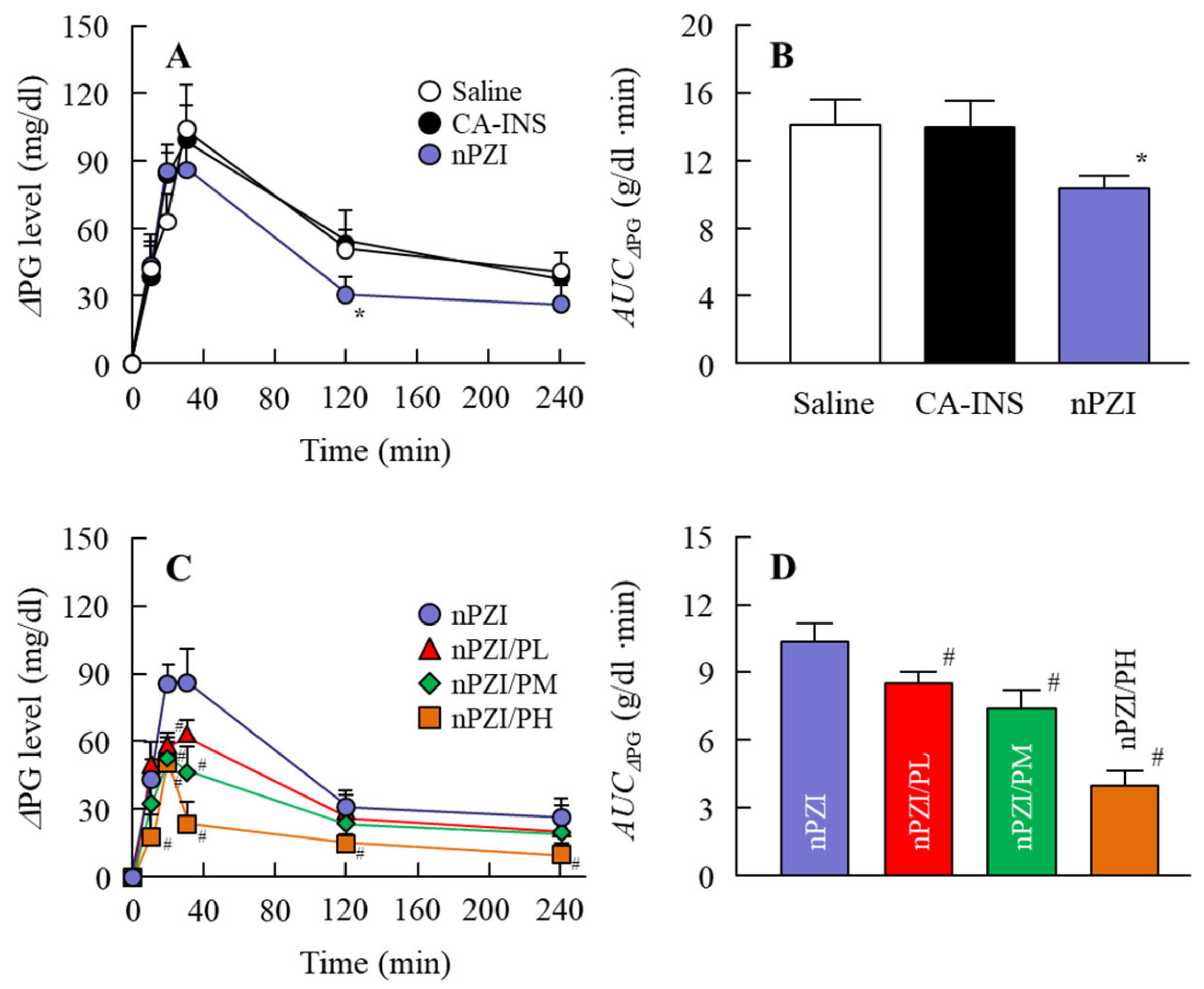
3.3. Preventive Effect of the INS Ophthalmic Formulations on Blood Glucose Spike in the Rabbit OGTT Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baron, A.D. Postprandial hyperglycaemia and a-glucosidase inhibitors. Diabetes Res. Clin. Pract. 1998, 40, S51–S55. [Google Scholar] [CrossRef]
- Cavalot, F.; Pagliarino, A.; Valle, M.; Martino, L.D.; Bonomo, K.; Massucco, P.; Anfossi, G.; Trovati, M. Postprandial blood glucose predicts cardiovascular events and all-cause mortality in type 2 diabetes in a 14-year follow-up: Lessons from the San Luigi Gonzaga Diabetes Study. Diabetes Care 2011, 34, 2237–2243. [Google Scholar] [CrossRef] [Green Version]
- Cavalot, F.; Petrelli, A.; Traversa, M.; Bonomo, K.; Fiora, E.; Conti, M.; Anfossi, G.; Costa, G.; Trovati, M. Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: Lessons from the San Luigi Gonzaga Diabetes Study. J. Clin. Endocrinol. Metab. 2006, 91, 813–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceriello, A.; Hanefeld, M.; Leiter, L.; Monnier, L.; Moses, A.; Owens, D.; Tajima, N.; Tuomilehto, J. Postprandial glucose regulation and diabetic complications. Arch. Intern. Med. 2004, 164, 2090–2095. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A. Postprandial hyperglycemia and diabetes complications: Is it time to treat? Diabetes 2005, 54, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Meigs, J.B.; Nathan, D.M.; D’Agostino, R.B.; Wilson, P.W.F. Fasting and postchallenge glycemia and cardiovascular disease risk: The Framingham Offspring Study. Diabetes Care 2002, 25, 1845–1850. [Google Scholar] [CrossRef] [Green Version]
- De Vegt, F.; Dekker, J.M.; Ruhé, H.G.; Stehouwer, C.D.; Nijpels, G.; Bouter, L.M.; Heine, R.J. Hyperglycaemia is associated with all-cause and cardiovascular mortality in the Hoorn population: The Hoorn Study. Diabetologia 1999, 42, 926–931. [Google Scholar] [CrossRef] [Green Version]
- Khaw, K.-T.; Wareham, N.; Bingham, S.; Luben, R.; Welch, A.; Day, N. Association of hemoglobin A1C with cardiovascular disease and mortality in adults: The European Prospective Investigation into Cancer in Norfolk. Ann. Intern. Med. 2004, 141, 413–420. [Google Scholar] [CrossRef]
- Coutinho, M.; Gerstein, H.C.; Wang, Y.; Yusuf, S. The relationship between glucose and incident cardiovascular events: A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care 1999, 22, 233–240. [Google Scholar] [CrossRef]
- Standl, E.; Schnell, O.; Ceriello, A. Postprandial hyperglycemia and glycemic variability should we care? Diabetes Care 2011, 34, S120–S127. [Google Scholar] [CrossRef] [Green Version]
- Rizza, R.A. Pathogenesis of fasting and postprandial hyperglycemia in type 2 diabetes: Implications for therapy. Diabetes 2010, 59, 2697–2707. [Google Scholar] [CrossRef] [Green Version]
- Yaturu, S. Insulin therapies: Current and future trends at dawn. World. J. Diabetes 2013, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.V.; Huntzicker, M.A. Delivery of systemic regular insulin via the ocular route in dogs. J. Ocul. Pharmacol. Ther. 1996, 12, 515–526. [Google Scholar] [CrossRef]
- Morgan, R.V. Delivery of systemic regular insulin via the ocular route in cats. J. Ocul. Pharmacol. Ther. 1995, 11, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Tei, C.; Nishida, K.; Nakamura, J. Effect of ophthalmic preservatives on serum concentration and local irritation of ocularly applied insulin. Biol. Pharm. Bull. 1995, 18, 169–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, A.; Ziv, E. Pharmacokinetic considerations of new insulin formulations and routes of administration. Clin. Pharmacokinet. 1997, 33, 285–301. [Google Scholar] [CrossRef]
- Bartlett, J.D.; Slusser, T.G.; Turner-Henson, A.; Singh, K.P.; Atchison, J.A.; Pillion, D.J. Toxicity of insulin administration chronically to human eye in vivo. J. Ocul. Pharmacol. 1994, 10, 101–107. [Google Scholar] [CrossRef]
- Pillion, D.J.; Recchia, J.; Wang, P.; Marciani, D.J.; Kensil, C.R. DS-1, a modified quillaja saponin, enhances ocular and nasal absorption of insulin. J. Pharm. Sci. 1995, 84, 1276–1279. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.P.; Garg, A.; Singla, A.K.; Aggarwal, D. Vesicular ocular drug delivery: An overview. Int. J. Pharm. 2004, 269, 1–14. [Google Scholar] [CrossRef]
- Nagai, N.; Iwai, Y.; Sakamoto, A.; Otake, H.; Oaku, Y.; Abe, A.; Nagahama, T. Drug delivery system based on minoxidil nanoparticles promotes hair growth in C57BL/6 mice. Int. J. Nanomed. 2019, 14, 7921. [Google Scholar] [CrossRef] [Green Version]
- Nagai, N.; Ogata, F.; Otake, H.; Nakazawa, Y.; Kawasaki, N. Energy-dependent endocytosis is responsible for drug transcorneal penetration following the instillation of ophthalmic formulations containing indomethacin nanoparticles. Int. J. Nanomed. 2019, 14, 1213–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minami, M.; Seiriki, R.; Otake, H.; Nakazawa, Y.; Kanai, K.; Tanino, T.; Nagai, N. Development of sustained-release ophthalmic formulation based on tranilast solid nanoparticles. Materials 2020, 13, 1675. [Google Scholar] [CrossRef] [Green Version]
- Nagai, N.; Minami, M.; Deguchi, S.; Otake, H.; Sasaki, H.; Yamamoto, N. An in situ gelling system based on methylcellulose and tranilast solid nanoparticles enhances ocular residence time and drug absorption into the cornea and conjunctiva. Front. Bioeng. Biotechnol. 2020, 8, 764. [Google Scholar] [CrossRef]
- Nagai, N.; Isaka, T.; Deguchi, S.; Minami, M.; Yamaguchi, M.; Otake, H.; Okamoto, N.; Nakazawa, Y. In situ gelling systems using pluronic F127 enhance corneal permeability of indomethacin nanocrystals. Int. J. Mol. Sci. 2020, 21, 7083. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Umachi, K.; Otake, H.; Oka, M.; Hiramatsu, N.; Sasaki, H.; Yamamoto, N. Ophthalmic in situ gelling system containing lanosterol nanoparticles delays collapse of lens structure in shumiya cataract rats. Pharmaceutics 2020, 12, 629. [Google Scholar] [CrossRef]
- Yamamoto, A.; Luo, A.M.; Dodda-Kashi, S.; Lee, V.H. The ocular route for systemic insulin delivery in the albino rabbit. J. Pharm. Exp. Ther. 1989, 249, 249–255. [Google Scholar]
- Araki-Sasaki, K.; Ohashi, Y.; Sasabe, T.; Hayashi, K.; Watanabe, H.; Tano, Y.; Handa, H. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest. Ophthalmol. Vis. Sci. 1995, 36, 614–621. [Google Scholar]
- Nagai, N.; Ito, Y.; Okamoto, N.; Shimomura, Y. A nanoparticle formulation reduces the corneal toxicity of indomethacin eye drops and enhances its corneal permeability. Toxicology 2014, 319, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ito, Y.; Okamoto, N.; Shimomura, Y. Size effect of rebamipide ophthalmic nanodispersions on its therapeutic efficacy for corneal wound healing. Exp. Eye Res. 2016, 151, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, B.L.; Kasser, C.; Keuthage, W.; Huptas, M.; Dette, H.; Klute, A. Comparison of insulin aspart vs. regular human insulin with or without insulin detemir concerning adipozytokines and metabolic effects in patients with type 2 diabetes mellitus. Exp. Clin. Endocrinol. Diabetes 2013, 121, 210–213. [Google Scholar] [CrossRef] [PubMed]
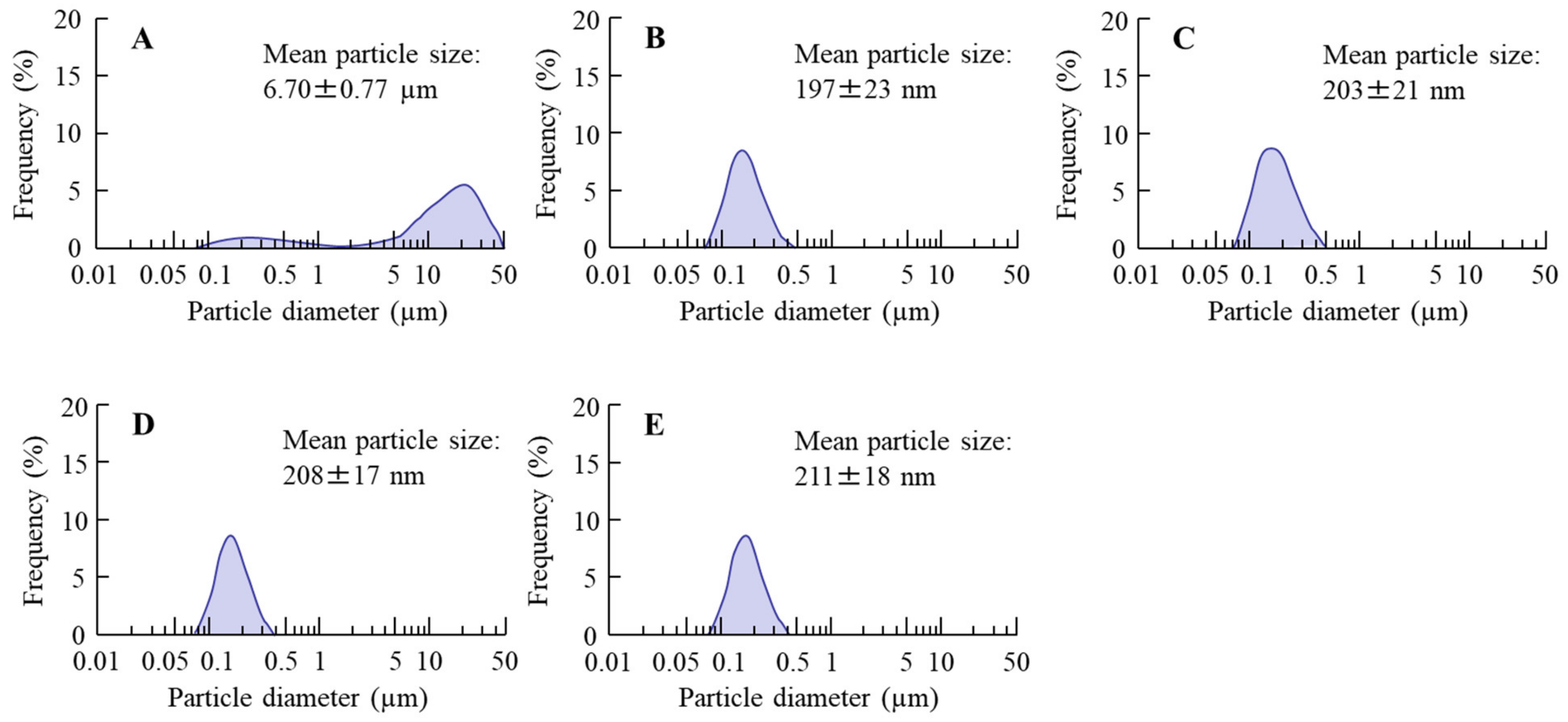


| Formulation | PZI | NaGC | MC | PAA | Treatment |
|---|---|---|---|---|---|
| PZI-NPs | 0.3% | 1% | 4% | — | Bead mill |
| PZI/PL | 0.3% | 1% | 4% | 0.001% | Bead mill |
| PZI/PM | 0.3% | 1% | 4% | 0.01% | Bead mill |
| PZI/PH | 0.3% | 1% | 4% | 0.1% | Bead mill |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deguchi, S.; Ogata, F.; Isaka, T.; Otake, H.; Nakazawa, Y.; Kawasaki, N.; Nagai, N. Prevention of Postprandial Hyperglycemia by Ophthalmic Nanoparticles Based on Protamine Zinc Insulin in the Rabbit. Pharmaceutics 2021, 13, 375. https://doi.org/10.3390/pharmaceutics13030375
Deguchi S, Ogata F, Isaka T, Otake H, Nakazawa Y, Kawasaki N, Nagai N. Prevention of Postprandial Hyperglycemia by Ophthalmic Nanoparticles Based on Protamine Zinc Insulin in the Rabbit. Pharmaceutics. 2021; 13(3):375. https://doi.org/10.3390/pharmaceutics13030375
Chicago/Turabian StyleDeguchi, Saori, Fumihiko Ogata, Takumi Isaka, Hiroko Otake, Yosuke Nakazawa, Naohito Kawasaki, and Noriaki Nagai. 2021. "Prevention of Postprandial Hyperglycemia by Ophthalmic Nanoparticles Based on Protamine Zinc Insulin in the Rabbit" Pharmaceutics 13, no. 3: 375. https://doi.org/10.3390/pharmaceutics13030375