Abstract
Adult human brains consume a disproportionate amount of energy substrates (2–3% of body weight; 20–25% of total glucose and oxygen). Adenosine triphosphate (ATP) is a universal energy currency in brains and is produced by oxidative phosphorylation (OXPHOS) using ATP synthase, a nano-rotor powered by the proton gradient generated from proton-coupled electron transfer (PCET) in the multi-complex electron transport chain (ETC). ETC catalysis rates are reduced in brains from humans with neurodegenerative diseases (NDDs). Declines of ETC function in NDDs may result from combinations of nitrative stress (NS)–oxidative stress (OS) damage; mitochondrial and/or nuclear genomic mutations of ETC/OXPHOS genes; epigenetic modifications of ETC/OXPHOS genes; or defects in importation or assembly of ETC/OXPHOS proteins or complexes, respectively; or alterations in mitochondrial dynamics (fusion, fission, mitophagy). Substantial free energy is gained by direct O2-mediated oxidation of NADH. Traditional ETC mechanisms require separation between O2 and electrons flowing from NADH/FADH2 through the ETC. Quantum tunneling of electrons and much larger protons may facilitate this separation. Neuronal death may be viewed as a local increase in entropy requiring constant energy input to avoid. The ATP requirement of the brain may partially be used for avoidance of local entropy increase. Mitochondrial therapeutics seeks to correct deficiencies in ETC and OXPHOS.
1. Introduction
A 70 kg human adult nominally makes ~70 kg of adenosine triphosphate (ATP) per 24 h. Under conditions of normal oxygen availability, most of this ATP is made in mitochondria by oxidative phosphorylation (OXPHOS) of energy substrates directly or indirectly created by solar photons through photosynthesis. This amount of ATP (8.31 × 1025 molecules/24 h) requires ~20.8 × 1025 electrons/24 h to be passed through the mitochondrial electron transport chain (ETC), when ~2.5 electrons are required for each ATP generated by ATP synthase under normal coupling. If the adult brain, which comprises 2–3% of body weight, but consumes at least ~20% of molecular oxygen and energy substrates [1], contains on average 86 billion (86 × 109) neurons [2], and if each neuron contains 1000 mitochondria (likely an overestimate), then each neuronal mitochondrion in the brain must pass on average 1.40 × 107 electrons/s to maintain ATP production. This estimation is based on glucose utilization/oxygen consumption being split 1–1 between neurons and nonneuronal cells in the brain and is not corrected for glial generation of lactate (from glucose) and neuronal metabolism of glial lactate.
If each neuronal mitochondrion has 104 ETC macrocomplexes (“respirasome”; likely an overestimate) along the inner membrane cristae, then each ETC macrocomplex passes ~1400 electrons/s (0.0007 s·electron−1) and at least ~7000 protons/s (0.00014 s·proton−1) to maintain ATP production. (Note that this estimate of proton translocation rate (1.4 × 10−4 s/proton) is comparable to the lower estimate (0.35 × 10−4 s) of proton tunneling time through an AT base pair in DNA at room temperature [3,4].) These calculations represent lower-limit averages with debatable assumptions (i.e., likely more protons are translocated), and they do not include corrections for electron or proton leakage/scavenging, variations in coupling between electron flow and ATP synthesis, variations in substrate availability, or other conditions of mitochondrial “health”.
What is apparent from these average estimates of electron and proton velocities (momenta, if particle masses are also considered) is that Nature has designed in mitochondria efficient and stereotyped mechanisms for controlling electron and proton flow to transform potential energies of solar photon-derived small molecules acquired by photosynthesis into ATP. One intriguing but unresolved question is whether mitochondria ultimately represent an organelle mediating a transition between quantum and classical behaviors of electrons and protons. Again, note that several-fold more protons are pumped than electrons are passed to maintain ATP production.
The ETC/OXPHOS process in mitochondria is therefore critical to energy metabolism in the brain. The mitochondrial ETC/OXPHOS process is also damaged during human aging, mainly as a result of consuming so much oxygen, leading to “oxidative stress”. Such damage is particularly meaningful for brain energy metabolism and may account for the increased incidence of degenerative brain diseases associated with aging.
2. Quantum Tunneling of Protons and Electrons in Mitochondria
Electrons (mass = 9.11 × 10−28 gm) and protons (mass = 1.67 × 10−24 gm) are both quantum entities that are best described as waves existing in probabilistic vector spaces (“quantum fields”) with “spin” one-half and are either elementary particles (electrons) or composed of quarks (protons) in the standard model. These “waves” become “particles” upon certain types of detection, potentially explaining the wave–particle duality universally observed in quantum entities since their descriptions in the early 20th century (see [5]).
According to the Heisenberg uncertainty principle, the locations and momenta of electrons and protons cannot be precisely known at the same time, at least when moving through empty space. Yet contemporary descriptions of mitochondrial function appear to violate this principle, and it is critical that the momentum-location constraints on electrons and protons be kept in mind as mitochondrial ETC activity is analyzed.
It is likely, though not proven, that proton pumping (mitochondrial proton-coupled electron transfer (PCET)) occurs in Complex I through protein subunits separable from those mediating electron transport [6]. These proton-pumping complexes appear to be composed of the seven hydrophobic Complex I subunits coded by the mitochondrial genome (mtDNA) [6]. If this formulation is correct, then damage/mutations to mtDNA (at least to the seven Complex I subunits) will selectively affect proton pumping rates and not directly alter ETC catalytic rates.
Although the electron acceptor molecule (ubiquinone) for electron flow in Complex I is well characterized, there does not appear to be a separate proton acceptor molecule in the intermembrane space, other than water molecules. Because the downstream ATP synthase rotor head (for OXPHOS) appears to accept only protons (not hydrated protons, [7]), this situation begs the question of how pumped protons are “protected” from hydration by water molecules in the mitochondrial intermembrane space. (Note that proton solvation by water is very energetically favorable with Free energy ~266 kcal/mol.). Perhaps there exists an as of yet unknown proton acceptor molecule in the intermembrane space (other than water) with different thermodynamics of proton binding? An alternative mechanism proposed by Leone, et al. [7] is that the rotor arms of ATP synthase operate using a gradient of un-hydrated protons bound to carboxylate anions, with water molecules separately bound to the rotor arms.
3. Decoherence
It is debatable as to whether decoherence occurs to a significant degree in mitochondria. Stated simplistically, decoherence (loss of “quantum-ness”) occurs when there is interaction of quantum entities with non-quantum, classical macroscopic objects such that quantum behavior is reduced or lost [8]. Mitochondria may properly be considered macroscopic entities; whether Complex I iron–sulfur centers with low energy molecular orbitals that are separated by 14 angstroms or less, and thus form “wires” for conducting electrons, meet the same criterion is debatable. The addition of nearby water molecules arranged in tandem appears to provide a pathway for electron tunneling through these wires, which reduces activation energies (thus increasing rates) but theoretically has no effect on the energetics of electron movement ([9] and references therein).
Tunneling is the phenomenon of quantum entities appearing to pass through energy barriers due to their wave properties and small (nonzero) probabilities (wave function (Ψ2)) of existing on either side of barriers defining an energy well [10] (also, see Figure 1). Tunneling is considered to be a quantum phenomenon; thus, if decoherence is dominant in mitochondrial ETC function, then tunneling is less probable. Contrarily stated, the greater the quantum-ness of ETC behavior, the greater the probability that tunneling may occur.
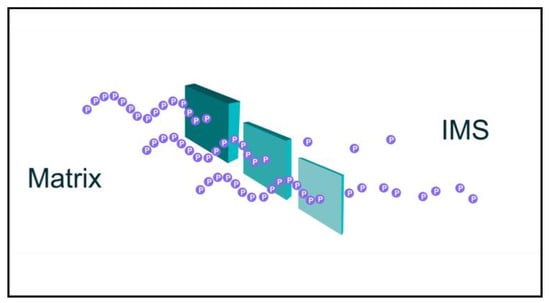
Figure 1.
Cartoon of proton tunneling. Protons pumped from the mitochondrial matrix into the intermembrane space (IMS) must overcome electrostatic repulsion of other protons (already in the IMS?) and likely avoid “irreversible” solvation by water. These are but two of likely several energy barriers that protons must overcome, and “quantum tunneling” may provide a mechanism to overcome energy barriers experienced by protons moving into the IMS and increase rates of proton pumping.
Shown in Figure 1 are protons (purple spheres) moving as a sine wave through barriers of variable thickness. Mathematically, quantum tunneling may be viewed as follows: Let P be the probability of a particle with mass m and energy E passing through a barrier with energy V:
where V is the energy of the potential barrier, E is the kinetic energy possessed by the particle, α is the thickness of the barrier, m is mass of the particle (in the case of protons, mass = 1.67 × 10−24 gm), and h is Plank’s constant (6.626 × 10−34 m2·kg/s). (Above taken from ChemLibre Texts: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry/Quantum_Mechanics/02._Fundamental_Concepts_of_Quantum_Mechanics/Tunneling (accessed on 21 February 2021)). (The above are taken from [9]).
By this formula, decreasing barrier thickness (α) will increase the probability of tunneling through an energy barrier. This is presented in Figure 1.
Electron tunneling in Complex I was recently reviewed [9] (and references therein). Proton tunneling has been described in laboratory experiments, typically performed at very cold temperatures and high pressures [11]. Proton tunneling in mitochondrial ETC has not been described but has been proposed to occur through hydrogen bonds in replicating DNA molecules as a mechanism of spontaneous DNA mutation [3]. Proton tunneling may also occur in the “Grotthuss” mechanism of “proton jumping”, whereby protons move through a hydrogen bond network of adjacent water molecules [12,13].
PCET [14] has been proposed (at least in X-ray resolved crystals of bacterial Complex I) to occur by the combination of electrostatic charge-mediated conformational change in tertiary structure of proton channels following reduction of ubiquinone [15,16]. By this “action at a distance” mechanism, channels in proton-pumping Complex I subunits (see Figure 2) are opened as a result of two electron reduction of ubiquinone following attachment of NADH to its binding site in the matrix side of Complex I and oxidation of NADH to NAD+ with reduction of attached FMN cofactor, followed by passage of the two resulting electrons to ubiquinone to form ubiquinol.
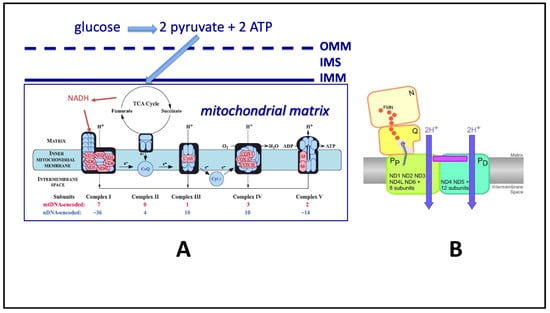
Figure 2.
Overview of Mitochondrial Electron Transport Chain ETC/Oxidative Phosphorylation (OXPHOS) and ATP Production. (A). (left) Linear representation of the mitochondrial ETC/OXPHOS system. Shown are the 5 mitochondrial complexes involved in ETC/OXPHOS. In pink are the 13 individual protein subunits derived by transcription of mtDNA genes (usually maternally derived) and translated in the mitochondrial matrix. In blue are the protein subunits derived from nDNA (paternally and maternally derived) that are synthesized in the cytosol and imported into mitochondria. Mammals are now thought to have a total of 45 subunits in Complex I (7 from mtDNA, 38 from nDNA). In the brain, glucose is believed to be the major carbon energy source that is transformed to ATP. In other tissues, mitochondria can metabolize fatty acids and amino acids. Glucose (6 carbons) is broken down outside of mitochondria into 2 pyruvate molecules (3 carbons each) by glycolysis, and the resulting pyruvate is imported into the mitochondrial matrix by specific pyruvate carrier proteins that span the relatively protein-rich outer mitochondrial membrane (OMM), the intermembrane space (IMS), and the relatively lipid-rich inner mitochondrial membrane (IMM). Once in the matrix, pyruvate is oxidatively decarboxylated by the tricarboxylic acid cycle (TCA cycle), yielding reducing electrons that in pairs reduce the electron carrier NAD+ to NADH. NADH is subsequently oxidized back to NAD+ at Complex I (thus its name, NADH-NAD oxidoreductase) and transfers its two electrons to flavin mononucleotide (FMN) that is embedded in the hydrophilic (matrix) arm of Complex I. FMN then passes these two electrons through the Fe–S centers of Complex I to reduce the electron carrier ubiquinone to ubiquinol. This reaction provides the initial free energy that is used for proton pumping. Ubiquinol is reoxidized to ubiquinone at Complex III, where a separate electron carrier in the IMS, cytochrome C, is reduced. Reduced cytochrome C is reoxidized at Complex IV, giving up electrons that participate in the reduction of molecular oxygen to water. Protons are pumped into the IMS at Complexes I, III, and IV (none at Complex II, now regarded as a component of the TCA cycle), and the resulting proton gradient is used to drive ATP production by Complex V. (B). (right) Cartoon showing separate ETC and proton-pumping components of Complex I (taken from Figure 6 of [6]). Shown are the embedded FMN moiety and the nine Fe–S centers that pass electrons through Complex I (orange circles), leading to reduction of ubiquinone to ubiquinol. Additionally shown are the proposed separate proton-pumping subunits of Complex I that consist of proximal (PP) and distal (PD) subunits that are both believed to be located primarily in the lipid IMM and are in turn composed of the 7 hydrophobic proteins coded by mtDNA and 8 or 12 proteins coded by nDNA, respectively. Proton tunneling into the IMS may occur at these sites.
The energetics of ubiquinone reduction theoretically drive the translocation of protons across the inner membrane through proton channels in Complex I subunits “opened” by ubiquinone reduction, but a simpler proton tunneling mechanism may be operative and contribute to the overall PCET rate of Complex I (please also see [6,15,16,17]). Note that the proposal of proton tunneling does not change the bioenergetics, which depend on the free energy of ubiquinone reduction to ubiquinol, but proton tunneling could lower activation energy of proton passage and thus increase the rate of proton pumping. Ubiquinone reduction-induced conformational changes (“action at a distance”) and proton tunneling could synergistically work together to induce Complex I proton displacement, such that proton pumping would not be rate limiting in Complex I PCET. (Please see Figure 2 for a current model of Complex I subunits mediating proton pumping).
Which mechanism might be affected in neurodegenerative diseases (NDDs) (if either mechanism is even operative) is unknown. The possibility should also be considered that proton pumping is not directly affected by NDD pathobiology, and that the major deficit leading to a reduced rate of ATP synthesis is lowering of electron transport rate. Finally, we are aware of no data supporting or refuting the existence of electron quantum tunneling in the other ETC complexes (beyond Complex I, which has been crystallized) and no data supporting or refuting the existence of proton quantum tunneling in any ETC Complex.
4. Entropy and Neurodegeneration
Whatever origin of the observable universe hypothesis one subscribes to, all can agree that the cosmological evidence supports the ongoing expansion of the observable universe with a resulting steady increase in entropy. Earlier thermodynamic theorists (such as Clausius) describing the varying forms of this “Second Law of Thermodynamics” developed the concept that the entropy in the Universe may remain constant but is more likely constantly increased, regardless of what happens locally.
The human brain and its ~86 billion neurons display a marked (increase in order)/(decrease in disorder) that represents thermodynamically a decrease in entropy of cellular molecules. In fact, all cells, and life forms themselves, represent a reduction in molecular entropy; from this perspective, cell and organismal death can be viewed as an (inevitable) increase in molecular entropy.
Might neurodegeneration and neuronal death also be viewed as a local increase in entropy, driven ultimately by a reduction in energy input, whatever the “genesis” cause(s) of the bioenergetic deficit? By this paradigm, neuronal death and its attendant increase in molecular entropy would be thermodynamically favored, independent of whether it occurred during “life” or after organismal “death”. If occurring during life, then a clinical phenotype is generated that can be discerned (i.e., loss of cognitive capacity in Alzheimer’s disease (AD); loss of smooth voluntary movement in Parkinson’s disease (PD); loss of muscle mass and appearance of weakness in amyotrophic lateral sclerosis (ALS), etc.).
If this paradigm is true, then prevention of neuronal death (in NDDs) can be viewed as a thermodynamic problem with potential thermodynamic solutions. For example, energy input, which is already disproportionately elevated in adult human brain, could be increased by processes that stimulate mitochondrial energy transformation and ATP synthesis. One could also attempt to increase synaptogenesis and size/interactions of neuronal networks (which should also reduce neuronal molecular entropy). However, we wish to note that no reported therapeutic strategies derived from the above thermodynamic hypothesis of neuronal death have yet been published.
5. Oxidative Phosphorylation (OXPHOS) Alterations in NDDs
OXPHOS is an evolved process in which electron flow through the ETC is coupled to proton translocation from the mitochondrial matrix to the intermembrane space, creating a proton and pH gradient between the mitochondrial matrix and intermembrane space. The resulting proton gradient is used to rotate the arm of ATP synthase, an evolutionarily old enzyme [18] that appears to require non-hydrated protons to operate [7] (see above). As discussed previously, this PCET may utilize proton tunneling and/or structural alterations in proton-pumping subunits of the ETC (at least for Complex I).
Because electron flow (at least in Complex I) is believed to use a tunneling mechanism (for discussion see [9] and references therein), structural alterations to proteins critical to electron tunneling may result in reduced rates of electron flow, leading to reduced rates of proton pumping and ATP synthesis. This could result in a bioenergetic deficiency state, based on maintaining a minimum rate of ATP synthesis necessary for neuronal functions (see above). By this mechanism, proton pumping (PCET) would not be mechanistically impaired per se, just reduced in rate.
In NDDs variable reductions in ETC rates at one or more specific complexes have been described. Epigenetic modifications potentially responsible for these reductions include pre-transcriptional changes to genes such as gene methylation and histone modifications that affect gene promoter or repressor activities. Epigenetic alterations have been described in amyotrophic lateral sclerosis (ALS, [19]), Parkinson’s disease (PD, [20,21,22,23,24,25,26,27,28]), and Alzheimer’s disease (AD, [19,20,21,22,25,28,29,30,31,32,33,34,35]). In many studies, cell or animal models of NDDs are utilized, with the understanding that similar phenomena may occur in the more common sporadic forms of each NDD.
Reductions in bioenergetics may also derive from nitrative damage to proteins, particularly to nitration of tyrosine residues by peroxynitrite anion (ONOO−). So-called “nitrative stress”, which is frequently found in models that demonstrate “oxidative stress”, have been described in ALS [36,37,38,39,40,41,42,43], AD [36,37,40,44,45,46,47,48,49,50,51,52,53,54,55], and PD [36,37,40,42,44,53,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73] tissues.
Oxidative stress (OS) is the condition where production rates of oxidizing species exceed rates of inactivation. Oxidizing species may damage lipids, nucleic acids, and proteins and thus are potentially toxic to cells and energy production at several levels. Because most molecular oxygen is utilized by mitochondria for ETC activity (and is reduced to water), mitochondria are particularly susceptible to OS. OS damage has been described in ALS [74,75], AD [31,35,44,45,50,52,53,55,76,77,78,79,80,81,82,83,84,85,86,87,88,89], and PD [24,36,53,55,56,57,60,62,63,64,65,66,67,71,77,90,91,92,93,94,95,96,97] tissues and models.
6. Summary of ETC-OXPHOS
The human brain has disproportionately elevated (~10-fold, relative to mass) energy substrate and oxygen consumption rates. These elevated metabolic rates in brain likely depend on electron and proton tunneling in mitochondria, although neither of these processes has been conclusively demonstrated to occur. Mitochondria can be viewed as transitional organelles that bridge the quantum world of very small wave-particle behavior and the classical world of decoherent larger, more macroscopic structures such as cells.
Photosynthesis yields both the small molecules that directly or indirectly drive mitochondrial electron transport and the toxic by-product (molecular oxygen) that is an excellent electron acceptor (oxidant) for terrestrial life. The highly electrophilic nature of molecular oxygen requires “protection” of reducing equivalents (as mainly NADH) and competes with ETC thermodynamics to yield oxygen free radicals. These free radicals must be detoxified or they will damage cellular constituents (proteins, lipids, nucleic acids) and can combine in several ways with other molecules to yield nitrogen–oxygen toxins (“nitrative stress”).
Protons pumped into the intermembrane space theoretically require protection from thermodynamically favorable hydration, since non-hydrated protons appear to be favored for driving the ATP synthase rotor [7]. How this occurs is presently unknown but may require anatomic proximity of ATP synthase rotor proton-binding sites to proton-pumping sites or an as yet unknown proton solvation system other than water alone. An alternative mechanism presented by Leone et al., involves carboxylate protonation (by non-hydrated protons) and binding of water (from hydronium ions) to ATP synthase [7]. By this mechanism, hydrated protons could drive ATP synthase, but the H3O+ ions would dissociate rapidly into H+ and H2O that would separately bind to ATP synthase.
These massive energy transformation systems appear to be damaged in neurodegenerative diseases (NDDs), at least in terms of ease of detecting epigenetic alterations/oxidative stress damage/nitrative stress damage. The result could be a reduction in neuronal ATP synthesis rate, with neuronal dysfunction leading to emergence of early clinical phenotypes and an ultimate increase in entropy following neuronal death or even autophagic digestion of organelles (i.e., mitophagy).
Nature was tasked with producing large quantities of ATP that are used by neurons for many purposes, including the lifetime (usually over many decades) maintenance of nondividing state and recharging of neuronal potentials where rapid potential swings are necessary for functions of both individual neurons and neuronal networks. A truly remarkable system resulted, which appears to fade as organisms age and accumulate biochemical damages over a lifetime. Whether these aging phenomena can be more successfully controlled and the burden of NDD reduced are future challenges to mitochondrial therapeutics.
7. Brain Mitochondrial Therapeutics
The human brain is one of several tissues that are “non-mitotic”, meaning that the majority of its cells do not undergo cell division during most of the organism’s lifetime. In fact, entering the cell cycle is considered a lethal event for mature neurons, compared to “mitotic” cells of mesodermal and endodermal origins that regularly die, divide, and are thus replaced.
Even in non-mitotic neurons, mitochondria undergo their own cycles of DNA (mitochondrial DNA, mtDNA) replication. While the mechanistic details of mtDNA replication remain debated, all agree that mtDNA replication is independent of host cell division in both non-mitotic (should be small or nonexistent) and mitotic tissues.
We have yet to learn how mtDNA replication is regulated, although some knowledge exists about the molecules and their hierarchy of control for mtDNA replication. For instance, mtDNA replication utilizes a DNA polymerase specially synthesized by nuclear genes for mtDNA replication, coded for by host cells (DNA polymerase gamma) and imported into mitochondria. There appear to be multiple copies of mtDNA within each mitochondrion, but it remains unclear how that number is regulated. Additionally, not all copies of mtDNA within each mitochondrion, and thus within each cell, are necessarily identical, a condition known as heteroplasmy. In addition, mtDNA appears to have a higher mutation rate than does nuclear DNA, ascribed to both the relative lack of protective proteins and limited DNA repair mechanisms.
The thirteen genes encoded by mtDNA (all for ETC/OXPHOS function) are believed to be translated within the mitochondrial matrix using a genetic code similar to but not identical with the code used in nuclear DNA–nuclear mRNA translation. Special mitochondrial chaperone proteins (again provided by the host cell) appear to assist assembly of the ETC/OXPHOS complexes that are characterized by many nuclear DNA-encoded subunits and lesser numbers of more hydrophobic mtDNA-encoded subunits. Again, several of the regulatory proteins for mtDNA transcription (synthesized from nuclear DNA genes and imported into mitochondria) are known, but the complete details of regulation of mtDNA transcription and translation/assembly into functioning ETC/OXPHOS complexes remain unclear.
Mitochondrial therapeutics strategies, in terms of ATP production, are difficult to implement currently, due mainly to ignorance about details of how mitochondria within brain neurons (and many other cell types) regulate/are regulated in terms of ETC/OXPHOS and thus ATP production capacities. Several approaches can be discussed, and this list is by no means complete:
- Correction of mtDNA mutations
- Correction of mtRNA and/or mitochondrial mRNA errors
- Increase in mitochondrial mass leading to increased ETC/OXPHOS capacity to make ATP
- Prevention of epigenetic, nitrative stress (NS) and oxidative stress (OS) damage to ETC/OXPHOS genes or proteins
The above potential strategies relate solely to mitochondrial ETC/OXPHOS function and not directly to regulation of mitochondrial calcium signaling or cell death initiation, important mitochondrial functions not addressed in this review.
7.1. Correction of mtDNA Mutations
The development of rapid and relatively inexpensive “next-generation” DNA sequencing has allowed the development of “3-parent babies” as a viable strategy for prevention of mtDNA-transmitted mutations. If precautions are taken to screen out mitochondrial “pseudogenes” (stretches of nuclear DNA containing variable amounts of mtDNA sequences [98]), then specific mtDNA mutations can be defined in oocytes of mothers who have given birth to a child with a mtDNA mutation-derived disease. Because the mother’s oocytes may contain variable proportions of mutant compared to wild-type mtDNA (recall heteroplasmy), and because maternal transmission of mtDNA is the rule, implantation and growth of oocytes containing only wild-type mtDNA and both maternal and paternal nuclear genomes is now possible with mitochondrial replacement therapy. This can be accomplished by transferring the maternal meiotic nuclear spindle into a donor oocyte that contains only wild-type mtDNA (and has its own nuclear meiotic spindle removed), followed by fertilization with paternal sperm. This technique is referred to as maternal spindle transfer (MST). An alternative approach is to fertilize a donor oocyte with paternal sperm, then remove the paternal-donor pronuclei and replace them with pre-fusion maternal and paternal pronuclei. This approach is known as pronuclei transfer (PNT). See [99] for details. These approaches to a 3-parent baby have been developed, discussed, and implemented in the UK by the Newcastle group [99,100,101,102,103,104] and by the Mitalipov group at Oregon Health Sciences University [100,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119].
7.2. Correction of mtRNA and/or Mitochondrial mRNA Errors
The mtDNA genome contains 13 sequences/genes for ETC/OXPHOS proteins that are made in the mitochondrial matrix, 22 tRNA sequences for ribosomal protein synthesis, and 2 rRNA’s that assist in making ETC/OXPHOS proteins from mtDNA genome sequences (that first must be transcribed into mtRNAs). Many of the mtDNA mutational errors that impact translation of mtRNA sequences are mutations in one or more of the tRNA genes in circular mtDNA [120,121]. In addition, it remains unclear how mitochondria maintain an adequate supply of tRNAs needed for synthesis of multiple proteins [122]. Post-transcriptional mt-tRNA gene modifications may also play a role in mitochondrial RNA-based diseases [123].
There are several published reports of correcting mt-tRNA mutation defects, usually by rescuing the respiratory phenotypes of cells harboring specific mt-tRNA mutations (for example, see [124]). These are successful but appear to be restricted to specific mutations, although a more “generic” approach has been reported [125]. This approach utilizes the mitochondrial importation of wild-type tRNAs fused with a mitochondrial importation signal. Using this approach, the authors were able to partially correct metabolic abnormalities of cybrid cells carrying mutations for MELAS (mitochondrial encephalopathy lactic acidosis and stroke) or MERRF (mitochondrial encephalopathy and ragged red fiber disease) [125].
7.3. Increase in Mitochondrial Mass Leading to Increased ETC/OXPHOS Capacity to Make ATP
Mitochondrial mass is controlled by the processes of mitochondrial biogenesis (also known as mitobiogenesis, increases mitochondrial mass) and mitochondrial autophagy (also known as mitophagy, decreases mitochondrial mass). Both processes are important for maintaining overall neuronal and cellular bioenergetic function, are operative under normal circumstances, and can be impaired in certain disease phenotypes. Several key pathways are known for mitobiogenesis [126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218] and mitophagy [56,61,64,93,139,145,151,163,192,194,195,205,209,213,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248]. The reader is directed to comprehensive reviews of these two important subjects (for mitobiogenesis: [129,130,136,164,167,192,193], and [211]; for mitophagy: [226,233], and [243]).
An obvious question relates to increasing mass of mitochondria containing damaged mtDNA. There are at least two related issues to consider. First, increasing mass of impaired mtDNA-containing mitochondria may improve bioenergetics for the host cell, which is a desired therapeutic goal. The same argument can be applied to cells containing mutated nuclear DNA coding for mitochondrial genes. Second, it remains unclear whether stimulation of mitobiogenesis (or manipulation of mitophagy) will yield net positive or negative effects on cells harboring a heterogenous collection of mitochondria. It must be remembered that effects on peripheral tissues do not necessarily extend into brain tissues.
It is likely that such experiments will need to be tested on an individual’s cells before being applied to that individual. Stimulating mitobiogenesis or manipulating mitophagy could be performed on white blood cells or muscle cells, both readily accessible tissues that are mitotic and non-mitotic, respectively. Improvements in respiration or ATP synthesis rates can be assayed in response to several agents.
7.4. Prevention of Epigenetic, Nitrative Stress (NS) and Oxidative Stress (OS) Damage to ETC/OXPHOS Genes (Epigenetics) or Proteins (NS and OS)
This final approach represents decades of investigation by many scientists, is potentially applicable to both specific clinical phenotypes and the broad area of aging, and likely will continue to be popular in the future. Mitochondrial respiration, particularly in brain neurons and generally throughout the body, suffers from taking place in environments with relatively high levels of oxygen molecules and oxygen–nitrogen adducts (such as peroxynitrite anion, ONOO-), or nitric oxide (NO) itself). In addition, it remains unclear how the > 80 genes responsible for mitochondrial proteins of the ETC and OXPHOS systems are regulated by epigenetics, but recent studies suggest that this does occur and that mitochondrial metabolism can affect nuclear epigenetics [107,249,250,251,252].
There have been many attempts to develop therapies directed toward reduction of OS and/or NS. The most promising utilize molecules that are either organic cations at physiological pH, such as pramipexole [253,254], or are attached to “inactive” organic cationic groups such as triphenylphosphonium (TPP) [255,256,257,258] or rhodamine [256,259,260,261,262]. The underlying concepts are that by virtue of lipophilicity, such potential therapeutics can pass through cell and mitochondrial membranes, and the cationic nature suggests that such molecules will be concentrated into the relatively negative mitochondrial matrix (a result of proton pumping).
The OS/NS scavenging molecules must have intrinsic activity and their concentration into the mitochondrial matrix adds organelle specificity. It is striking that the capacity of mitochondrially-targeted ROS and RNS ultimately derive from proton pumping across the inner membrane (responsible for the mitochondria membrane potential), which may involve several mechanisms of PCET, including proton tunneling.
Both NS and OS have been reduced in brain and specifically human disease models [39,50,52,53,55,56,67,76,78,80,84,91,94,95,96,257,262] by such approaches. This therapeutic area appears to be popular in attempting to improve mitochondrial bioenergetics in nervous tissues, as well as other organs.
8. Conclusions
Mitochondria have evolved, likely from protobacterial precursors through endosymbiosis [263], and now inhabit cells of almost all terrestrial and marine plants and animals, including humans. In addition to their critical roles in modulating cellular calcium signaling and cell death initiation, mitochondria through ETC/OXPHOS appear to supply most of the substantial daily ATP requirement for humans. Adult human brain has a ~10-fold disproportionate (relative to mass) ATP production rate and depends on the stereotyped movement of reducing electrons down an energy gradient in the ETC and conservation of this ETC energy decrease by proton displacement across the mitochondrial inner membrane. This electron movement and proton displacement, however they occur, must respect quantum mechanical constraints.
Both electrons moving through the ETC and proton displacement from the matrix to the intermembrane space (IMS) may utilize quantum tunneling in addition to other mechanisms. It is not yet clear whether tunneling occurs at all, but it is a theoretically appealing mechanism for quantum entities to pass through energy barriers and reduce activation energies (thus increasing rates of proton transfer).
Mitochondria must likely segregate electrons from electrophilic molecular oxygen and protons from solvation by water. How these feats are accomplished remains unclear, but our daily ATP requirements likely require these biochemical gymnastics. Mitochondria may represent a necessary transitional organelle between the quantum world of elementary particles and energy-releasing catabolism of molecules created from absorbed solar-derived photons. Decoherence (loss of quantum-ness) may assist ATP production in mitochondria, and theoretically its presence may vary with energy needs.
Many neurodegenerative diseases (NDDs) afflicting humans may be viewed thermodynamically as increases in molecular entropy during life of the organism as neurons die. Such local entropy increases may arise from decreased neuronal energy production traceable to decline of OXPHOS rates. OXPHOS rates in turn depend on availability of intact ATP synthase complexes and (likely) non-hydrated protons in the intermembrane space or at the proton-binding sites of the ATP synthase rotor.
Mitochondrial therapeutics can address bioenergetic deficiencies at multiple levels, from epigenetic changes in mitochondrial and/or nuclear genomes, through measures to reduce post-translational damage to ETC/OXPHOS proteins. Many such approaches have been/are being developed, and optimism exists for varied solutions to the human problems of mitochondrial ETC/OP dysfunction in NDDs.
Author Contributions
J.P.B.J. wrote the manuscript draft, which was reviewed by both J.P.B.J. and I.G.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
I.G.O. was supported by the international Clinical Research Center, St. Anne’s University Hospital, CZ-6569i Brno, Czech Republic.
Conflicts of Interest
J.P.B. is the inventor on patents related to the use of R(+) pramipexole as an antioxidant/neuroprotectant in NDD and aging.
References
- Blazey, T.; Snyder, A.Z.; Goyal, M.S.; Vlassenko, A.G.; Raichle, M.E. A systematic meta-analysis of oxygen-to-glucose and oxygen-to-carbohydrate ratios in the resting human brain. PLoS ONE 2018, 13, e0204242. [Google Scholar] [CrossRef] [PubMed]
- Von Bartheld, C.S.; Bahney, J.; Herculano-Houzel, S. The search for true numbers of neurons and glial cells in the human brain: A review of 150 years of cell counting. J. Comp. Neurol. 2016, 524, 3865–3895. [Google Scholar] [CrossRef]
- Löwdin, P.-O. Isotope effect in tunneling and its influence on mutation rates. Mutat. Res. Mol. Mech. Mutagen. 1965, 2, 218–221. [Google Scholar] [CrossRef]
- Srivastava, R. The Role of Proton Transfer on Mutations. Front. Chem. 2019, 7, 536. [Google Scholar] [CrossRef]
- Maudlin, T. Philosophy of Physics: Quantum Theory; Princeton Foundations of Contemporary Philosophy Book, 33; Princeton University Press: Princeton, NJ, USA, 2019. [Google Scholar]
- Dröse, S.; Krack, S.; Sokolova, L.; Zwicker, K.; Barth, H.-D.; Morgner, N.; Heide, H.; Steger, M.; Nübel, E.; Zickermann, V.; et al. Functional Dissection of the Proton Pumping Modules of Mitochondrial Complex I. PLoS Biol. 2011, 9, e1001128. [Google Scholar] [CrossRef] [PubMed]
- Leone, V.; Krah, A.; Faraldo-Gómez, J.D. On the Question of Hydronium Binding to ATP-Synthase Membrane Rotors. Biophys. J. 2010, 99, L53–L55. [Google Scholar] [CrossRef][Green Version]
- Schlosshauer, M. Decoherence and the Quantum-to-Classical Transition; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Bennett, J.P. Medical hypothesis: Neurodegenerative diseases arise from oxidative damage to electron tunneling proteins in mitochondria. Med. Hypotheses 2019, 127, 1–4. [Google Scholar] [CrossRef]
- Trixler, F. Quantum Tunnelling to the Origin and Evolution of Life. Curr. Org. Chem. 2013, 17, 1758–1770. [Google Scholar] [CrossRef]
- Yen, F.; Gao, T. Dielectric Anomaly in Ice near 20 K: Evidence of Macroscopic Quantum Phenomena. J. Phys. Chem. Lett. 2015, 6, 2822–2825. [Google Scholar] [CrossRef] [PubMed]
- Cukierman, S. Et tu, Grotthuss! and other unfinished stories. Biochim. Biophys. Acta (BBA) Bioenerg. 2006, 1757, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Gerwert, K.; Freier, E.; Wolf, S. The role of protein-bound water molecules in microbial rhodopsins. Biochim. Biophys. Acta (BBA) Bioenerg. 2014, 1837, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Hammes-Schiffer, S. Proton-Coupled Electron Transfer: Moving Together and Charging Forward. J. Am. Chem. Soc. 2015, 137, 8860–8871. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Belevich, G.; Gamiz-Hernandez, A.P.; Róg, T.; Vattulainen, I.; Verkhovskaya, M.L.; Wikström, M.; Hummer, G.; Kaila, V.R.I. Redox-induced activation of the proton pump in the respiratory complex I. Proc. Natl. Acad. Sci. USA 2015, 112, 11571–11576. [Google Scholar] [CrossRef] [PubMed]
- Wikstrom, M.; Sharma, V.; Kaila, V.R.; Hosler, J.P.; Hummer, G. New Perspectives on Proton Pumping in Cellular Respiration. Chem. Rev. 2015, 115, 2196–2221. [Google Scholar] [CrossRef] [PubMed]
- Ripple, M.O.; Kim, N.; Springett, R. Mammalian Complex I Pumps 4 Protons per 2 Electrons at High and Physiological Proton Motive Force in Living Cells. J. Biol. Chem. 2013, 288, 5374–5380. [Google Scholar] [CrossRef]
- Signes, A.; Fernandez-Vizarra, E. Assembly of Mammalian Oxidative Phosphorylation Complexes I-V and Supercomplexes. Essays Biochem. 2018, 62, 255–270. [Google Scholar] [PubMed]
- Bennett, S.A.; Tanaz, R.; Cobos, S.N.; Torrente, M.P. Epigenetics in amyotrophic lateral sclerosis: A role for histone post-translational modifications in neurodegenerative disease. Transl. Res. 2019, 204, 19–30. [Google Scholar] [CrossRef]
- Consales, C.; Merla, C.; Marino, C.; Benassi, B. The Epigenetic Component of the Brain Response to Electromagnetic Stimulation in Parkinson’s Disease Patients: A Literature Overview. Bioelectromagnetics 2018, 39, 3–14. [Google Scholar] [CrossRef]
- Phillipson, O.T. Alpha-Synuclein, Epigenetics, Mitochondria, Metabolism, Calcium Traffic, & Circadian Dysfunction in Parkinson’s Disease. An Integrated Strategy for Management. Ageing Res. Rev. 2017, 40, 149–167. [Google Scholar]
- Irwin, M.H.; Moos, W.H.; Faller, D.V.; Steliou, K.; Pinkert, C.A. Epigenetic Treatment of Neurodegenerative Disorders: Alzheimer and Parkinson Diseases. Drug Dev. Res. 2016, 77, 109–123. [Google Scholar] [CrossRef]
- Phang, J.M.; Liu, W.; Hancock, C.N.; Fischer, J.W. Proline Metabolism and Cancer: Emerging Links to Glutamine and Collagen. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Parlato, R.; Liss, B. How Parkinson’s Disease Meets Nucleolar Stress. Biochim. Biophys. Acta 2014, 1842, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Valente, A.X.; Das Neves, R.P.; Oliveira, P.J. Epigenetic engineering to reverse the Parkinson’s expression state. Parkinsonism Relat. Disord. 2012, 18, 717–721. [Google Scholar] [CrossRef]
- Hurley, M.J.; Dexter, D.T. Voltage-Gated Calcium Channels and Parkinson’s Disease. Pharmacol. Ther. 2012, 133, 324–333. [Google Scholar] [CrossRef]
- Diederich, N.J.; Parent, A. Parkinson’s Disease: Acquired Frailty of Archaic Neural Networks? J. Neurol. Sci. 2012, 314, 143–151. [Google Scholar] [CrossRef]
- Mendelsohn, A.R.; Larrick, J.W. Rapamycin As an Antiaging Therapeutic?: Targeting Mammalian Target of Rapamycin to Treat Hutchinson–Gilford Progeria and Neurodegenerative Diseases. Rejuvenation Res. 2011, 14, 437–441. [Google Scholar] [CrossRef]
- Dos Santos, S.M.; Romeiro, C.F.R.; Rodrigues, C.A.; Cerqueira, A.R.L.; Monteiro, M.C. Mitochondrial Dysfunction and Alpha-Lipoic Acid: Beneficial or Harmful in Alzheimer’s Disease? Oxidative Med. Cell. Longev. 2019, 2019, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ellison, E.M.; Bradley-Whitman, M.A.; Lovell, M.A. Single-Base Resolution Mapping of 5-Hydroxymethylcytosine Modifications in Hippocampus of Alzheimer’s Disease Subjects. J. Mol. Neurosci. 2017, 63, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Vachharajani, V.T.; Liu, T.; Wang, X.; Hoth, J.J.; Yoza, B.K.; McCall, C.E. Sirtuins Link Inflammation and Metabolism. J. Immunol. Res. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kamps, J.J.A.G.; Huang, J.; Poater, J.; Xu, C.; Pieters, B.J.G.E.; Dong, A.; Jasmin, M.; Sherman, W.; Beuming, T.; Bickelhaupt, F.M.; et al. Chemical basis for the recognition of trimethyllysine by epigenetic reader proteins. Nat. Commun. 2015, 6, 8911. [Google Scholar] [CrossRef]
- Salminen, A.; Haapasalo, A.; Kauppinen, A.; Kaarniranta, K.; Soininen, H.; Hiltunen, M. Impaired Mitochondrial Energy Metabolism in Alzheimer’s Disease: Impact on Pathogenesis via Disturbed Epigenetic Regulation of Chromatin Landscape. Prog. Neurobiol. 2015, 131, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Daulatzai, M.A. “Boomerang Neuropathology“ of Late-Onset Alzheimer’s Disease Is Shrouded in Harmful “BDDS“: Breathing, Diet, Drinking, and Sleep during Aging. Neurotox. Res. 2015, 28, 55–93. [Google Scholar] [CrossRef] [PubMed]
- Brewer, G.J. Epigenetic oxidative redox shift (EORS) theory of aging unifies the free radical and insulin signaling theories. Exp. Gerontol. 2010, 45, 173–179. [Google Scholar] [CrossRef]
- Hata, Y.; Ma, N.; Yoneda, M.; Morimoto, S.; Okano, H.; Murayama, S.; Kawanishi, S.; Kuzuhara, S.; Kokubo, Y. Nitrative Stress and Tau Accumulation in Amyotrophic Lateral Sclerosis/Parkinsonism-Dementia Complex (Als/Pdc) in the Kii Penin-sula, Japan. Front. Neurosci. 2017, 11, 751. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Mackie, K. Interplay of cannabinoid 2 (CB2) receptors with nitric oxide synthases, oxidative and nitrative stress, and cell death during remote neurodegeneration. J. Mol. Med. 2012, 90, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Nardo, G.; Pozzi, S.; Pignataro, M.; Lauranzano, E.; Spano, G.; Garbelli, S.; Mantovani, S.; Marinou, K.; Papetti, L.; Monteforte, M.; et al. Amyotrophic Lateral Sclerosis Multiprotein Biomarkers in Peripheral Blood Mononuclear Cells. PLoS ONE 2011, 6, e25545. [Google Scholar] [CrossRef]
- Chen, K.; Northington, F.J.; Martin, L.J. Inducible nitric oxide synthase is present in motor neuron mitochondria and Schwann cells and contributes to disease mechanisms in ALS mice. Anat. Embryol. 2010, 214, 219–234. [Google Scholar] [CrossRef]
- Basso, M.; Samengo, G.; Nardo, G.; Massignan, T.; D’Alessandro, G.; Tartari, S.; Cantoni, L.; Marino, M.; Cheroni, C.; De Biasi, S.; et al. Characterization of Detergent-Insoluble Proteins in ALS Indicates a Causal Link between Nitrative Stress and Aggregation in Pathogenesis. PLoS ONE 2009, 4, e8130. [Google Scholar] [CrossRef]
- Martin, L.J.; Gertz, B.; Pan, Y.; Price, A.C.; Molkentin, J.D.; Chang, Q. The mitochondrial permeability transition pore in motor neurons: Involvement in the pathobiology of ALS mice. Exp. Neurol. 2009, 218, 333–346. [Google Scholar] [CrossRef]
- Martin, L.J. Transgenic Mice with Human Mutant Genes Causing Parkinson’s Disease and Amyotrophic Lateral Sclerosis Provide Common Insight into Mechanisms of Motor Neuron Selective Vulnerability to Degeneration. Rev. Neurosci. 2007, 18, 115–136. [Google Scholar] [CrossRef]
- Kochman, A.; Kośka, C.; Metodiewa, D. Submolecular adventures of brain tyrosine: What are we searching for now? Amino Acids 2002, 23, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Li, H.; Gao, Z. Copper Binding Induces Nitration of NPY under Nitrative Stress: Complicating the Role of NPY in Alzheimer’s Disease. Chem. Res. Toxicol. 2018, 31, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Majkutewicz, I.; Kurowska, E.; Podlacha, M.; Myślińska, D.; Grembecka, B.; Ruciński, J.; Pierzynowska, K.; Wrona, D. Age-dependent effects of dimethyl fumarate on cognitive and neuropathological features in the streptozotocin-induced rat model of Alzheimer’s disease. Brain Res. 2018, 1686, 19–33. [Google Scholar] [CrossRef]
- Guivernau, B.; Bonet, J.; Valls-Comamala, V.; Bosch-Morato, M.; Godoy, J.A.; Inestrosa, N.C.; Peralvarez-Marin, A.; Fernan-dez-Busquets, X.; Andreu, D.; Oliva, B.; et al. Amyloid-Beta Peptide Nitrotyrosination Stabilizes Oligomers and En-hances Nmdar-Mediated Toxicity. J. Neurosci. 2016, 36, 11693–11703. [Google Scholar] [CrossRef]
- Lu, N.; Li, J.; Gao, Z. Key Roles of Tyr 10 in Cu Bound Abeta Complexes and Its Relevance to Alzheimer’s Disease. Arch. Biochem. Biophys. 2015, 584, 1–9. [Google Scholar] [CrossRef]
- Lu, N.; Li, J.; Tian, R.; Peng, Y.Y. Key Roles of Arg(5), Tyr(10) and His Residues in Abeta-Heme Peroxidase: Relevance to Alzheimer’s Disease. Biochem. Biophys. Res. Commun. 2014, 452, 676–681. [Google Scholar] [CrossRef]
- Weinreb, O.; Amit, T.; Bar-Am, O.; Youdim, M.B. Ladostigil: A Novel Multimodal Neuroprotective Drug with Cholines-terase and Brain-Selective Monoamine Oxidase Inhibitory Activities for Alzheimer’s Disease Treatment. Curr. Drug. Targets 2012, 13, 483–494. [Google Scholar] [CrossRef]
- Giridharan, V.V.; Thandavarayan, R.A.; Konishi, T. Effect of Shengmai-san on Cognitive Performance and Cerebral Oxidative Damage in BALB/c Mice. J. Med. Food 2011, 14, 601–609. [Google Scholar] [CrossRef]
- Quinn, J.F.; Bussiere, J.R.; Hammond, R.S.; Montine, T.J.; Henson, E.; Jones, R.E.; Stackman, R.W., Jr. Chronic Dietary Alpha-Lipoic Acid Reduces Deficits in Hippocampal Memory of Aged Tg2576 Mice. Neurobiol. Aging 2007, 28, 213–225. [Google Scholar] [CrossRef]
- Calabrese, V.; Sultana, R.; Scapagnini, G.; Guagliano, E.; Sapienza, M.; Bella, R.; Kanski, J.; Pennisi, G.; Mancuso, C.; Stella, A.M.; et al. Nitrosative Stress, Cellular Stress Response, and Thiol Homeostasis in Patients with Alzheimer’s Disease. Antioxid. Redox Signal. 2006, 8, 1975–1986. [Google Scholar] [CrossRef] [PubMed]
- Giasson, B.I.; Ischiropoulos, H.; Lee, V.M.; Trojanowski, J.Q. The Relationship between Oxidative/Nitrative Stress and Pathological Inclusions in Alzheimer’s and Parkinson’s Diseases. Free Radic. Biol. Med. 2002, 32, 1264–1275. [Google Scholar] [CrossRef]
- Williamson, K.S.; Gabbita, S.P.; Mou, S.; West, M.; Pye, Q.N.; Markesbery, W.R.; Cooney, R.V.; Grammas, P.; Reimann-Philipp, U.; Floyd, R.A.; et al. The Nitration Product 5-Nitro-Gamma-Tocopherol Is Increased in the Alzheimer Brain. Nitric Oxide 2002, 6, 221–227. [Google Scholar] [CrossRef]
- Giasson, B.I.; Duda, J.E.; Murray, I.V.; Chen, Q.; Souza, J.M.; Hurtig, H.I.; Ischiropoulos, H.; Trojanowski, J.Q.; Lee, V.M. Oxidative Damage Linked to Neurodegeneration by Selective Alpha-Synuclein Nitration in Synucleinopathy Lesions. Science 2000, 290, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wen, X.; Jiang, H.; Wang, J.; Song, N.; Xie, J. Interactions between Iron and Alpha-Synuclein Pathology in Parkinson’s Disease. Free Radic. Biol. Med. 2019, 141, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Maki, R.A.; Holzer, M.; Motamedchaboki, K.; Malle, E.; Masliah, E.; Marsche, G.; Reynolds, G.F. Human Myeloperoxidase (Hmpo) Is Expressed in Neurons in the Substantia Nigra in Parkinson’s Disease and in the Hmpo-Alpha-Synuclein-A53t Mouse Model, Correlating with Increased Nitration and Aggregation of Alpha-Synuclein and Exacerbation of Motor Impairment. Free Radic. Biol. Med. 2019, 141, 115–140. [Google Scholar]
- He, Y.; Yu, Z.; Chen, S. Alpha-Synuclein Nitration and Its Implications in Parkinson’s Disease. ACS Chem. Neurosci. 2019, 10, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.Q.; Wang, Y.L.; Yuan, B.S.; Yuan, X.; Hou, X.O.; Bian, Y.S.; Liu, C.F.; Hu, L.F. Impaired Cbs-H2s Signaling Axis Contributes to Mptp-Induced Neurodegeneration in a Mouse Model of Parkinson’s Disease. Brain Behav. Immun. 2018, 67, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Collier, T.J.; Kanaan, N.M.; Kordower, J.H. Aging and Parkinson’s Disease: Different Sides of the Same Coin? Mov. Disord. 2017, 32, 983–990. [Google Scholar] [CrossRef]
- Kleinknecht, A.; Popova, B.; Lazaro, D.F.; Pinho, R.; Valerius, O.; Outeiro, T.F.; Braus, G.H. C-Terminal Tyrosine Residue Modifications Modulate the Protective Phosphorylation of Serine 129 of Alpha-Synuclein in a Yeast Model of Parkinson’s Disease. PLoS Genet. 2016, 12, e1006098. [Google Scholar] [CrossRef]
- Dias, V.; Junn, E.; Mouradian, M.M. The Role of Oxidative Stress in Parkinson’s Disease. J. Parkinson’s Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef]
- Schildknecht, S.; Gerding, H.R.; Karreman, C.; Drescher, M.; Lashuel, H.A.; Outeiro, T.F.; Di Monte, D.A.; Leist, M. Oxidative and Nitrative Alpha-Synuclein Modifications and Proteostatic Stress: Implications for Disease Mechanisms and Interventions in Synucleinopathies. J. Neurochem. 2013, 125, 491–511. [Google Scholar] [CrossRef]
- Lee, J.; Giordano, S.; Zhang, J. Autophagy, mitochondria and oxidative stress: Cross-talk and redox signalling. Biochem. J. 2011, 441, 523–540. [Google Scholar] [CrossRef] [PubMed]
- Sian-Hulsmann, J.; Mandel, S.; Youdim, M.B.; Riederer, P. The Relevance of Iron in the Pathogenesis of Parkinson’s Disease. J. Neurochem. 2011, 118, 939–957. [Google Scholar] [CrossRef]
- Kupershmidt, L.; Okun, Z.; Amit, T.; Mandel, S.; Saltsman, I.; Mahammed, A.; Bar-Am, O.; Gross, Z.; Youdim, M.B. Metal-locorroles as Cytoprotective Agents against Oxidative and Nitrative Stress in Cellular Models of Neurodegeneration. J. Neurochem. 2010, 113, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Danielson, S.R.; Andersen, J.K. Oxidative and Nitrative Protein Modifications in Parkinson’s Disease. Free Radic. Biol. Med. 2008, 44, 1787–1794. [Google Scholar] [CrossRef]
- Trostchansky, A.; Rubbo, H. Lipid nitration and formation of lipid-protein adducts: Biological insights. Amino Acids 2006, 32, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, M.; Luques, L.; Bejar, C.; Shoham, S. Ladostigil, a novel multifunctional drug for the treatment of dementia co-morbid with depression. Focus Extrapyramidal Dysfunct. 2006, 70, 443–446. [Google Scholar] [CrossRef]
- Jenner, P.; Olanow, C.W. The Pathogenesis of Cell Death in Parkinson’s Disease. Neurology 2006, 66 (Suppl. 4), S24–S36. [Google Scholar] [CrossRef]
- Norris, E.H.; Giasson, B.I. Role of Oxidative Damage in Protein Aggregation Associated with Parkinson’s Disease and Related Disorders. Antioxid. Redox Signal. 2005, 7, 672–684. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A. Postmortem Studies in Parkinson’s Disease. Dialogues Clin. Neurosci. 2004, 6, 281–293. [Google Scholar] [PubMed]
- Yamin, G.; Uversky, V.N.; Fink, A.L. Nitration Inhibits Fibrillation of Human Alpha-Synuclein in Vitro by Formation of Soluble Oligomers. FEBS Lett. 2003, 542, 147–152. [Google Scholar] [CrossRef]
- Bozzo, F.; Mirra, A.; Carri, M.T. Oxidative Stress and Mitochondrial Damage in the Pathogenesis of Als: New Perspectives. Neurosci. Lett. 2017, 636, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Bai, Z.; Qin, X.; Cheng, Y. Aberrations in Oxidative Stress Markers in Amyotrophic Lateral Sclerosis: A Systematic Review and Meta-Analysis. Oxid. Med. Cell. Longev. 2019, 2019, 1712323. [Google Scholar] [CrossRef]
- Butterfield, D.A. Amyloid Beta-Peptide (1-42)-Induced Oxidative Stress and Neurotoxicity: Implications for Neurodegener-ation in Alzheimer’s Disease Brain. A Review. Free Radic. Res. 2002, 36, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Castegna, A.; Drake, J.; Scapagnini, G.; Calabrese, V. Vitamin E and Neurodegenerative Disorders Associated with Oxidative Stress. Nutr. Neurosci. 2002, 5, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Griffin, S.; Munch, G.; Pasinetti, G.M. Amyloid Beta-Peptide and Amyloid Pathology Are Central to the Oxidative Stress and Inflammatory Cascades under Which Alzheimer’s Disease Brain Exists. J. Alzheimer’s Dis. 2002, 4, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Kanski, J. Methionine Residue 35 Is Critical for the Oxidative Stress and Neurotoxic Properties of Alzheimer’s Amyloid Beta-Peptide 1-42. Peptides 2002, 23, 1299–1309. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Lauderback, C.M. Lipid Peroxidation and Protein Oxidation in Alzheimer’s Disease Brain: Potential Causes and Consequences Involving Amyloid Beta-Peptide-Associated Free Radical Oxidative Stress. Free Radic. Biol. Med. 2002, 32, 1050–1060. [Google Scholar] [CrossRef]
- Chang, Y.-T.; Chang, W.-N.; Tsai, N.-W.; Huang, C.-C.; Kung, C.-T.; Su, Y.-J.; Lin, W.-C.; Cheng, B.-C.; Su, C.-M.; Chiang, Y.-F.; et al. The Roles of Biomarkers of Oxidative Stress and Antioxidant in Alzheimer’s Disease: A Systematic Review. BioMed Res. Int. 2014, 2014, 1–14. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Boil. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, C. Oxidative Stress in Alzheimer’s Disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef]
- Drake, J.; Kanski, J.; Varadarajan, S.; Tsoras, M.; Butterfield, D.A. Elevation of Brain Glutathione by Gamma-Glutamylcysteine Ethyl Ester Protects against Peroxynitrite-Induced Oxidative Stress. J. Neurosci. Res. 2002, 68, 776–784. [Google Scholar] [CrossRef]
- Kanski, J.; Aksenova, M.; Schoneich, C.; Butterfield, D.A. Substitution of Isoleucine-31 by Helical-Breaking Proline Abol-ishes Oxidative Stress and Neurotoxic Properties of Alzheimer’s Amyloid Beta-Peptide. Free Radic. Biol. Med. 2002, 32, 1205–1211. [Google Scholar] [CrossRef]
- Kanski, J.; Varadarajan, S.; Aksenova, M.; Butterfield, D.A. Role of Glycine-33 and Methionine-35 in Alzheimer’s Amyloid Beta-Peptide 1-42-Associated Oxidative Stress and Neurotoxicity. Biochim. Biophys. Acta 2002, 1586, 190–198. [Google Scholar] [CrossRef]
- LaFontaine, M.A.; Mattson, M.P.; Butterfield, D.A. Oxidative Stress in Synaptosomal Proteins from Mutant Presenilin-1 Knock-in Mice: Implications for Familial Alzheimer’s Disease. Neurochem. Res. 2002, 27, 417–421. [Google Scholar] [CrossRef]
- Tönnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.G.; Zhu, X. Oxidative Stress and Mitochondrial Dysfunction in Alzheimer’s Disease. Biochim. Biophys. Acta 2014, 1842, 1240–1247. [Google Scholar] [CrossRef]
- Guo, J.D.; Zhao, X.; Li, Y.; Li, G.R.; Liu, X.L. Damage to Dopaminergic Neurons by Oxidative Stress in Parkinson’s Disease (Review). Int. J. Mol. Med. 2018, 41, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T. Oxidative Stress and Mitochondrial Dysfunction-Linked Neurodegenerative Disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Ali, S.A. Oxidative Stress-Related Biomarkers in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Iran. J. Neurol. 2018, 17, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.; Wong, Y.C.; Ysselstein, D.; Severino, A.; Krainc, D. Synaptic, Mitochondrial, and Lysosomal Dysfunction in Parkinson’s Disease. Trends Neurosci. 2019, 42, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson Disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Raza, C.; Anjum, R.; Shakeel, N.U.A. Parkinson’s Disease: Mechanisms, Translational Models and Management Strate-gies. Life Sci. 2019, 226, 77–90. [Google Scholar] [CrossRef]
- Trist, B.G.; Hare, D.J.; Double, K.L. Oxidative Stress in the Aging Substantia Nigra and the Etiology of Parkinson’s Disease. Aging Cell 2019, 18, e13031. [Google Scholar] [CrossRef]
- Wei, Z.; Li, X.; Li, X.; Liu, Q.; Cheng, Y. Oxidative Stress in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front. Mol. Neurosci. 2018, 11, 236. [Google Scholar] [CrossRef]
- Santibanez-Koref, M.; Griffin, H.; Turnbull, D.M.; Chinnery, P.F.; Herbert, M.; Hudson, F. Assessing Mitochondrial Heteroplasmy Using Next Generation Sequencing: A Note of Caution. Mitochondrion 2019, 46, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Craven, L.; Murphy, J.; Turnbull, D.M.; Taylor, R.W.; Gorman, G.S.; McFarland, R. Scientific and Ethical Issues in Mitochondrial Donation. New Bioeth. 2018, 24, 57–73. [Google Scholar] [CrossRef]
- Chinnery, P.F.; Craven, L.; Mitalipov, S.; Stewart, J.B.; Herbert, M.; Turnbull, D.M. The challenges of mitochondrial replacement. PLoS Genet. 2014, 10, e1004315. [Google Scholar] [CrossRef]
- Craven, L.; Murphy, J.L.; Turnbull, D.M. Mitochondrial donation—Hope for families with mitochondrial DNA disease. Emerg. Top. Life Sci. 2020, 4, 151–154. [Google Scholar] [CrossRef]
- Hyslop, L.A.; Blakeley, P.; Craven, L.; Richardson, J.; Fogarty, N.M.E.; Fragouli, E.; Lamb, M.; Wamaitha, S.E.; Prathalingam, N.; Zhang, Q.; et al. Towards clinical application of pronuclear transfer to prevent mitochondrial DNA disease. Nature 2016, 534, 383–386. [Google Scholar] [CrossRef]
- Pickett, S.J.; Blain, A.; Ng, Y.S.; Wilson, I.J.; Taylor, R.W.; McFarland, R.; Turnbull, D.M.; Gorman, G.S. Mitochondrial Donation—Which Women Could Benefit? New Engl. J. Med. 2019, 380, 1971–1972. [Google Scholar] [CrossRef]
- Richardson, J.; Irving, L.; Hyslop, L.A.; Choudhary, M.; Murdoch, A.; Turnbull, D.M.; Herbert, M. Concise Reviews: Assisted Reproductive Technologies to Prevent Transmission of Mitochondrial DNA Disease. Stem Cells 2015, 33, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Amato, P.; Tachibana, M.; Sparman, M.; Mitalipov, S. Three-Parent in Vitro Fertilization: Gene Replacement for the Prevention of Inherited Mitochondrial Diseases. Fertil. Steril. 2014, 101, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Byrne, J.A.; Pedersen, D.A.; Clepper, L.L.; Nelson, M.; Sanger, W.G.; Gokhale, S.; Wolf, D.P.; Mitalipov, S.M. Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature 2007, 450, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Folmes, C.D.L.; Ma, H.; Mitalipov, S.; Terzic, A. Mitochondria in pluripotent stem cells: Stemness regulators and disease targets. Curr. Opin. Genet. Dev. 2016, 38, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.; Wu, J.; Gutierrez, N.M.; Koski, A.; Tippner-Hedges, R.; Agaronyan, K.; Platero-Luengo, A.; Martinez-Redondo, P.; Ma, H.; Lee, Y.; et al. Mitochondrial Re-placement in Human Oocytes Carrying Pathogenic Mitochondrial DNA Mutations. Nature 2016, 540, 270–275. [Google Scholar] [CrossRef]
- Ma, H.; Folmes, C.D.L.; Wu, J.; Morey, R.; Mora-Castilla, S.; Ocampo, A.; Ma, L.; Poulton, J.; Wang, X.; Ahmed, R.; et al. Metabolic rescue in pluripotent cells from patients with mtDNA disease. Nat. Cell Biol. 2015, 524, 234–238. [Google Scholar] [CrossRef]
- Ma, H.; Marti Gutierrez, N.; Morey, R.; Van Dyken, C.; Kang, E.; Hayama, T.; Lee, Y.; Li, Y.; Tippner-Hedges, R.; Wolf, D.P.; et al. Incompatibility between Nuclear and Mitochondrial Genomes Contributes to an Interspecies Re-productive Barrier. Cell Metab. 2016, 24, 283–294. [Google Scholar] [CrossRef]
- Ma, H.; O’Neil, R.C.; Gutierrez, N.M.; Hariharan, M.; Zhang, Z.Z.; He, Y.; Cinnioglu, C.; Kayali, R.; Kang, E.; Lee, Y.; et al. Functional Human Oocytes Generated by Transfer of Polar Body Genomes. Cell Stem Cell 2017, 20, 112–119. [Google Scholar] [CrossRef]
- Mitalipov, S.; Wolf, D.P. Clinical and Ethical Implications of Mitochondrial Gene Transfer. Trends Endocrinol. Metab. 2014, 25, 5–7. [Google Scholar] [CrossRef][Green Version]
- Tachibana, M.; Amato, P.; Sparman, M.; Gutierrez, N.M.; Tippner-Hedges, R.; Ma, H.; Kang, E.; Fulati, A.; Lee, H.-S.; Sritanaudomchai, H.; et al. Human Embryonic Stem Cells Derived by Somatic Cell Nuclear Transfer. Cell 2013, 153, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, M.; Amato, P.; Sparman, M.; Woodward, J.; Sanchis, D.M.; Ma, H.; Gutierrez, N.M.; Tippner-Hedges, R.; Kang, E.; Lee, H.-S.; et al. Towards germline gene therapy of inherited mitochondrial diseases. Nat. Cell Biol. 2013, 493, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, M.; Sparman, M.; Mitalipov, S. Chromosome transfer in mature oocytes. Fertil. Steril. 2012, 97, e16. [Google Scholar] [CrossRef]
- Tachibana, M.; Sparman, M.; Sritanaudomchai, H.; Ma, H.; Clepper, L.; Woodward, J.; Li, Y.; Ramsey, C.; Kolotushkina, O.; Mitalipov, S. Mitochondrial gene replacement in primate offspring and embryonic stem cells. Nature 2009, 461, 367–372. [Google Scholar] [CrossRef]
- Wolf, D.P.; Mitalipov, N.; Mitalipov, S. Mitochondrial replacement therapy in reproductive medicine. Trends Mol. Med. 2015, 21, 68–76. [Google Scholar] [CrossRef]
- Wolf, D.P.; Mitalipov, S. Mitochondrial Replacement Therapies Can Circumvent Mtdna-Based Disease Transmission. Cell Metab. 2014, 20, 6–8. [Google Scholar] [CrossRef][Green Version]
- Zhao, M.T.; Chen, H.; Liu, Q.; Shao, N.Y.; Sayed, N.; Wo, H.T.; Zhang, J.Z.; Ong, S.G.; Liu, C.; Kim, Y.; et al. Molecular and Functional Resemblance of Differentiated Cells Derived from Isogenic Human Ipscs and Scnt-Derived Escs. Proc. Natl. Acad. Sci. USA 2017, 114, E11111–E11120. [Google Scholar] [CrossRef] [PubMed]
- Elson, J.L.; Swalwell, H.; Blakely, E.L.; McFarland, R.; Taylor, R.W.; Turnbull, D.M. Pathogenic mitochondrial tRNA mutations—Which mutations are inherited and why? Hum. Mutat. 2009, 30, E984–E992. [Google Scholar] [CrossRef] [PubMed]
- Yarham, J.W.; Elson, J.L.; Blakely, E.L.; McFarland, R.; Taylor, R.W. Mitochondrial tRNA mutations and disease. Wiley Interdiscip. Rev. RNA 2010, 1, 304–324. [Google Scholar] [CrossRef]
- Ribas de Pouplana, L. The Mitochondrial Trna Conundrum. Nat. Rev. Mol. Cell Biol. 2020, 21, 361. [Google Scholar] [CrossRef]
- Richter, U.; Evans, M.E.; Clark, W.C.; Marttinen, P.; Shoubridge, E.A.; Suomalainen, A.; Wredenberg, A.; Wedell, A.; Pan, T.; Battersby, B.J. Rna Modification Landscape of the Human Mitochondrial Trna(Lys) Regulates Protein Synthesis. Nat. Commun. 2018, 9, 3966. [Google Scholar] [CrossRef]
- Karicheva, O.Z.; Kolesnikova, O.A.; Schirtz, T.; Vysokikh, M.Y.; Mager-Heckel, A.-M.; Lombès, A.; Boucheham, A.; Krasheninnikov, I.A.; Martin, R.P.; Entelis, N.; et al. Correction of the consequences of mitochondrial 3243A>G mutation in the MT-TL1 gene causing the MELAS syndrome by tRNA import into mitochondria. Nucleic Acids Res. 2011, 39, 8173–8186. [Google Scholar] [CrossRef]
- Wang, G.; Shimada, E.; Zhang, J.; Hong, J.S.; Smith, G.M.; Teitell, M.A.; Koehler, C.M. Correcting human mitochondrial mutations with targeted RNA import. Proc. Natl. Acad. Sci. USA 2012, 109, 4840–4845. [Google Scholar] [CrossRef]
- Alfadhel, M.; Nashabat, M.; Abu Ali, Q.; Hundallah, K. Mitochondrial iron-sulfur cluster biogenesis from molecular understanding to clinical disease. Neuroscience 2017, 22, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Artika, I.M. Current understanding of structure, function and biogenesis of yeast mitochondrial ATP synthase. J. Bioenerg. Biomembr. 2019, 51, 315–328. [Google Scholar] [CrossRef]
- Babbitt, S.E.; Sutherland, M.C.; San Francisco, B.; Mendez, D.L.; Kranz, R.G. Mitochondrial cytochrome c biogenesis: No longer an enigma. Trends Biochem. Sci. 2015, 40, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.H.; McStay, G.P. Modular biogenesis of mitochondrial respiratory complexes. Mitochondrion 2020, 50, 94–114. [Google Scholar] [CrossRef] [PubMed]
- Bishop, D.J.; Botella, J.; Genders, A.J.; Lee, M.J.-C.; Saner, N.J.; Kuang, J.; Yan, X.; Granata, C. High-Intensity Exercise and Mitochondrial Biogenesis: Current Controversies and Future Research Directions. Physiology 2019, 34, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Bouchez, C.; Devin, A. Mitochondrial Biogenesis and Mitochondrial Reactive Oxygen Species (Ros): A Complex Rela-tionship Regulated by the Camp/Pka Signaling Pathway. Cells 2019, 8, 287. [Google Scholar] [CrossRef] [PubMed]
- Bragoszewski, P.; Turek, M.; Chacinska, A. Control of mitochondrial biogenesis and function by the ubiquitin–proteasome system. Open Biol. 2017, 7. [Google Scholar] [CrossRef]
- Bykov, Y.S.; Rapaport, D.; Herrmann, J.M.; Schuldiner, M. Cytosolic Events in the Biogenesis of Mitochondrial Proteins. Trends Biochem. Sci. 2020, 45, 650–667. [Google Scholar] [CrossRef]
- Cameron, R.B.; Beeson, C.C.; Schnellmann, R.G. Development of Therapeutics that Induce Mitochondrial Biogenesis for the Treatment of Acute and Chronic Degenerative Diseases. J. Med. Chem. 2016, 59, 10411–10434. [Google Scholar] [CrossRef]
- Carelli, V.; Maresca, A.; Caporali, L.; Trifunov, S.; Zanna, C.; Rugolo, M. Mitochondria: Biogenesis and mitophagy balance in segregation and clonal expansion of mitochondrial DNA mutations. Int. J. Biochem. Cell Biol. 2015, 63, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Cherry, A.D.; Piantadosi, C.A. Regulation of Mitochondrial Biogenesis and Its Intersection with Inflammatory Responses. Antioxid. Redox Signal. 2015, 22, 965–976. [Google Scholar] [CrossRef]
- Craig, D.M.; Ashcroft, S.P.; Belew, M.Y.; Stocks, B.; Currell, K.; Baar, K.; Philp, A. Utilizing small nutrient compounds as enhancers of exercise-induced mitochondrial biogenesis. Front. Physiol. 2015, 6, 296. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; De Stefani, D.; De Vivo, I.; Scapagnini, G. Polyphenols as Caloric Restriction Mimetics Regulating Mitochondrial Biogenesis and Mitophagy. Trends Endocrinol. Metab. 2020, 31, 536–550. [Google Scholar] [CrossRef]
- De Oliveira, M.R.; Jardim, F.R.; Setzer, W.N.; Nabavi, S.M. Curcumin, mitochondrial biogenesis, and mitophagy: Exploring recent data and indicating future needs. Biotechnol. Adv. 2016, 34, 813–826. [Google Scholar] [CrossRef]
- De Oliveira, M.R.; Nabavi, S.F.; Manayi, A.; Daglia, M.; Hajheydari, Z. Resveratrol and the mitochondria: From triggering the intrinsic apoptotic pathway to inducing mitochondrial biogenesis, a mechanistic view. Biochim. Biophys. Acta (BBA) Gen. Subj. 2016, 1860, 727–745. [Google Scholar] [CrossRef]
- Doan, K.N.; Ellenrieder, L.; Becker, T. Mitochondrial porin links protein biogenesis to metabolism. Curr. Genet. 2019, 65, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Dorn, G.W., 2nd; Vega, R.B.; Kelly, D.P. Mitochondrial Biogenesis and Dynamics in the Developing and Diseased Heart. Genes Dev. 2015, 29, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
- Drwesh, L.; Rapaport, D. Biogenesis Pathways of Alpha-Helical Mitochondrial Outer Membrane Proteins. Biol. Chem. 2020, 401, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Dutkiewicz, R.; Nowak, M. Molecular chaperones involved in mitochondrial iron–sulfur protein biogenesis. JBIC J. Biol. Inorg. Chem. 2018, 23, 569–579. [Google Scholar] [CrossRef]
- Edens, B.M.; Miller, N.; Ma, Y.-C. Impaired Autophagy and Defective Mitochondrial Function: Converging Paths on the Road to Motor Neuron Degeneration. Front. Cell. Neurosci. 2016, 10, 44. [Google Scholar] [CrossRef]
- Edwards, R.; Gerlich, S.; Tokatlidis, K. The biogenesis of mitochondrial intermembrane space proteins. Biol. Chem. 2020, 401, 737–747. [Google Scholar] [CrossRef]
- Ellenrieder, L.; Martensson, C.U.; Becker, T. Biogenesis of Mitochondrial Outer Membrane Proteins, Problems and Diseases. Biol. Chem. 2015, 396, 1199–1213. [Google Scholar] [CrossRef]
- Erlich, A.T.; Tryon, L.D.; Crilly, M.J.; Memme, J.M.; Moosavi, Z.S.M.; Oliveira, A.N.; Beyfuss, K.; Hood, D.A. Function of specialized regulatory proteins and signaling pathways in exercise-induced muscle mitochondrial biogenesis. Integr. Med. Res. 2016, 5, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J.; Scorza, F.A. Effects of antiepileptic drugs on mitochondrial functions, morphology, kinetics, biogenesis, and survival. Epilepsy Res. 2017, 136, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Fontecha-Barriuso, M.; Martin-Sanchez, D.; Martinez-Moreno, J.M.; Monsalve, M.; Ramos, A.M.; Sanchez-Niño, M.D.; Ruiz-Ortega, M.; Ortiz, A.; Sanz, A.B. The Role of PGC-1α and Mitochondrial Biogenesis in Kidney Diseases. Biomolecules 2020, 10, 347. [Google Scholar] [CrossRef] [PubMed]
- Franz, A.; Kevei, É.; Hoppe, T. Double-edged alliance: Mitochondrial surveillance by the UPS and autophagy. Curr. Opin. Cell Biol. 2015, 37, 18–27. [Google Scholar] [CrossRef]
- Fu, W.; Liu, Y.; Yin, H. Mitochondrial Dynamics: Biogenesis, Fission, Fusion, and Mitophagy in the Regulation of Stem Cell Behaviors. Stem Cells Int. 2019, 2019, 1–15. [Google Scholar] [CrossRef]
- Golpich, M.; Amini, E.; Mohamed, Z.; Ali, R.A.; Ibrahim, N.M.; Ahmadiani, A. Mitochondrial Dysfunction and Biogenesis in Neurodegenerative diseases: Pathogenesis and Treatment. CNS Neurosci. Ther. 2017, 23, 5–22. [Google Scholar] [CrossRef]
- Gomes, J.V.P.; Rigolon, T.C.B.; da Silveira Souza, M.S.; Alvarez-Leite, J.I.; Lucia, C.M.D.; Martino, H.S.D.; Rosa, C.D.O.B. Anti-obesity Effects of Anthocyanins on Mitochondrial Biogenesis, Inflammation, and Oxidative Stress: A Systematic Review. Nutrition 2019, 66, 192–202. [Google Scholar] [CrossRef]
- Granata, C.; Jamnick, N.A.; Bishop, D.J. Principles of Exercise Prescription, and How They Influence Exercise-Induced Changes of Transcription Factors and Other Regulators of Mitochondrial Biogenesis. Sports Med. 2018, 48, 1541–1559. [Google Scholar] [CrossRef]
- Grevel, A.; Pfanner, N.; Becker, T. Coupling of import and assembly pathways in mitochondrial protein biogenesis. Biol. Chem. 2019, 401, 117–129. [Google Scholar] [CrossRef]
- Groennebaek, T.; Vissing, K. Impact of Resistance Training on Skeletal Muscle Mitochondrial Biogenesis, Content, and Function. Front. Physiol. 2017, 8, 713. [Google Scholar] [CrossRef]
- Gupta, A.; Becker, T. Mechanisms and pathways of mitochondrial outer membrane protein biogenesis. Biochim. Biophys. Acta (BBA) Bioenerg. 2021, 1862, 148323. [Google Scholar] [CrossRef] [PubMed]
- Gureev, A.P.; Shaforostova, E.A.; Popov, V.N. Regulation of Mitochondrial Biogenesis as a Way for Active Longevity: Interaction between the Nrf2 and PGC-1α Signaling Pathways. Front. Genet. 2019, 10, 435. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Duan, Y.; Yao, K.; Li, F.; Hou, Y.; Wu, G.; Yin, Y. Beta-Hydroxy-Beta-Methylbutyrate, Mitochondrial Biogenesis, and Skeletal Muscle Health. Amino Acids 2016, 48, 653–664. [Google Scholar] [CrossRef]
- Herrmann, J.M.; Riemer, J. Apoptosis inducing factor and mitochondrial NADH dehydrogenases: Redox-controlled gear boxes to switch between mitochondrial biogenesis and cell death. Biol. Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hey-Mogensen, M.; Clausen, T.R. Targeting Mitochondrial Biogenesis and Mitochondrial Substrate Utilization to Treat Obesity and Insulin Resistance, Respectively—Two Data-Driven Hypotheses. Curr. Diabetes Rev. 2017, 13, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Hiraumi, Y.; Huang, C.; Andres, A.M.; Xiong, Y.; Ramil, J.; Gottlieb, R.A. Myogenic Progenitor Cell Differentiation Is Dependent on Modulation of Mitochondrial Biogenesis through Autophagy. In Etiology and Morphogenesis of Congenital Heart Disease: From Gene Function and Cellular Interaction to Morphology; Nakanishi, T., Markwald, R.R., Baldwin, H.S., Keller, B.B., Srivastava, D., Yamagishi, H., Eds.; Springer: Tokyo, Japan, 2016. [Google Scholar]
- Hood, D.A.; Tryon, L.D.; Carter, H.N.; Kim, Y.; Chen, C.C. Unravelling the Mechanisms Regulating Muscle Mitochondrial Biogenesis. Biochem. J. 2016, 473, 2295–2314. [Google Scholar] [CrossRef]
- Horten, P.; Colina-Tenorio, L.; Rampelt, H. Biogenesis of Mitochondrial Metabolite Carriers. Biomolecules 2020, 10, 1008. [Google Scholar] [CrossRef]
- Islam, H.; Edgett, B.A.; Gurd, B.J. Coordination of mitochondrial biogenesis by PGC-1α in human skeletal muscle: A reevaluation. Metabolism 2018, 79, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Islam, H.; Hood, D.A.; Gurd, B.J. Looking beyond PGC-1α: Emerging regulators of exercise-induced skeletal muscle mitochondrial biogenesis and their activation by dietary compounds. Appl. Physiol. Nutr. Metab. 2020, 45, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, S.; Blackburn, J.K.; Elsworth, J.D. PPARgamma/Pgc1alpha Signaling as a Potential Therapeutic Target for Mitochondrial Biogenesis in Neurodegenerative Disorders. Pharmacol. Ther. 2020, 107705. [Google Scholar] [CrossRef]
- Kandezi, N.; Mohammadi, M.; Ghaffari, M.; Gholami, M.; Motaghinejad, M.; Safari, S. Novel Insight to Neuroprotective Potential of Curcumin: A Mechanistic Review of Possible Involvement of Mitochondrial Biogenesis and PI3/Akt/ GSK3 or PI3/Akt/CREB/BDNF Signaling Pathways. Int. J. Mol. Cell. Med. 2020, 9, 1–32. [Google Scholar]
- Kiyama, T.; Chen, C.K.; Wang, S.W.; Pan, P.; Ju, Z.; Wang, J.; Takada, S.; Klein, W.H.; Mao, C.A. Essential Roles of Mitochondrial Biogenesis Regulator Nrf1 in Retinal Development and Homeostasis. Mol. Neurodegener. 2018, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Kondadi, A.K.; Anand, R.; Reichert, A.S. Functional Interplay between Cristae Biogenesis, Mitochondrial Dynamics and Mitochondrial DNA Integrity. Int. J. Mol. Sci. 2019, 20, 4311. [Google Scholar] [CrossRef]
- Krämer, L.; Groh, C.; Herrmann, J.M. The proteasome: Friend and foe of mitochondrial biogenesis. FEBS Lett. 2020. [Google Scholar] [CrossRef]
- Li, P.A.; Hou, X.; Hao, S. Mitochondrial biogenesis in neurodegeneration. J. Neurosci. Res. 2017, 95, 2025–2029. [Google Scholar] [CrossRef]
- Lill, R.; Freibert, S.-A. Mechanisms of Mitochondrial Iron-Sulfur Protein Biogenesis. Annu. Rev. Biochem. 2020, 89, 471–499. [Google Scholar] [CrossRef]
- Lima, T.I.; Araujo, H.N.; Menezes, E.S.; Sponton, C.H.; Araújo, M.B.; Bomfim, L.H.; Queiroz, A.L.; Passos, M.A.; DE Sousa, T.A.; Hirabara, S.M.; et al. Role of microRNAs on the Regulation of Mitochondrial Biogenesis and Insulin Signaling in Skeletal Muscle. J. Cell. Physiol. 2016, 232, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Widlund, H.R.; Puigserver, P. PGC-1 Coactivators: Shepherding the Mitochondrial Biogenesis of Tumors. Trends Cancer 2016, 2, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Manganas, P.; MacPherson, L.; Tokatlidis, K. Oxidative protein biogenesis and redox regulation in the mitochondrial intermembrane space. Cell Tissue Res. 2017, 367, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Mansilla, N.; Racca, S.; Gras, D.E.; Gonzalez, D.H.; Welchen, E. The Complexity of Mitochondrial Complex IV: An Update of Cytochrome c Oxidase Biogenesis in Plants. Int. J. Mol. Sci. 2018, 19, 662. [Google Scholar] [CrossRef]
- McKie, G.L.; Wright, D.C. Biochemical Adaptations in White Adipose Tissue Following Aerobic Exercise: From Mitochondrial Biogenesis to Browning. Biochem. J. 2020, 477, 1061–1081. [Google Scholar] [CrossRef] [PubMed]
- Melber, A.; Winge, D.R. Steps toward Understanding Mitochondrial Fe/S Cluster Biogenesis. Methods Enzym. 2018, 599, 265–292. [Google Scholar] [CrossRef]
- Miao, L.; Shen, X.; Whiteman, M.; Xin, H.; Shen, Y.; Xin, X.; Moore, P.K.; Zhu, Y.-Z. Hydrogen Sulfide Mitigates Myocardial Infarction via Promotion of Mitochondrial Biogenesis-Dependent M2 Polarization of Macrophages. Antioxid. Redox Signal. 2016, 25, 268–281. [Google Scholar] [CrossRef]
- Moulin, C.; Caumont-Sarcos, A.; Ieva, R. Mitochondrial Presequence Import: Multiple Regulatory Knobs Fine-Tune Mitochondrial Biogenesis and Homeostasis. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 930–944. [Google Scholar] [CrossRef]
- Ndi, M.; Marin-Buera, L.; Salvatori, R.; Singh, A.P.; Ott, M. Biogenesis of the bc1 Complex of the Mitochondrial Respiratory Chain. J. Mol. Biol. 2018, 430, 3892–3905. [Google Scholar] [CrossRef]
- Nirwane, A.; Majumdar, A. Understanding Mitochondrial Biogenesis through Energy Sensing Pathways and Its Translation in Cardio-Metabolic Health. Arch. Physiol. Biochem. 2018, 124, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Nowinski, S.M.; Van Vranken, J.G.; Dove, K.K.; Rutter, J. Impact of Mitochondrial Fatty Acid Synthesis on Mitochondrial Biogenesis. Curr. Biol. 2018, 28, R1212–R1219. [Google Scholar] [CrossRef] [PubMed]
- Ogunbona, O.B.; Claypool, S.M. Emerging Roles in the Biogenesis of Cytochrome C Oxidase for Members of the Mitochondrial Carrier Family. Front. Cell. Dev. Biol. 2019, 7, 3. [Google Scholar] [CrossRef]
- Ortega, S.P.; Chouchani, E.T.; Boudina, S. Stress turns on the heat: Regulation of mitochondrial biogenesis and UCP1 by ROS in adipocytes. Adipocyte 2016, 6, 56–61. [Google Scholar] [CrossRef]
- Panajatovic, M.; Singh, F.; Duthaler, U.; Krahenbuhl, S.; Bouitbir, J. Role of Pgc-1-Alpha-Associated Mitochondrial Biogenesis in Statin-Induced Myotoxicity. Eur. Cardiol. 2020, 15, e35. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.G.; Hawley, J.A. Molecular Basis of Exercise-Induced Skeletal Muscle Mitochondrial Biogenesis: Historical Advances, Current Knowledge, and Future Challenges. Cold Spring Harb. Perspect. Med. 2017, 8, a029686. [Google Scholar] [CrossRef]
- Pfanner, N.; Warscheid, B.; Wiedemann, N. Mitochondrial proteins: From biogenesis to functional networks. Nat. Rev. Mol. Cell Biol. 2019, 20, 267–284. [Google Scholar] [CrossRef]
- Picca, A.; Lezza, A.M. Regulation of Mitochondrial Biogenesis through Tfam-Mitochondrial DNA Interactions: Useful Insights from Aging and Calorie Restriction Studies. Mitochondrion 2015, 25, 67–75. [Google Scholar] [CrossRef]
- Ploumi, C.; Daskalaki, I.; Tavernarakis, N. Mitochondrial biogenesis and clearance: A balancing act. FEBS J. 2016, 284, 183–195. [Google Scholar] [CrossRef]
- Popov, L. Mitochondrial biogenesis: An update. J. Cell. Mol. Med. 2020, 24, 4892–4899. [Google Scholar] [CrossRef] [PubMed]
- Praharaj, P.P.; Panigrahi, D.P.; Bhol, C.S.; Patra, S.; Mishra, S.R.; Mahapatra, K.K.; Behera, B.P.; Singh, A.; Patil, S.; Bhutia, S.K. Mitochondrial rewiring through mitophagy and mitochondrial biogenesis in cancer stem cells: A potential target for anti-CSC cancer therapy. Cancer Lett. 2021, 498, 217–228. [Google Scholar] [CrossRef]
- Rainey, N.E.; Moustapha, A.; Petit, P.X. Curcumin, a Multifaceted Hormetic Agent, Mediates an Intricate Crosstalk between Mitochondrial Turnover, Autophagy, and Apoptosis. Oxidative Med. Cell. Longev. 2020, 2020, 1–23. [Google Scholar] [CrossRef]
- Scholpa, N.E.; Schnellmann, R.G. Mitochondrial-Based Therapeutics for the Treatment of Spinal Cord Injury: Mitochondrial Biogenesis as a Potential Pharmacological Target. J. Pharmacol. Exp. Ther. 2017, 363, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Sheremet, N.L.; Andreeva, N.A.; Shmel’kova, M.S.; Tsigankova, P.G. Mitochondrial biogenesis in hereditary optic neuropathies. Вестник Офтальмологии 2019, 135, 85–91. [Google Scholar] [CrossRef]
- Simmons, E.C.; Scholpa, N.E.; Schnellmann, R.G. Mitochondrial biogenesis as a therapeutic target for traumatic and neurodegenerative CNS diseases. Exp. Neurol. 2020, 329, 113309. [Google Scholar] [CrossRef]
- Skuratovskaia, D.; Komar, A.; Vulf, M.; Litvinova, L. Mitochondrial destiny in type 2 diabetes: The effects of oxidative stress on the dynamics and biogenesis of mitochondria. PeerJ 2020, 8, e9741. [Google Scholar] [CrossRef] [PubMed]
- Smiles, W.J.; Camera, D.M. The Guardian of the Genome P53 Regulates Exercise-Induced Mitochondrial Plasticity Beyond Organelle Biogenesis. Acta Physiol. 2018, 222, e13004. [Google Scholar] [CrossRef]
- Tamura, Y.; Endo, T. Role of Intra- and Inter-mitochondrial Membrane Contact Sites in Yeast Phospholipid Biogenesis. Single Mol. Single Cell Seq. 2017, 997, 121–133. [Google Scholar] [CrossRef]
- Tang, J.X.; Thompson, K.; Taylor, R.W.; Olahova, M. Mitochondrial Oxphos Biogenesis: Co-Regulation of Protein Synthesis, Import, and Assembly Pathways. Int. J. Mol. Sci. 2020, 21, 3820. [Google Scholar] [CrossRef] [PubMed]
- Thornton, C.; Jones, A.; Nair, S.; Aabdien, A.; Mallard, C.; Hagberg, H. Mitochondrial dynamics, mitophagy and biogenesis in neonatal hypoxic-ischaemic brain injury. FEBS Lett. 2017, 592, 812–830. [Google Scholar] [CrossRef]
- Timón-Gómez, A.; Nývltová, E.; Abriata, L.A.; Vila, A.J.; Hosler, J.; Barrientos, A. Mitochondrial cytochrome c oxidase biogenesis: Recent developments. Semin. Cell Dev. Biol. 2018, 76, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.; Sadoshima, J. Mitochondrial autophagy in cardiomyopathy. Curr. Opin. Genet. Dev. 2016, 38, 8–15. [Google Scholar] [CrossRef]
- Van Haute, L.; Powell, C.A.; Minczuk, M. Dealing with an Unconventional Genetic Code in Mitochondria: The Biogenesis and Pathogenic Defects of the 5-Formylcytosine Modification in Mitochondrial tRNAMet. Biomolecules 2017, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- VanderVeen, B.N.; Fix, D.K.; Carson, J.A. Disrupted Skeletal Muscle Mitochondrial Dynamics, Mitophagy, and Biogenesis during Cancer Cachexia: A Role for Inflammation. Oxid. Med. Cell. Longev. 2017, 2017, 3292087. [Google Scholar] [CrossRef] [PubMed]
- Vega, R.B.; Horton, J.L.; Kelly, D.P. Maintaining Ancient Organelles: Mitochondrial Biogenesis and Maturation. Circ. Res. 2015, 116, 1820–1834. [Google Scholar] [CrossRef]
- Paz, M.V.; Cotán, D.; Garrido-Maraver, J.; Cordero, M.D.; Oropesa-Ávila, M.; De La Mata, M.; Pavón, A.D.; De Lavera, I.; Alcocer-Gómez, E.; Sánchez-Alcázar, J.A. Targeting autophagy and mitophagy for mitochondrial diseases treatment. Expert Opin. Ther. Targets 2015, 20, 487–500. [Google Scholar] [CrossRef]
- Wang, J.; Wen, X.; Liu, J.; Sun, H. Mitochondrial biogenesis Inhibitors for Anticancer Therapy: A Review of Recent Patents. Recent Pat. Anti-Cancer Drug Discov. 2016, 11, 332–341. [Google Scholar] [CrossRef]
- Whitaker, R.M.; Corum, D.; Beeson, C.C.; Schnellmann, R.G. Mitochondrial Biogenesis as a Pharmacological Target: A New Approach to Acute and Chronic Diseases. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 229–249. [Google Scholar] [CrossRef]
- Wideman, J.G.; Muñoz-Gómez, S.A. The evolution of ERMIONE in mitochondrial biogenesis and lipid homeostasis: An evolutionary view from comparative cell biology. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2016, 1861, 900–912. [Google Scholar] [CrossRef]
- Williams, J.A.; Zhao, K.; Jin, S.; Ding, W.-X. New methods for monitoring mitochondrial biogenesis and mitophagy in vitro and in vivo. Exp. Biol. Med. 2017, 242, 781–787. [Google Scholar] [CrossRef]
- Dos Santos, T.W.; Pereira, Q.C.; Teixeira, L.; Gambero, A.; Villena, J.A.; Ribeiro, M.L. Effects of Polyphenols on Thermogenesis and Mitochondrial Biogenesis. Int. J. Mol. Sci. 2018, 19, 2757. [Google Scholar] [CrossRef]
- Yambire, K.F.; Fernandez-Mosquera, L.; Steinfeld, R.; Mühle, C.; Ikonen, E.; Milosevic, I.; Raimundo, N. Mitochondrial biogenesis is transcriptionally repressed in lysosomal lipid storage diseases. eLife 2019, 8. [Google Scholar] [CrossRef]
- Yuan, Y.; Cruzat, V.F.; Newsholme, P.; Cheng, J.; Chen, Y.; Lu, Y. Regulation of SIRT1 in aging: Roles in mitochondrial function and biogenesis. Mech. Ageing Dev. 2016, 155, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Zamora, M.; Pardo, R.; Villena, J.A. Pharmacological induction of mitochondrial biogenesis as a therapeutic strategy for the treatment of type 2 diabetes. Biochem. Pharmacol. 2015, 98, 16–28. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, H. Translational regulation of mitochondrial biogenesis. Biochem. Soc. Trans. 2016, 44, 1717–1724. [Google Scholar] [CrossRef] [PubMed]
- Andhavarapu, S.; Katuri, A.; Bryant, J.; Patel, V.; Gupta, U.; Asemu, G.; Makar, T.K. Intersecting roles of ER stress, mitochondrial dysfunction, autophagy, and calcium homeostasis in HIV-associated neurocognitive disorder. J. NeuroVirol. 2020, 26, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Baquero, P.; Dawson, A.; Helgason, G.V. Autophagy and mitochondrial metabolism: Insights into their role and therapeutic potential in chronic myeloid leukaemia. FEBS J. 2019, 286, 1271–1283. [Google Scholar] [CrossRef]
- Chen, S.Y.; Gao, Y.; Sun, J.Y.; Meng, X.L.; Yang, D.; Fan, L.H.; Xiang, L.; Wang, P. Traditional Chinese Medicine: Role in Reducing Beta-Amyloid, Apoptosis, Autophagy, Neuroinflammation, Oxidative Stress, and Mitochondrial Dysfunction of Alzheimer’s Disease. Front. Pharmacol. 2020, 11, 497. [Google Scholar] [CrossRef]
- Cheng, J.; Wei, L.; Li, M. Progress in Regulation of Mitochondrial Dynamics and Mitochondrial Autophagy. Sheng Li Xue Bao 2020, 72, 475–487. [Google Scholar]
- Furukawa, K.; Innokentev, A.; Kanki, T. Regulatory Mechanisms of Mitochondrial Autophagy: Lessons from Yeast. Front. Plant. Sci. 2019, 10, 1479. [Google Scholar] [CrossRef]
- Gafar, A.A.; Draz, H.M.; Goldberg, A.A.; Bashandy, M.A.; Bakry, S.; Khalifa, M.A.; AbuShair, W.; Titorenko, V.I.; Sanderson, J.T. Lithocholic acid induces endoplasmic reticulum stress, autophagy and mitochondrial dysfunction in human prostate cancer cells. PeerJ 2016, 4, e2445. [Google Scholar] [CrossRef] [PubMed]
- Go, K.L.; Lee, S.; Zendejas, I.; Behrns, K.E.; Kim, J.S. Mitochondrial Dysfunction and Autophagy in Hepatic Ischemia/Reperfusion Injury. Biomed. Res. Int. 2015, 2015, 183469. [Google Scholar] [CrossRef]
- Hamacher-Brady, A.; Brady, N.R. Mitophagy programs: Mechanisms and physiological implications of mitochondrial targeting by autophagy. Cell. Mol. Life Sci. 2016, 73, 775–795. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.; Shi, Y. Regulation of autophagy by mitochondrial phospholipids in health and diseases. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2017, 1862, 114–129. [Google Scholar] [CrossRef]
- Huang, M.L.-H.; Chiang, S.; Kalinowski, D.S.; Bae, D.-H.; Sahni, S.; Richardson, D.R. The Role of the Antioxidant Response in Mitochondrial Dysfunction in Degenerative Diseases: Cross-Talk between Antioxidant Defense, Autophagy, and Apoptosis. Oxidative Med. Cell. Longev. 2019, 2019, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Katuri, A.; Bryant, J.L.; Patel, D.; Patel, V.; Andhavarapu, S.; Asemu, G.; Davis, H.; Makar, T.K. HIVAN associated tubular pathology with reference to ER stress, mitochondrial changes, and autophagy. Exp. Mol. Pathol. 2019, 106, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Kubli, D.A.; Gustafsson, Å.B. Unbreak My Heart: Targeting Mitochondrial Autophagy in Diabetic Cardiomyopathy. Antioxid. Redox Signal. 2015, 22, 1527–1544. [Google Scholar] [CrossRef]
- Madhavi, Y.V.; Gaikwad, N.; Yerra, V.G.; Kalvala, A.K.; Nanduri, S.; Kumar, A. Targeting AMPK in Diabetes and Diabetic Complications: Energy Homeostasis, Autophagy and Mitochondrial Health. Curr. Med. Chem. 2019, 26, 5207–5229. [Google Scholar] [CrossRef]
- Miyamoto, S. Hexokinase 2 mediated cellular protection: Interaction with Akt/mTORC1 to regulate mitochondrial protection and autophagy. Seikagaku. J. Jpn. Biochem. Soc. 2017, 89, 199–210. [Google Scholar]
- Ney, P.A. Mitochondrial Autophagy: Origins, Significance, and Role of Bnip3 and Nix. Biochim. Biophys. Acta 2015, 1853, 2775–2783. [Google Scholar] [CrossRef]
- Roca-Agujetas, V.; De Dios, C.; Lestón, L.; Marí, M.; Morales, A.; Colell, A. Recent Insights into the Mitochondrial Role in Autophagy and Its Regulation by Oxidative Stress. Oxidative Med. Cell. Longev. 2019, 2019, 1–16. [Google Scholar] [CrossRef]
- Saito, T.; Sadoshima, J. Molecular Mechanisms of Mitochondrial Autophagy/Mitophagy in the Heart. Circ. Res. 2015, 116, 1477–1490. [Google Scholar] [CrossRef]
- Shah, S.Z.A.; Zhao, D.; Hussain, T.; Sabir, N.; Mangi, M.H.; Yang, L. p62-Keap1-NRF2-ARE Pathway: A Contentious Player for Selective Targeting of Autophagy, Oxidative Stress and Mitochondrial Dysfunction in Prion Diseases. Front. Mol. Neurosci. 2018, 11, 310. [Google Scholar] [CrossRef]
- Sifuentes-Franco, S.; Pacheco-Moisés, F.P.; Rodríguez-Carrizalez, A.D.; Miranda-Díaz, A.G. The Role of Oxidative Stress, Mitochondrial Function, and Autophagy in Diabetic Polyneuropathy. J. Diabetes Res. 2017, 2017, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.A.; Yen, P.M. Thyroid hormone-mediated autophagy and mitochondrial turnover in NAFLD. Cell Biosci. 2016, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tagaya, M.; Arasaki, K. Regulation of Mitochondrial Dynamics and Autophagy by the Mitochondria-Associated Membrane. Adv. Exp. Med. Biol. 2017, 997, 33–47. [Google Scholar] [PubMed]
- Tricarico, P.M.; Crovella, S.; Celsi, F. Mevalonate Pathway Blockade, Mitochondrial Dysfunction and Autophagy: A Possible Link. Int. J. Mol. Sci. 2015, 16, 16067–16084. [Google Scholar] [CrossRef]
- Van Houten, B.; Hunter, S.E.; Meyer, J.N. Mitochondrial DNA Damage Induced Autophagy, Cell Death, and Disease. Front. Biosci. (Landmark Ed.) 2016, 21, 42–54. [Google Scholar] [CrossRef]
- Wang, B.; Abraham, N.; Gao, G.; Yang, Q. Dysregulation of Autophagy and Mitochondrial Function in Parkinson’s Disease. Transl. Neurodegener. 2016, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, G. Autophagy in Mitochondrial Quality Control. In Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Swtizerland, 2019; Volume 1206, pp. 421–434. [Google Scholar]
- Yamano, K.; Matsuda, N.; Tanaka, K. The Ubiquitin Signal and Autophagy: An Orchestrated Dance Leading to Mitochondrial Degradation. EMBO Rep. 2016, 17, 300–316. [Google Scholar] [CrossRef]
- Yamashita, S.I.; Kanki, T. How Autophagy Eats Large Mitochondria: Autophagosome Formation Coupled with Mitochondrial Fragmentation. Autophagy 2017, 13, 980–981. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Gao, G.; Mao, Z.; Yang, Q. Chaperone-Mediated Autophagy and Mitochondrial Homeostasis in Parkinson’s Disease. Park. Dis. 2016, 2016, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Culp, M.L.; Craver, J.G.; Darley-Usmar, V. Mitochondrial Function and Autophagy: Integrating Proteotoxic, Redox, and Metabolic Stress in Parkinson’s Disease. J. Neurochem. 2018, 144, 691–709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zheng, Y.; Chen, Z. Autophagy and Mitochondrial Encephalomyopathies. In Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2020; pp. 103–110. [Google Scholar]
- Kopinski, P.K.; Janssen, K.A.; Schaefer, P.M.; Trefely, S.; Perry, C.E.; Potluri, P.; Tintos-Hernandez, J.A.; Singh, L.N.; Karch, K.R.; Campbell, S.L.; et al. Regulation of nuclear epigenome by mitochondrial DNA heteroplasmy. Proc. Natl. Acad. Sci. USA 2019, 116, 16028–16035. [Google Scholar] [CrossRef]
- Matilainen, O.; Quirós, P.M.; Auwerx, J. Mitochondria and Epigenetics—Crosstalk in Homeostasis and Stress. Trends Cell Biol. 2017, 27, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Schell, J.C.; Rutter, J. Mitochondria link metabolism and epigenetics in haematopoiesis. Nat. Cell Biol. 2017, 19, 589–591. [Google Scholar] [CrossRef]
- Sharma, N.; Pasala, M.S.; Prakash, A. Mitochondrial DNA: Epigenetics and environment. Environ. Mol. Mutagen. 2019, 60, 668–682. [Google Scholar] [CrossRef]
- Danzeisen, R.; Schwalenstoecker, B.; Gillardon, F.; Buerger, E.; Krzykalla, V.; Klinder, K.; Schild, L.; Hengerer, B.; Ludolph, A.C.; Dorner-Ciossek, C.; et al. Targeted Antioxidative and Neuroprotective Properties of the Dopamine Agonist Pramipexole and Its Nondopaminergic Enantiomer SND919CL2x [(+)2-Amino-4,5,6,7-tetrahydro-6-lpropylamino-benzathiazole Dihydrochloride]. J. Pharmacol. Exp. Ther. 2005, 316, 189–199. [Google Scholar] [CrossRef]
- Ferrari-Toninelli, G.; Maccarinelli, G.; Uberti, D.L.; Buerger, E.; Memo, M. Mitochondria-targeted antioxidant effects of S(-) and R(+) pramipexole. BMC Pharmacol. 2010, 10, 2. [Google Scholar] [CrossRef]
- Akhmadishina, R.A.; Garifullin, R.; Petrova, N.V.; Kamalov, M.I.; Abdullin, T.I. Triphenylphosphonium Moiety Modulates Proteolytic Stability and Potentiates Neuroprotective Activity of Antioxidant Tetrapeptides in Vitro. Front. Pharmacol. 2018, 9, 115. [Google Scholar] [CrossRef]
- Battogtokh, G.; Choi, Y.S.; Kang, D.S.; Park, S.J.; Shim, M.S.; Huh, K.M.; Cho, Y.-Y.; Lee, J.Y.; Lee, H.S.; Kang, H.C. Mitochondria-targeting drug conjugates for cytotoxic, anti-oxidizing and sensing purposes: Current strategies and future perspectives. Acta Pharm. Sin. B 2018, 8, 862–880. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Liaw, K.; Sharma, R.; Zhang, Z.; Kannan, S.; Kannan, R.M. Targeting Mitochondrial Dysfunction and Oxidative Stress in Activated Microglia Using Dendrimer-Based Therapeutics. Theranostics 2018, 8, 5529–5547. [Google Scholar] [CrossRef]
- Titova, E.; Shagieva, G.; Ivanova, O.; Domnina, L.; Domninskaya, M.; Strelkova, O.; Khromova, N.; Kopnin, P.; Chernyak, B.; Skulachev, V.; et al. Mitochondria-targeted antioxidant SkQ1 suppresses fibrosarcoma and rhabdomyosarcoma tumour cell growth. Cell Cycle 2018, 17, 1797–1811. [Google Scholar] [CrossRef]
- Apostolova, N.; Victor, V.M. Molecular Strategies for Targeting Antioxidants to Mitochondria: Therapeutic Implications. Antioxid. Redox Signal. 2015, 22, 686–729. [Google Scholar] [CrossRef]
- Fetisova, E.K.; Antoschina, M.M.; Cherepanynets, V.D.; Izumov, D.S.; Kireev, I.I.; Kireev, R.I.; Lyamzaev, K.G.; Riabchenko, N.I.; Chernyak, B.V.; Skulachev, V.P. Radioprotective Effects of Mitochondria-Targeted Antioxidant SkQR. Radiat. Res. 2015, 183, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Genrikhs, E.E.; Stelmashook, E.V.; Popova, O.V.; Kapay, N.A.; Korshunova, G.A.; Sumbatyan, N.V.; Skrebitsky, V.G.; Skulachev, V.P.; Isaev, N.K. Mitochondria-Targeted Antioxidant Skqt1 Decreases Trauma-Induced Neurological Deficit in Rat and Prevents Amyloid-Beta-Induced Impairment of Long-Term Potentiation in Rat Hippocampal Slices. J. Drug Target. 2015, 23, 347–352. [Google Scholar] [CrossRef]
- Silachev, D.N.; Plotnikov, E.Y.; Zorova, L.D.; Pevzner, I.B.; Sumbatyan, N.V.; Korshunova, G.A.; Gulyaev, M.V.; Pirogov, Y.A.; Skulachev, V.P.; Zorov, D.B. Neuroprotective Effects of Mitochondria-Targeted Plastoquinone and Thymoquinone in a Rat Model of Brain Ischemia/Reperfusion Injury. Molecules 2015, 20, 14487–14503. [Google Scholar] [CrossRef]
- Margulis, L. Symbiotic theory of the origin of eukaryotic organelles; criteria for proof. Symp. Soc. Exp. Biol. 1975, 29, 21–38. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).