G Protein-Coupled Receptor Signaling and Regulation
A topical collection in International Journal of Molecular Sciences (ISSN 1422-0067). This collection belongs to the section "Biochemistry".
Viewed by 504522Editors
Interests: arrestin proteins; structure-function; protein engineering; GPCR signaling; GPCR trafficking; receptor regulation; cell proliferation; apoptosis; MAP kinases
* From Volume 18, Issue 7, 2017
Special Issues, Collections and Topics in MDPI journals
Interests: GPCR signaling and regulation; epigenetic regulation; anti-inflammatory strategies with less ‘side-effects’
Topical Collection Information
Dear Colleagues,
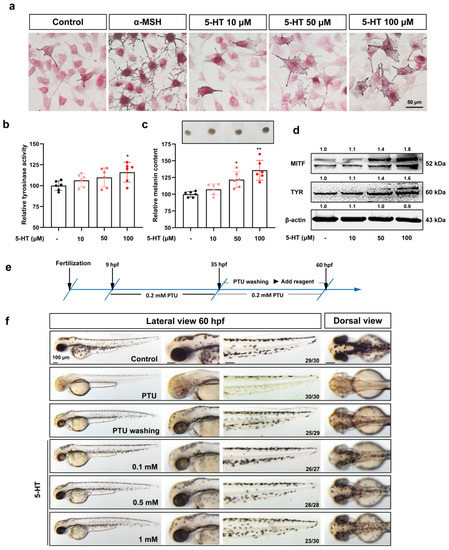
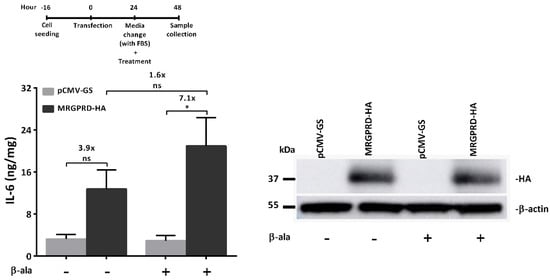
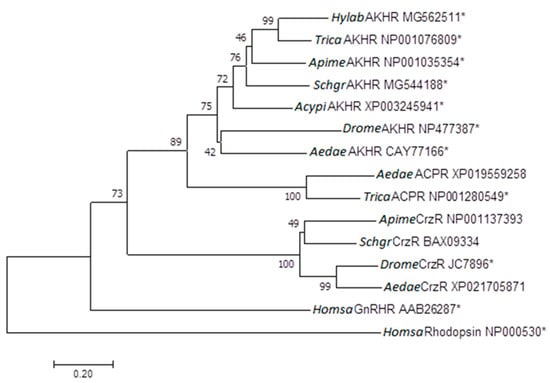
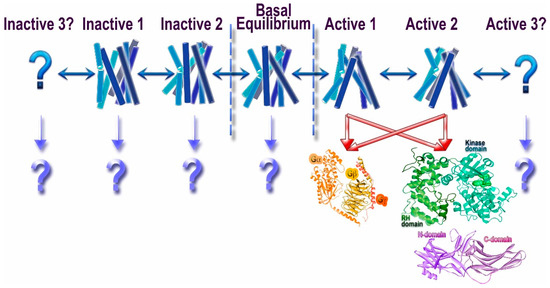
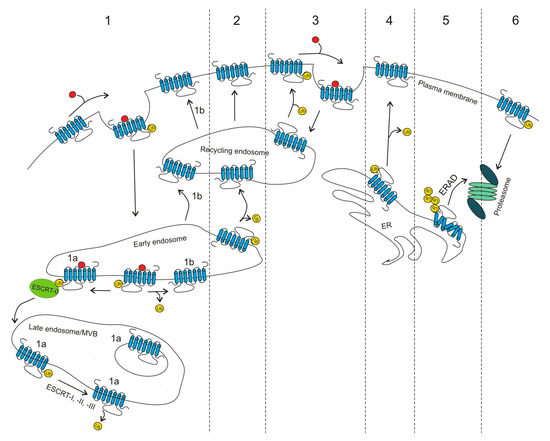
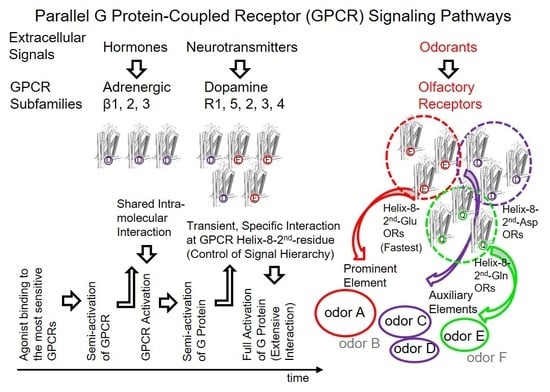
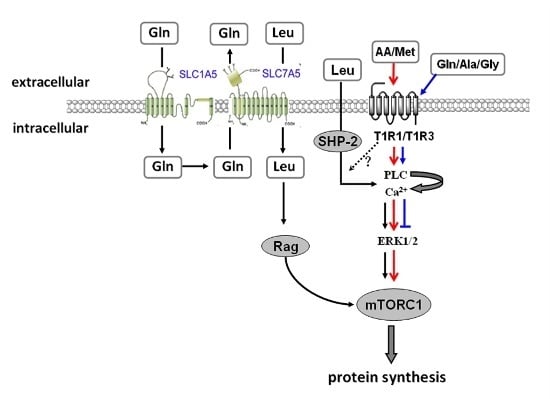
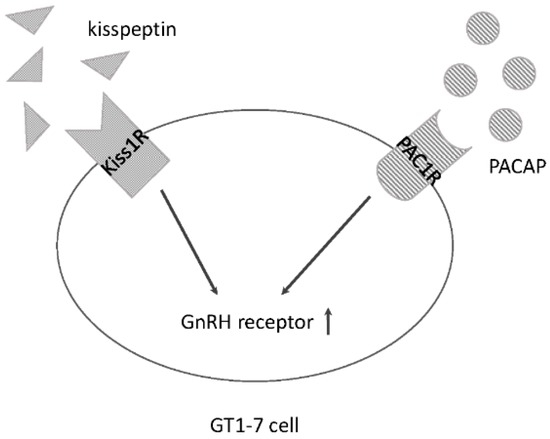
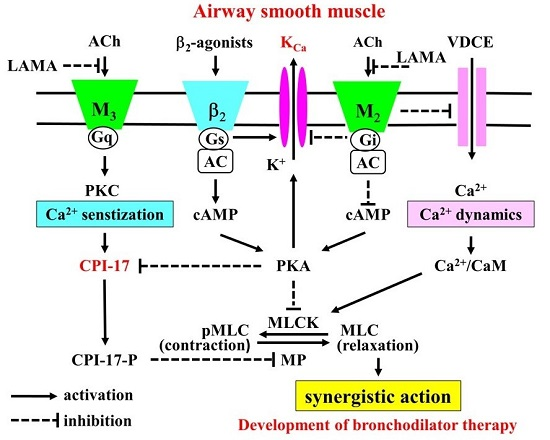
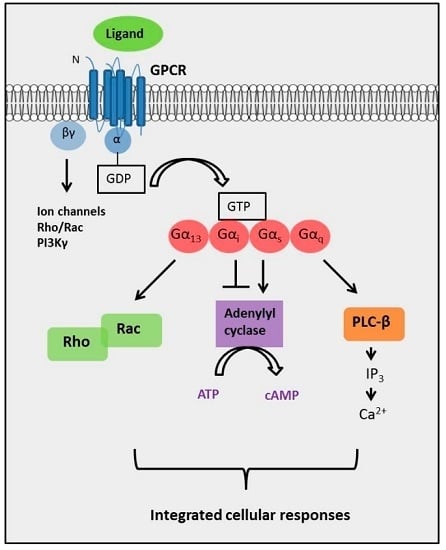
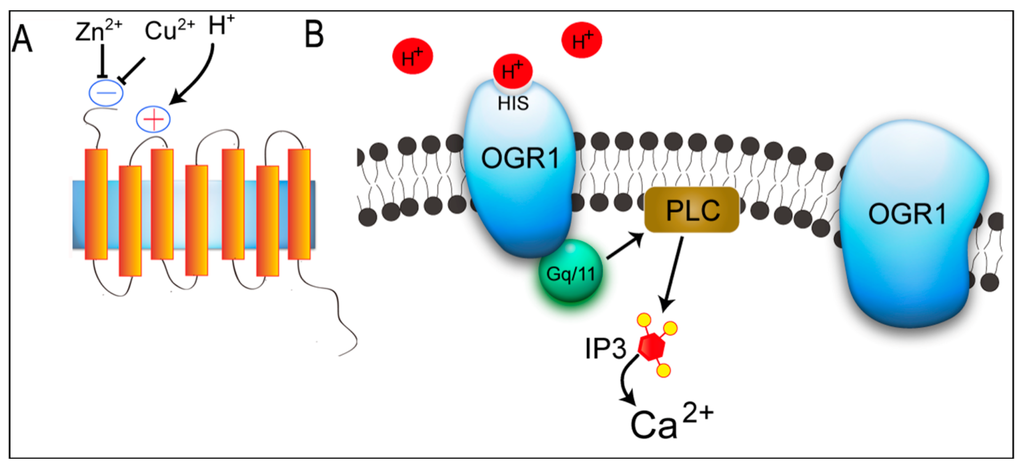
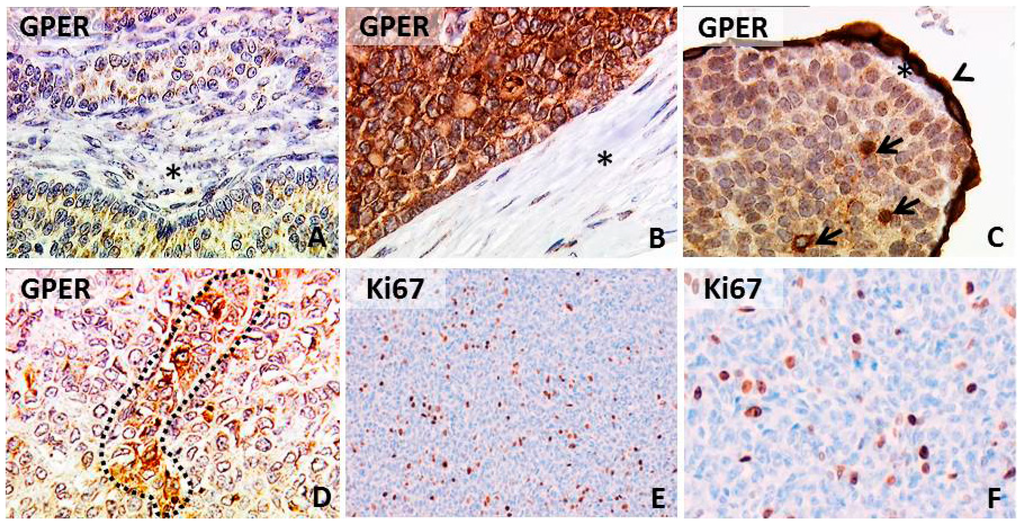
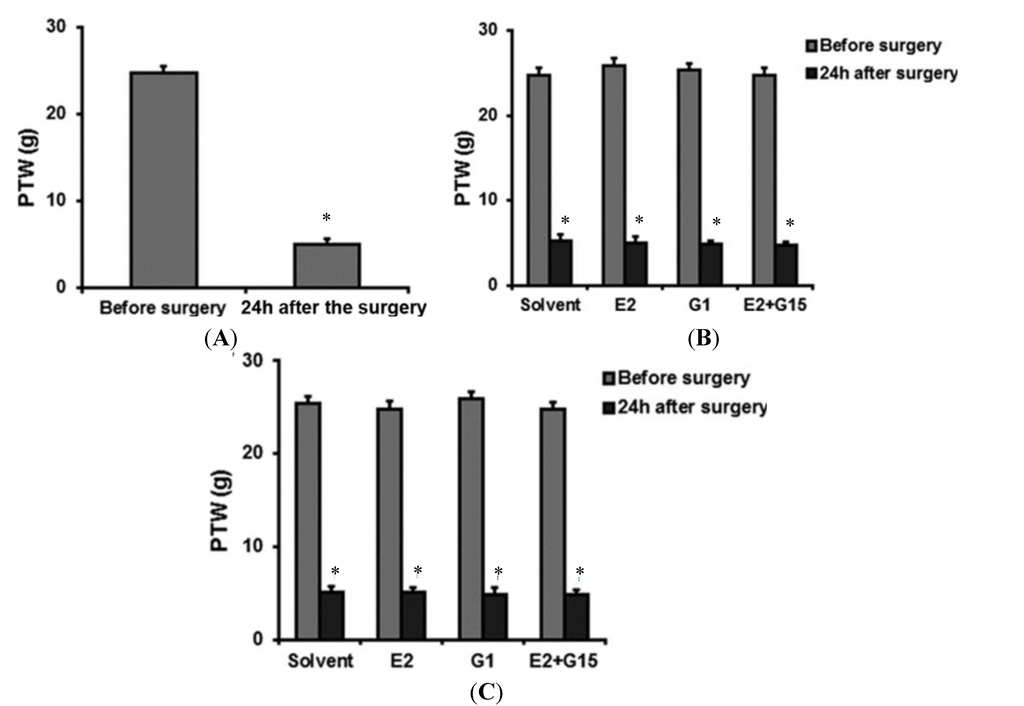
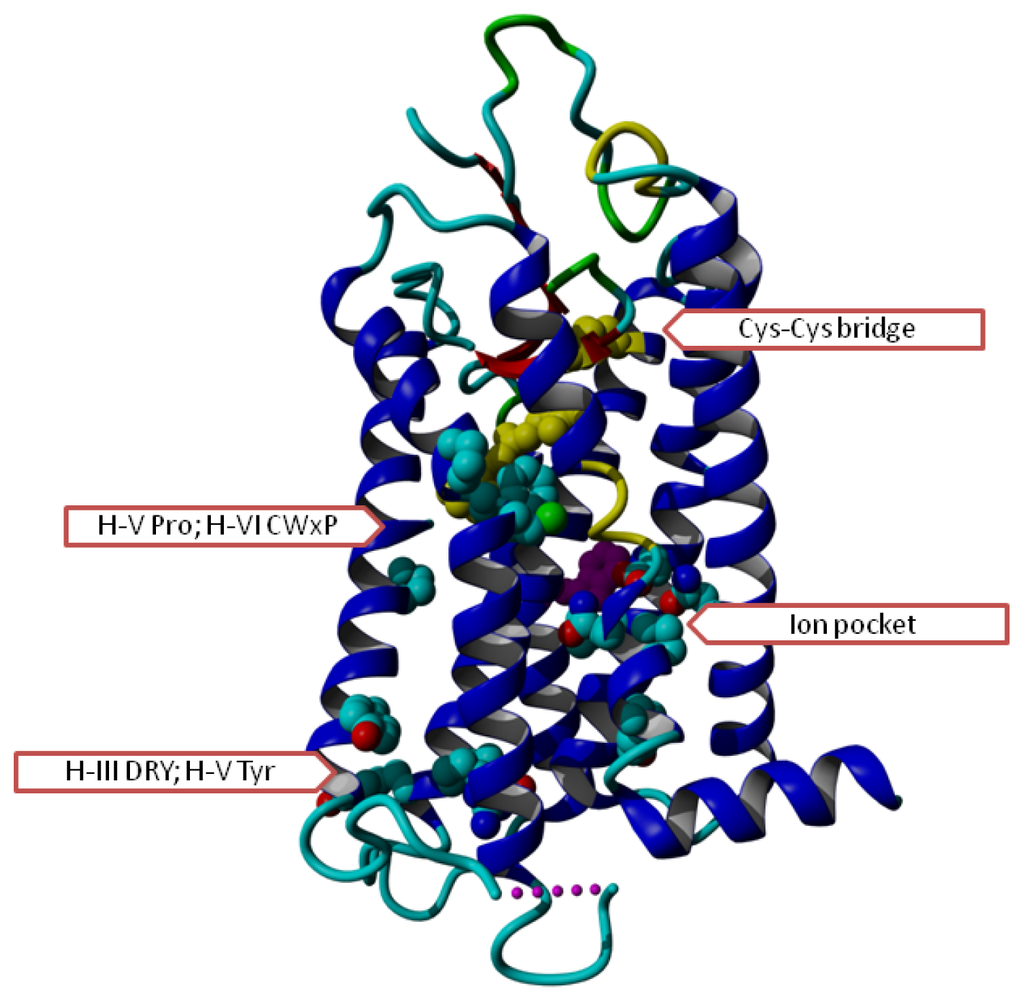
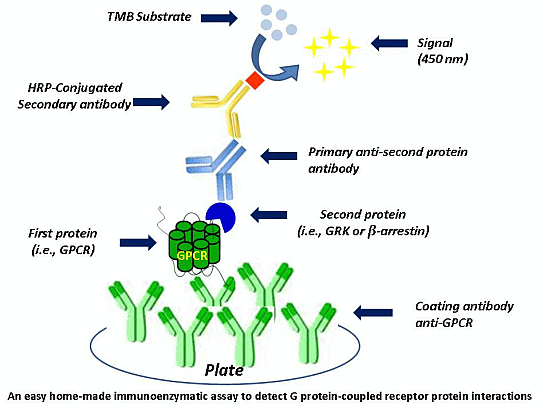
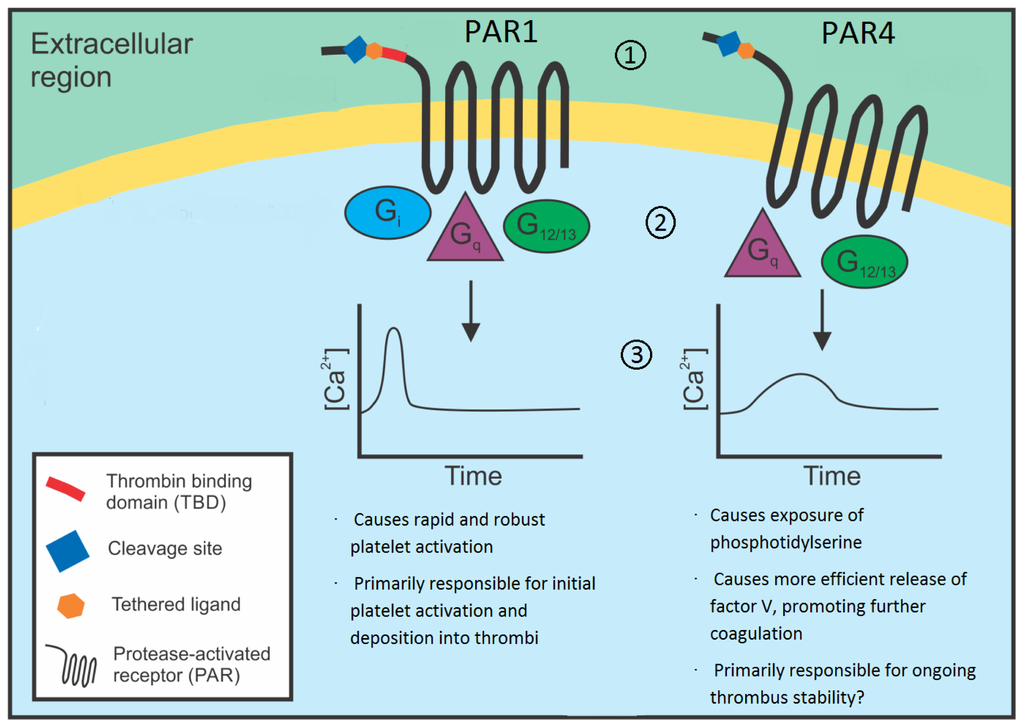
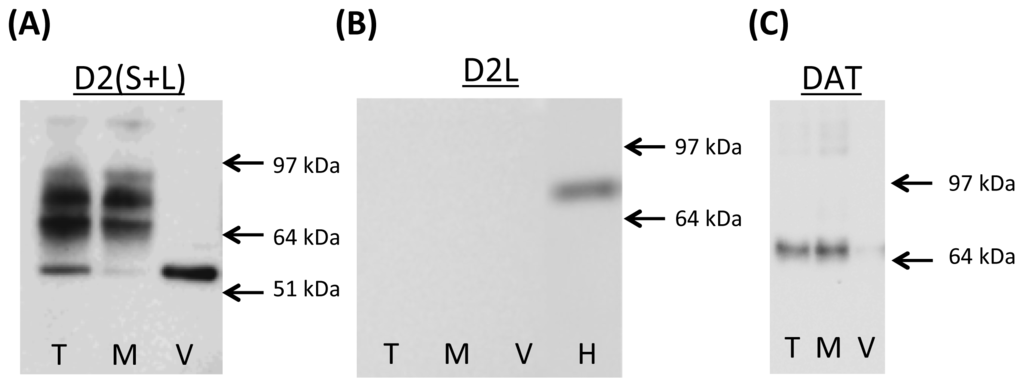
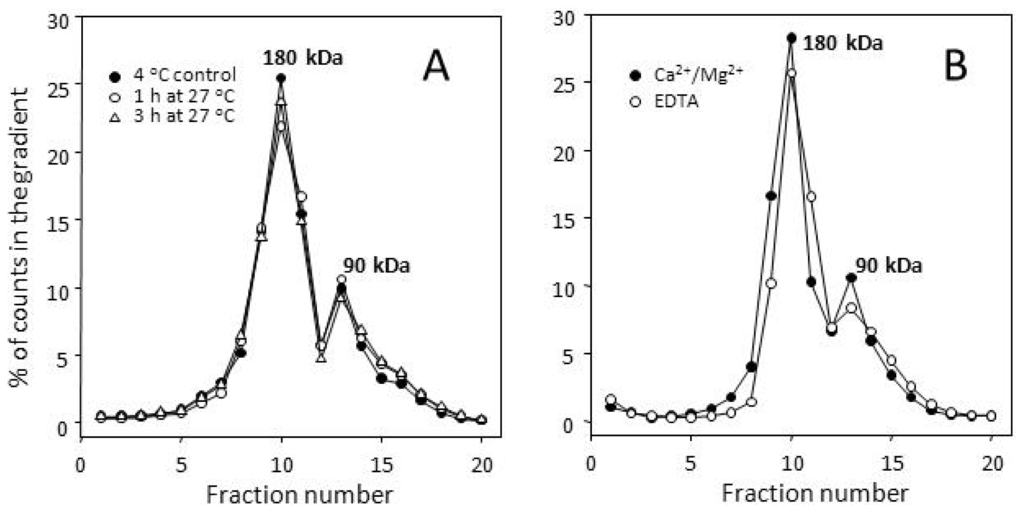
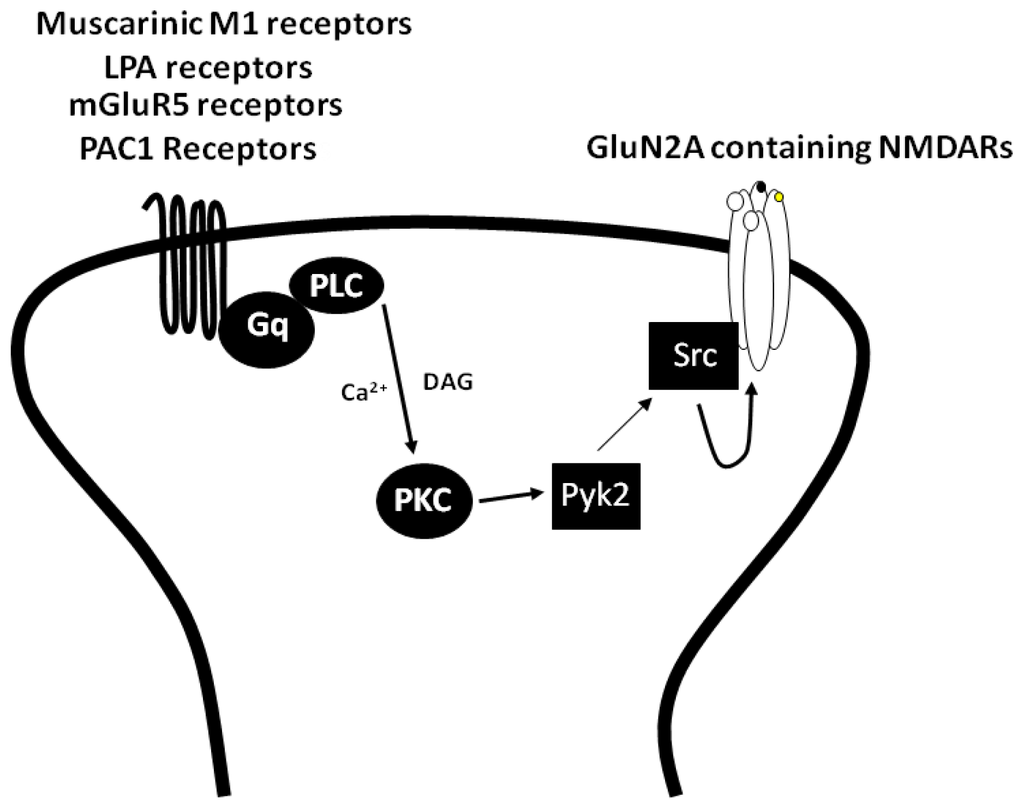
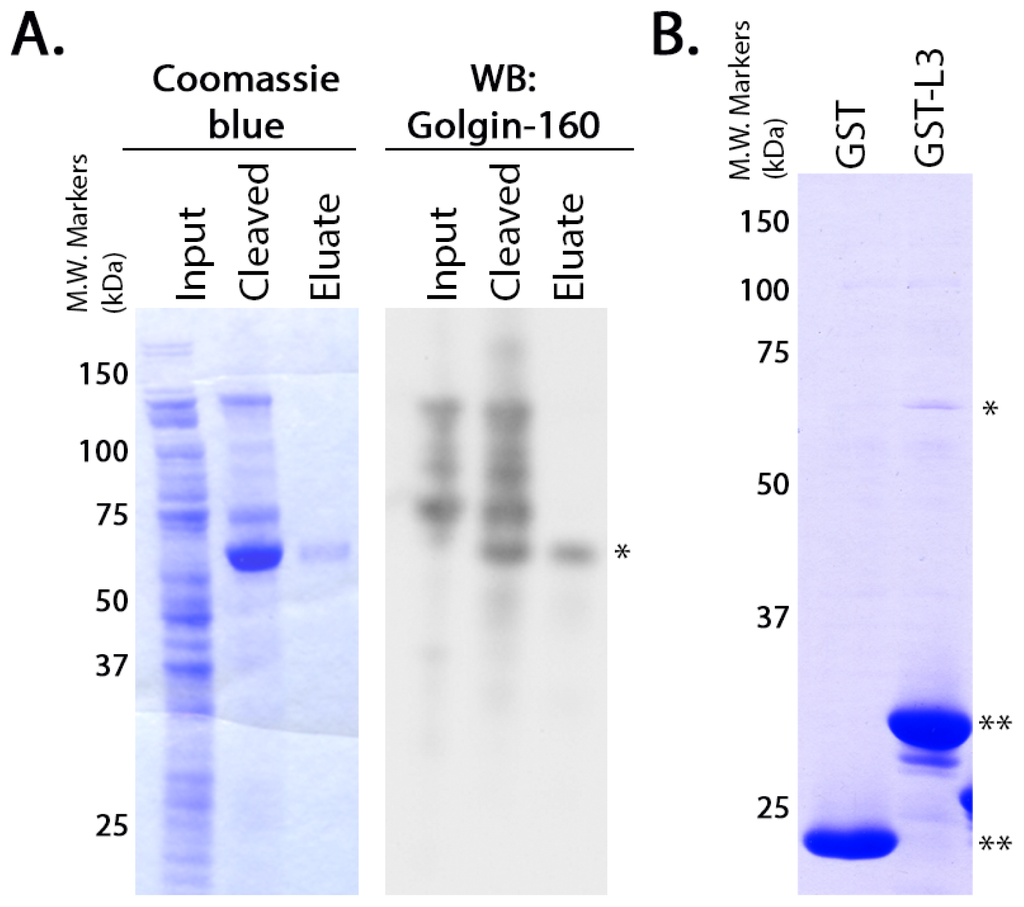
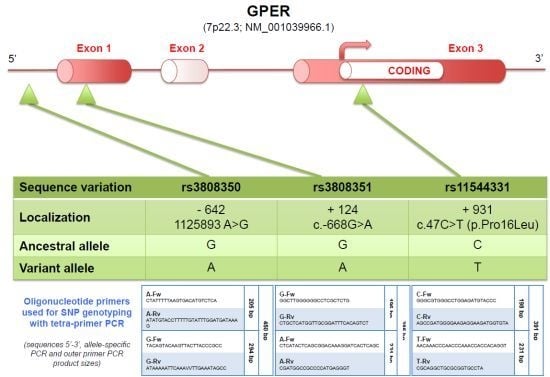
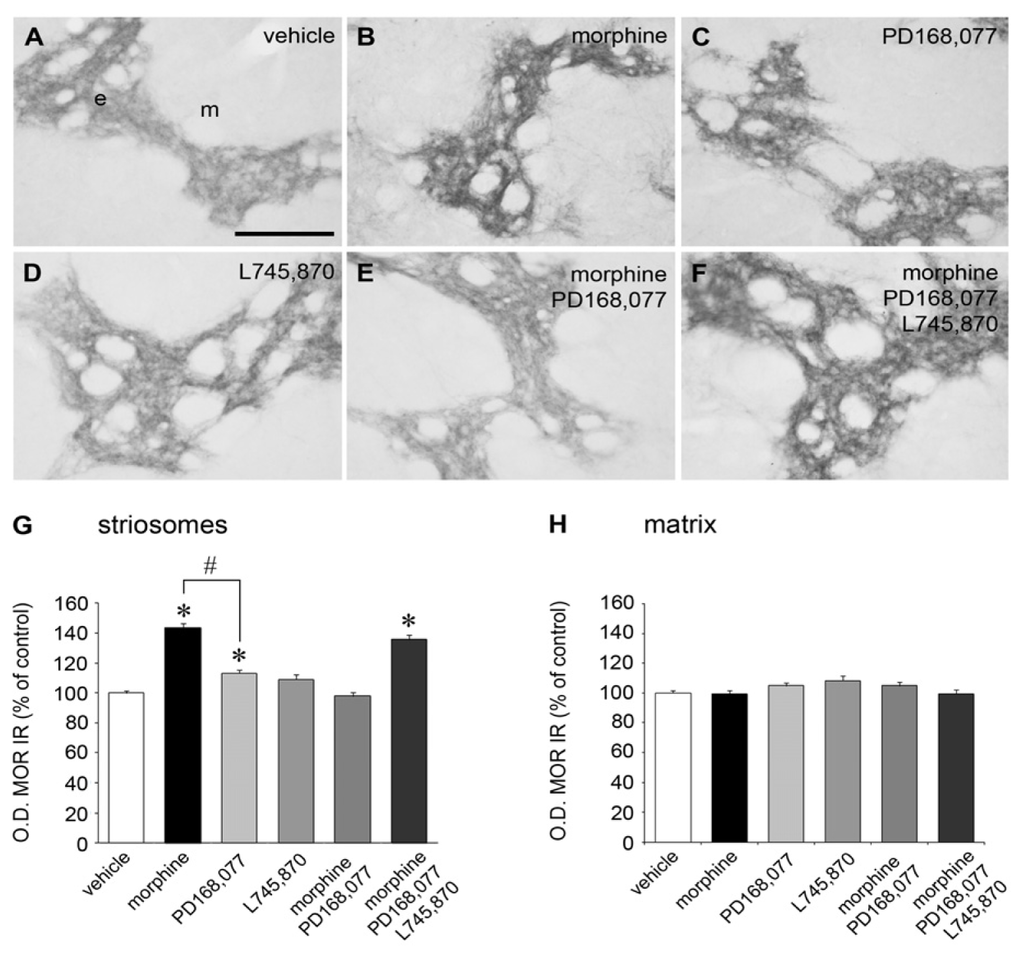
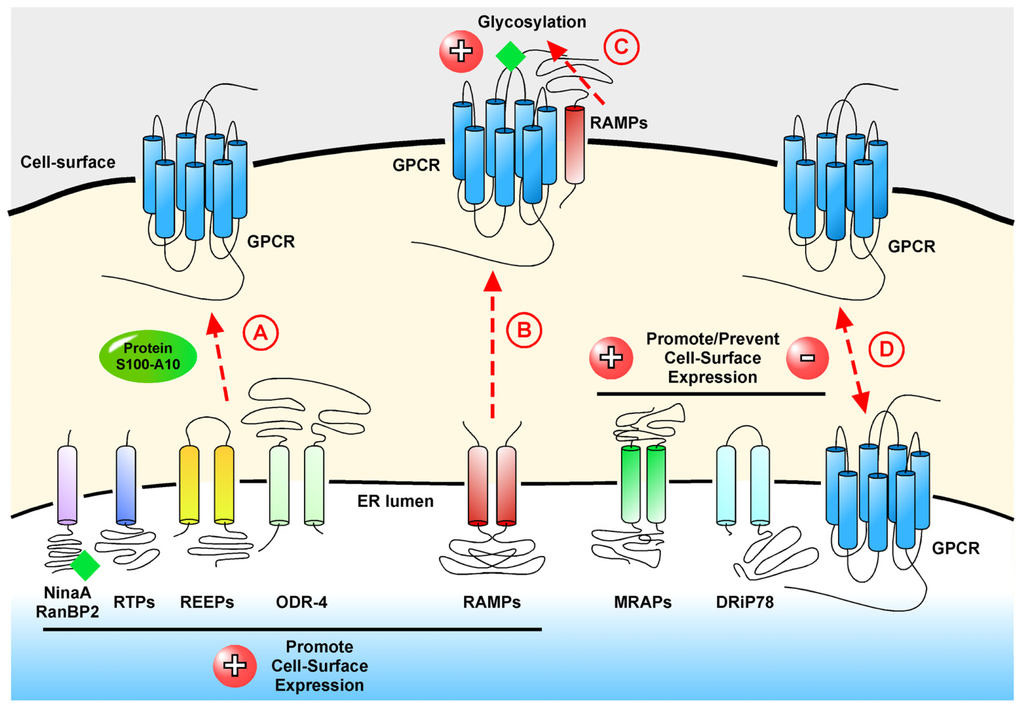
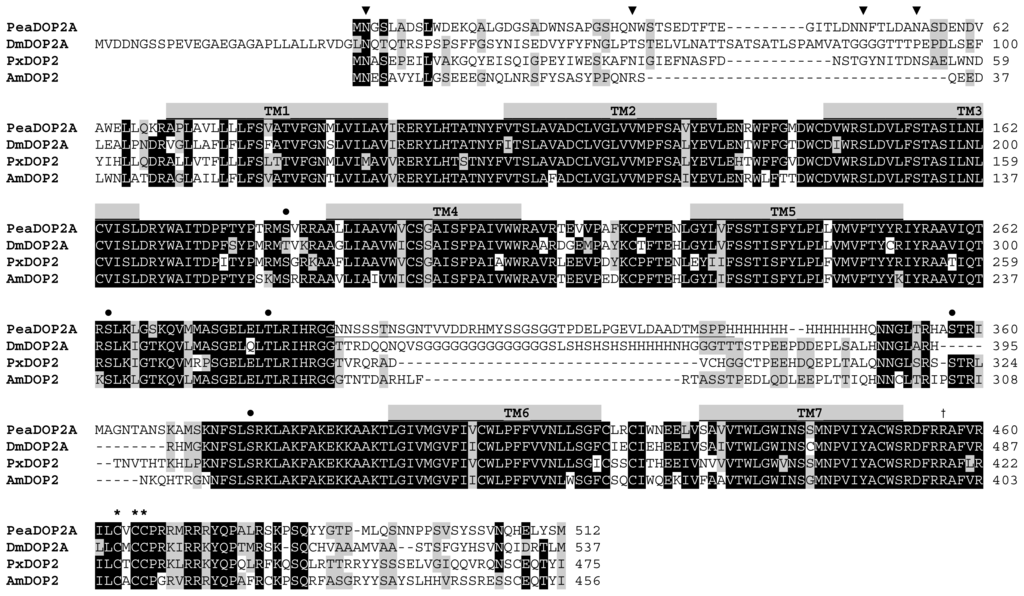
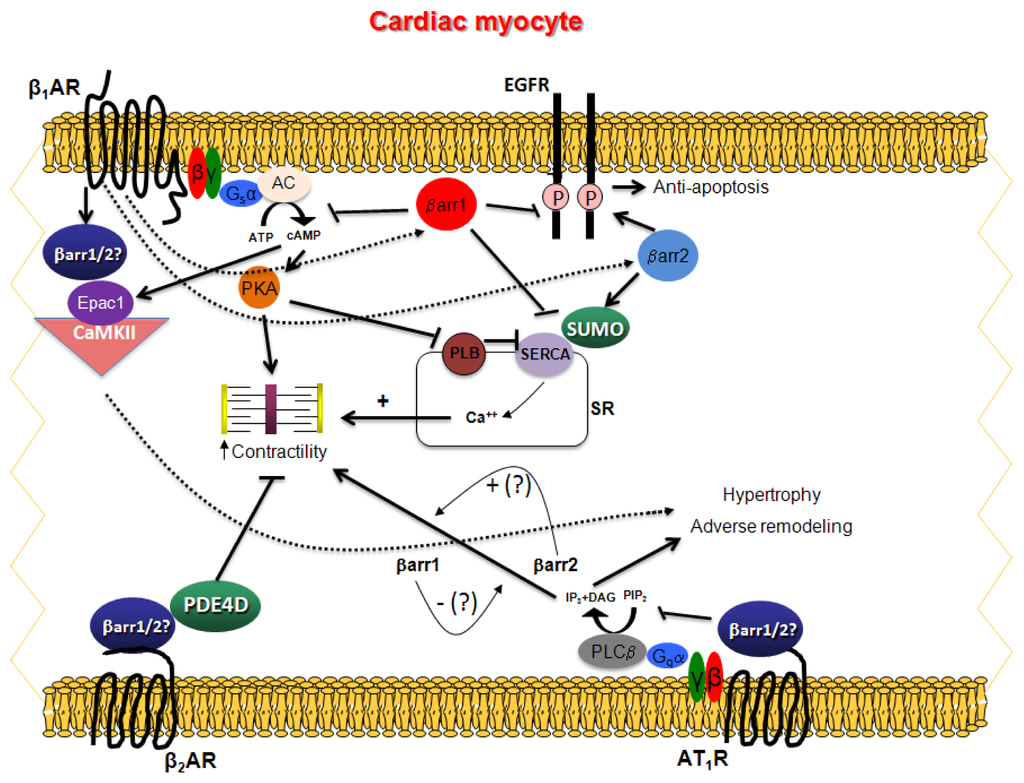
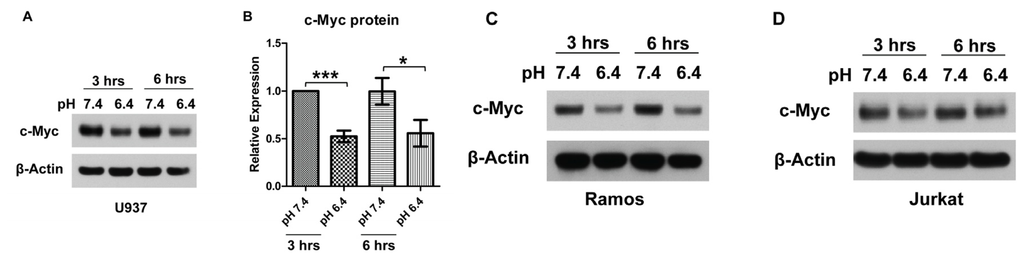
G protein-coupled receptors (GPCRs) are the largest family of proteins in animals, with more than 800 distinct subtypes in humans. GPCRs are targeted by a larger percentage of clinically-used drugs than any other protein family. All GPCRs have seven α-helical transmembrane domains (that is why they are also called seven transmembrane domain receptors, or 7TMRs) and, upon stimulation, change their conformation. Different GPCRs respond to light, odorants, pheromones, taste molecules, hormones, neurotransmitters, extracellular calcium, and many other stimuli. Most GPCRs couple to the G proteins (hence the name). GPCRs serve as guanyl nucleotide exchange factors (GEFs) for heterotrimeric G proteins, facilitating the release of bound GDP and the binding of GTP abundant in the cytoplasm. GTP-liganded G protein α-subunit dissociates from the βγ-subunit, whereupon both subunits interact with various effectors, regulating their activity. Active GPCR during its lifetime activates several G protein molecules, thereby amplifying the signal. Most effectors are enzymes or ion channels, ensuring additional signal amplification. G protein α-subunits self-inactivate via intrinsic GTPase activity. RGS (Regulators of G protein signaling) proteins facilitate this self-inactivation. Many GPCRs are regulated by the phosphorylation by GPCR kinases (GRKs) that specifically target active receptors. Active phosphorylated GPCRs bind arrestin proteins with high affinity. Arrestin binding precludes GPCR coupling to G proteins, often mediates the recruitment of the receptor to the coated pits for internalization, and initiates arrestin-mediated signaling.
Research articles, review articles, and communications on every aspect of GPCR activation and regulation, as well as on proteins participating in GPCR signal transduction, are invited.
Prof. Dr. Vsevolod V. Gurevich
Dr. Kathleen Van Craenenbroeck
Collection Editors
Manuscript Submission Information
Manuscripts for the topical collection can be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. All papers will be peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on this website. The topical collection considers regular research articles, short communications and review articles. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page.
Please visit the Instructions for Authors page before submitting a manuscript. The article processing charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs).
Keywords
- GPCRs
- signaling
- trafficking
- G proteins
- arrestins
- RGS proteins
- GRKs