Analysis of Peptide Ligand Specificity of Different Insect Adipokinetic Hormone Receptors
Abstract
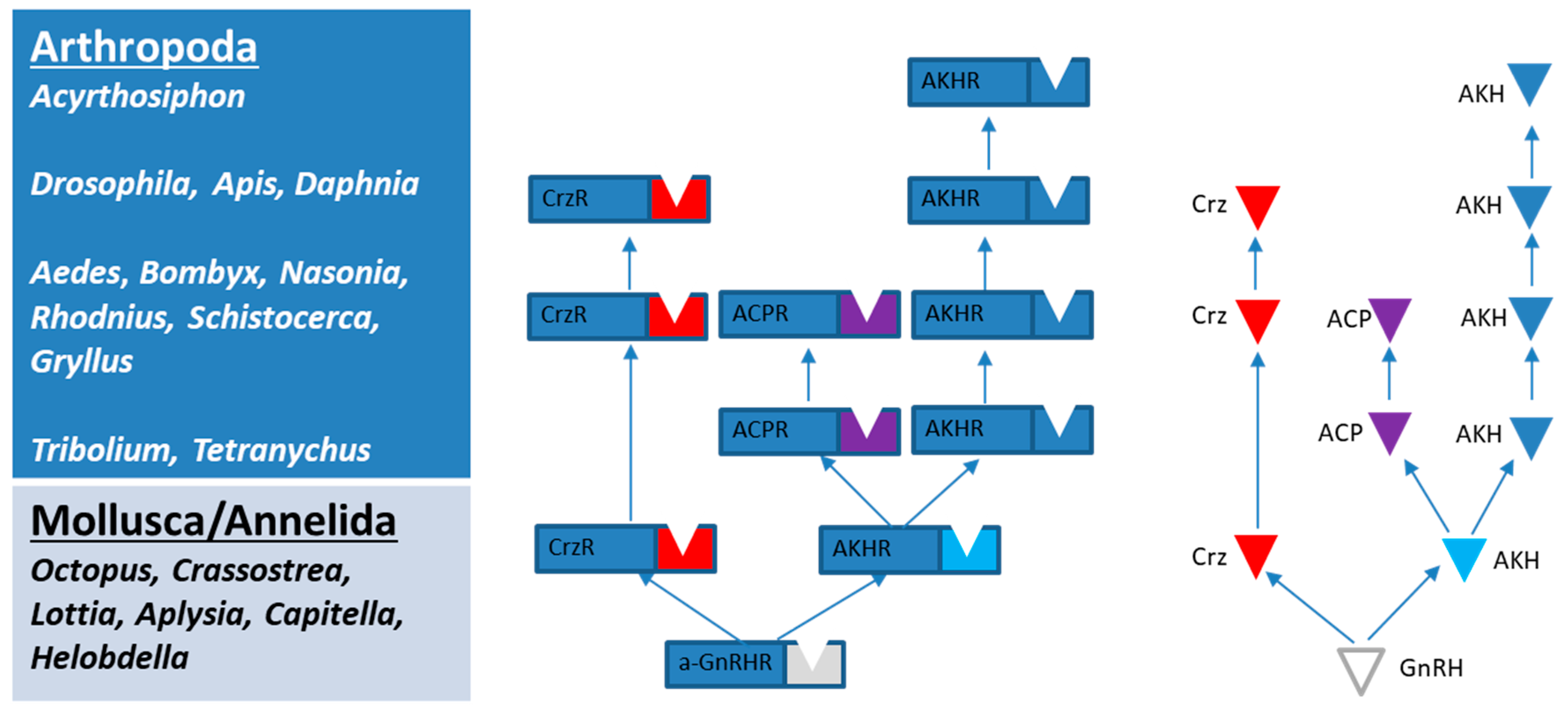
1. Introduction
2. Results and Discussion
2.1. Cloning of the Receptors
2.2. Phylogenetic Tree
2.3. Cell-Based Receptor Activity Assays
3. Materials and Methods
3.1. Molecular Cloning
3.2. Phylogenetic Analyses
3.3. Cell Culturing and Transfection
3.4. Calcium Reporter Assay
3.5. Synthetic Peptides
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stone, J.V.; Mordue, W.; Batley, K.E.; Morris, H.R. Structure of locust adipokinetic hormone, a neurohormone that regulates lipid utilisation during flight. Nature 1976, 263, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Gäde, G. Sequences of recently identified adipokinetic peptides: What do they tell us with respect to their hyperlipaemic activity in migratory locusts? Invertebr. Neurosci. 1997, 3, 217–222. [Google Scholar] [CrossRef]
- Gäde, G.; Auerswald, L. Mode of action of neuropeptides from the adipokinetic hormone family. Gen. Comp. Endocrinol. 2003, 132, 10–20. [Google Scholar] [CrossRef]
- Kodrík, D.; Krishnan, N.; Habuštová, O. Is the titer of adipokinetic peptides in Leptinotarsa decemlineata fed on genetically modified potatoes increased by oxidative stress? Peptides 2007, 28, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Večeřa, J.; Krishnan, N.; Alquicer, G.; Kodrík, D.; Socha, R. Adipokinetic hormone-induced enhancement of antioxidant capacity of Pyrrhocoris apterus hemolymph in response to oxidative stress. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 146, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Bharucha, K.N.; Tarr, P.; Zipursky, S.L. A glucagon-like endocrine pathway in Drosophila modulates both lipid and carbohydrate homeostasis. J. Exp. Biol. 2008, 211, 3103–3110. [Google Scholar] [CrossRef] [PubMed]
- Konuma, T.; Morooka, N.; Nagasawa, H.; Nagata, S. Knockdown of the adipokinetic hormone receptor increases feeding frequency in the two-spotted cricket Gryllus bimaculatus. Endocrinology 2012, 153, 3111–3122. [Google Scholar] [CrossRef] [PubMed]
- Caers, J.; Verlinden, H.; Zels, S.; Vandersmissen, H.P.; Vuerinckx, K.; Schoofs, L. More than two decades of research on insect neuropeptide GPCRs: An overview. Front. Endocrinol. 2012, 3, 151. [Google Scholar] [CrossRef] [PubMed]
- Zemanová, M.; Stašková, T.; Kodrík, D. Role of adipokinetic hormone and adenosine in the anti-stress response in Drosophila melanogaster. J. Insect Physiol. 2016, 91–92, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.; Hejníková, M.; Shaik, H.A.; Doležel, D.; Kodrík, D. Adipokinetic hormone activities in insect body infected by entomopathogenic nematode. J. Insect Physiol. 2017, 98, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Hauser, F.; Grimmelikhuijzen, C.J.P. Evolution of the AKH/corazonin/ACP/GnRH receptor superfamily and their ligands in the Protostomia. Gen. Comp. Endocrinol. 2014, 209, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Nässel, D.R.; Winther, Å.M.E. Drosophila neuropeptides in regulation of physiology and behavior. Prog. Neurobiol. 2010, 92, 42–104. [Google Scholar] [CrossRef] [PubMed]
- Gäde, G. Peptides of the adipokinetic hormone/red pigment-concentrating hormone family: A new take on biodiversity. Ann. N. Y. Acad. Sci. 2009, 1163, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Bednářová, A.; Kodrík, D.; Krishnan, N. Knockdown of adipokinetic hormone synthesis increases susceptibility to oxidative stress in Drosophila—A role for dFoxO? Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2015, 171, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Sajwan, S.; Sidorov, R.; Stašková, T.; Žaloudíková, A.; Takasu, Y.; Kodrík, D.; Zurovec, M. Targeted mutagenesis and functional analysis of adipokinetic hormone-encoding gene in Drosophila. Insect Biochem. Mol. Biol. 2015, 61, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Gáliková, M.; Diesner, M.; Klepsatel, P.; Hehlert, P.; Xu, Y.; Bickmeyer, I.; Predel, R.; Kühnlein, R.P. Energy homeostasis control in Drosophila adipokinetic hormone mutants. Genetics 2015, 201, 665–683. [Google Scholar] [CrossRef] [PubMed]
- Waterson, M.J.; Chung, B.Y.; Harvanek, Z.M.; Ostojic, I.; Alcedo, J.; Pletcher, S.D. Water sensor ppk28 modulates Drosophila lifespan and physiology through AKH signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 8137–8142. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hauser, F.; Skadborg, S.K.; Nielsen, S.V.; Kirketerp-Møller, N.; Grimmelikhuijzen, C.J.P. Adipokinetic hormones and their G protein-coupled receptors emerged in Lophotrochozoa. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Veenstra, J.A. The contribution of the genomes of a termite and a locust to our understanding of insect neuropeptides and neurohormones. Front. Physiol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Gäde, G.; Marco, H.G. The decapod red pigment-concentrating hormone (Panbo-RPCH) is the first identified neuropeptide of the order Plecoptera and is interpreted as homoplastic character state. Gen. Comp. Endocrinol. 2015, 221, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Lindemans, M.; Liu, F.; Janssen, T.; Husson, S.J.; Mertens, I.; Gäde, G.; Schoofs, L. Adipokinetic hormone signaling through the gonadotropin-releasing hormone receptor modulates egg-laying in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2009, 106, 1642–1647. [Google Scholar] [CrossRef] [PubMed]
- De Loof, A.; Lindemans, M.; Liu, F.; de Groef, B.; Schoofs, L. Endocrine archeology: Do insects retain ancestrally inherited counterparts of the vertebrate releasing hormones GnRH, GHRH, TRH, and CRF? Gen. Comp. Endocrinol. 2012, 177, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Roch, G.J.; Busby, E.R.; Sherwood, N.M. Evolution of GnRH: Diving deeper. Gen. Comp. Endocrinol. 2011, 171, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.W.; Gäde, G. Hormonal regulation of energy metabolism in insects as a driving force for performance. Integr. Comp. Biol. 2009, 49, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Hauser, F.; Søndergaard, L.; Grimmelikhuijzen, C.J. Molecular cloning, genomic organization and developmental regulation of a novel receptor from Drosophila melanogaster structurally related to gonadotropin-releasing hormone receptors for vertebrates. Biochem. Biophys. Res. Commun. 1998, 249, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Kim, Y.-J.J.; Adams, M.E. Identification of G protein-coupled receptors for Drosophila PRXamide peptides, CCAP, corazonin, and AKH supports a theory of ligand-receptor coevolution. Proc. Natl. Acad. Sci. USA 2002, 99, 11423–11428. [Google Scholar] [CrossRef] [PubMed]
- Staubli, F.; Jørgensen, T.J.D.; Cazzamali, G.; Williamson, M.; Lenz, C.; Søndergaard, L.; Roepstorff, P.; Grimmelikhuijzen, C.J.P. Molecular identification of the insect adipokinetic hormone receptors. Proc. Natl. Acad. Sci. USA 2002, 99, 3446–3451. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.K.; Hauser, F.; Cazzamali, G.; Williamson, M.; Grimmelikhuijzen, C.J.P. Cloning and characterization of the adipokinetic hormone receptor from the cockroach Periplaneta americana. Biochem. Biophys. Res. Commun. 2006, 343, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Wicher, D.; Agricola, H.J.; Söhler, S.; Gundel, M.; Heinemann, S.H.; Wollweber, L.; Stengl, M.; Derst, C. Differential receptor activation by cockroach adipokinetic hormones produces differential effects on ion currents, neuronal activity, and locomotion. J. Neurophysiol. 2005, 95, 2314–2325. [Google Scholar] [CrossRef] [PubMed]
- Belmont, M.; Cazzamali, G.; Williamson, M.; Hauser, F.; Grimmelikhuijzen, C.J.P. Identification of four evolutionarily related G protein-coupled receptors from the malaria mosquito Anopheles gambiae. Biochem. Biophys. Res. Commun. 2006, 344, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Huang, H.; Hua, R.; Li, G.; Yang, D.; Luo, J.; Zhang, C.; Shi, L.; Benovic, J.L.; Zhou, N. Molecular and functional characterization of adipokinetic hormone receptor and its peptide ligands in Bombyx mori. FEBS Lett. 2009, 583, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; He, X.; Deng, X.; Li, G.; Ying, G.; Sun, Y.; Shi, L.; Benovic, J.L.; Zhou, N. Bombyx adipokinetic hormone receptor activates extracellular signal-regulated kinase 1 and 2 via G protein-dependent PKA and PKC but β-arrestin-independent pathways. Biochemistry 2010, 49, 10862–10872. [Google Scholar] [CrossRef] [PubMed]
- Bil, M.; Timmermans, I.; Verlinden, H.; Huybrechts, R. Characterization of the adipokinetic hormone receptor of the anautogenous flesh fly, Sarcophaga crassipalpis. J. Insect Physiol. 2016, 89, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Caers, J.; Janssen, T.; van Rompay, L.; Broeckx, V.; van den Abbeele, J.; Gäde, G.; Schoofs, L.; Beets, I. Characterization and pharmacological analysis of two adipokinetic hormone receptor variants of the tsetse fly, Glossina morsitans morsitans. Insect Biochem. Mol. Biol. 2016, 70, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.K.; Stafflinger, E.; Schneider, M.; Hauser, F.; Cazzamali, G.; Williamson, M.; Kollmann, M.; Schachtner, J.; Grimmelikhuijzen, C.J.P. Discovery of a novel insect neuropeptide signaling system closely related to the insect adipokinetic hormone and corazonin hormonal systems. J. Biol. Chem. 2010, 285, 10736–10747. [Google Scholar] [CrossRef] [PubMed]
- Hauser, F.; Cazzamali, G.; Williamson, M.; Blenau, W.; Grimmelikhuijzen, C.J.P. A review of neurohormone GPCRs present in the fruitfly Drosophila melanogaster and the honey bee Apis mellifera. Prog. Neurobiol. 2006, 80, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Brent, C.S.; Fennern, E.; Amdam, G.V. Gustatory perception and fat body energy metabolism are jointly affected by vitellogenin and juvenile hormone in honey bees. PLoS Genet. 2012, 8. [Google Scholar] [CrossRef] [PubMed]
- Mayack, C.; Natsopoulou, M.E.; McMahon, D.P. Nosema ceranae alters a highly conserved hormonal stress pathway in honeybees. Insect Mol. Biol. 2015, 24, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Bordier, C.; Suchail, S.; Pioz, M.; Devaud, J.M.; Collet, C.; Charreton, M.; le Conte, Y.; Alaux, C. Stress response in honeybees is associated with changes in task-related physiology and energetic metabolism. J. Insect Physiol. 2017, 98, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Sturm, S.; Ramesh, D.; Brockmann, A.; Neupert, S.; Predel, R. Agatoxin-like peptides in the neuroendocrine system of the honey bee and other insects. J. Proteom. 2016, 132, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, C.; Merzendorfer, H.; Gäde, G. The adipokinetic hormone system in Culicinae (Diptera: Culicidae): Molecular identification and characterization of two adipokinetic hormone (AKH) precursors from Aedes aegypti and Culex pipiens and two putative AKH receptor variants from A. aegypti. Insect Biochem. Mol. Biol. 2009, 39, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Zandawala, M.; Hamoudi, Z.; Lange, A.B.; Orchard, I. Adipokinetic hormone signalling system in the Chagas disease vector, Rhodnius prolixus. Insect Mol. Biol. 2015, 24, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Jedličková, V.; Jedlička, P.; Lee, H.J. Characterization and expression analysis of adipokinetic hormone and its receptor in eusocial aphid Pseudoregma bambucicola. Gen. Comp. Endocrinol. 2015, 223, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbour-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The rapid generation of mutation data matrices from protein sequences. Bioinformatics 1992, 8, 275–282. [Google Scholar] [CrossRef]
- Zandawala, M.; Tian, S.; Elphick, M.R. The evolution and nomenclature of GnRH-type and corazonin-type neuropeptide signaling systems. Gen. Comp. Endocrinol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Verlinden, H.; Gijbels, M.; Lismont, E.; Lenaerts, C.; Vanden Broeck, J.; Marchal, E. The pleiotropic allatoregulatory neuropeptides and their receptors: A mini-review. J. Insect Physiol. 2015, 80. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.J.; Fukumura, K.; Nagata, S. Effects of adipokinetic hormone and its related peptide on maintaining hemolymph carbohydrate and lipid levels in the two-spotted cricket, Gryllus bimaculatus. Biosci. Biotechnol. Biochem. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Hummon, A.B.; Richmond, T.A.; Verleyen, P.; Baggerman, G.; Huybrechts, J.; Ewing, M.A.; Vierstraete, E.; Rodriguez-Zas, S.L.; Schoofs, L.; Robinson, G.E.; et al. From the genome to the proteome: Uncovering peptides in the Apis brain. Science 2006, 314, 647–649. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, G.M.; Robinson, G.E.; Gibbs, R.A.; Worley, K.C.; Evans, J.D.; Maleszka, R.; Robertson, H.M.; Weaver, D.B.; Beye, M.; Bork, P.; et al. Insights into social insects from the genome of the honeybee Apis mellifera. Nature 2006, 443, 931–949. [Google Scholar] [CrossRef]
- Caers, J.; Peeters, L.; Janssen, T.; De Haes, W.; Gäde, G.; Schoofs, L. Structure-activity studies of Drosophila adipokinetic hormone (AKH) by a cellular expression system of dipteran AKH receptors. Gen. Comp. Endocrinol. 2012, 177, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Marco, H.G.; Verlinden, H.; Vanden Broeck, J.; Gäde, G. Characterisation and pharmacological analysis of a crustacean G protein-coupled receptor: The red pigment-concentrating hormone receptor of Daphnia pulex. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, J.G.C.; Pandit, A.A.; Zandawala, M.; Nässel, D.R.; Davies, S.A.; Dow, J.A.T. DINeR: Database for Insect Neuropeptide Research. Insect Biochem. Mol. Biol. 2017, 86, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Zandawala, M.; Haddad, A.S.; Hamoudi, Z.; Orchard, I. Identification and characterization of the adipokinetic hormone/corazonin-related peptide signaling system in Rhodnius prolixus. FEBS J. 2015, 282, 3603–3617. [Google Scholar] [CrossRef] [PubMed]
- Poels, J.; Van Loy, T.; Vandersmissen, H.P.; Van Hiel, B.; Van Soest, S.; Nachman, R.J.; Vanden Broeck, J. Myoinhibiting peptides are the ancestral ligands of the promiscuous Drosophila sex peptide receptor. Cell. Mol. Life Sci. 2010, 67, 3511–3522. [Google Scholar] [CrossRef] [PubMed]
- Vandersmissen, H.P.; Nachman, R.J.; Vanden Broeck, J. Sex peptides and MIPs can activate the same G protein-coupled receptor. Gen. Comp. Endocrinol. 2012, 188, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Van Hiel, M.B.; Van Loy, T.; Poels, J.; Vandersmissen, H.P.; Verlinden, H.; Badisco, L.; Vanden Broeck, J. Neuropeptide Receptors as Possible Targets for Development of Insect Pest Control Agents. Adv. Exp. Med. Biol. 2010, 692, 211–226. [Google Scholar] [PubMed]
- Vanden Broeck, J.; Schoofs, L.; De Loof, A. Insect neuropeptides and their receptors: New leads for medical and agricultural applications. Trends Endocrinol. Metable 1997, 8, 321–326. [Google Scholar] [CrossRef]
- Verlinden, H.; Vleugels, R.; Zels, S.; Dillen, S.; Lenaerts, C.; Crabbé, K.; Spit, J.; Vanden Broeck, J. Receptors for neuronal or endocrine signalling molecules as potential targets for the control of insect pests. Adv. Insect Phys. 2014, 46, 167–303. [Google Scholar] [CrossRef]



| Neuropeptide Name | Amino Acid Sequence |
|---|---|
| AedaeAKH = SchgrAKH-III | pQLTF--TPSW--amide |
| DromeAKH | pQLTF--SPDW--amide |
| GlomoAKH | pQLTF--SPGW--amide |
| TricaAKH | pQLNF--STDW--amide |
| HylabAKH = PeramCAH-I | pQVNF--SPNW--amide |
| GrybiAKH | pQVNF--STGW--amide |
| SchgrAKH-II = ApimeAKH | pQLNF--STGW--amide |
| AcypiAKH | pQVNF--TPTWGQamide |
| AedaeACP | pQVTF--SRDWNAamide |
| LocmiCrz | pQ-TFQYSHGWTNamide |
| Species | Forward Primer | Reverse Primer | Tm |
|---|---|---|---|
| A. pisum | CACCATGGAAGTGATGGATTCTGACGCC | GTTAGTTCACAAATTGTACCAGATTACC | 63 |
| A. mellifera | CACCATGGAAAGCAGTATAAAAATAATCACC | TTAATCAAGAAGTTTGAGCGATAATGATAATGG | 66 |
| A. aegypti | CACCATGTCAAATGCAATTTTGAAAACAG | TCAGACTGGTTTGATTGCACAT | 61 |
| D. melanogaster | CACCATGGCAAAAGTAGCTGAG | TTACTTCTGGCGGATCGG | 64 |
| H. abietis | CACCATGAAGGAACTAAAAGATTCCCC | TCATTTTTCGATGTTGTTTCTTTGTAA | 60 |
| S. gregaria | CACCATGGCGGGCCTCGAATCGG | TCACCTTGCCTCCGTTGTTCTG | 59 |
| T. castaneum | CACCATGAACTTTAGTGAGACTCTTTGGA | CTATTCTAAAGTCTTCAGTGATATCTCA | 62 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchal, E.; Schellens, S.; Monjon, E.; Bruyninckx, E.; Marco, H.G.; Gäde, G.; Vanden Broeck, J.; Verlinden, H. Analysis of Peptide Ligand Specificity of Different Insect Adipokinetic Hormone Receptors. Int. J. Mol. Sci. 2018, 19, 542. https://doi.org/10.3390/ijms19020542
Marchal E, Schellens S, Monjon E, Bruyninckx E, Marco HG, Gäde G, Vanden Broeck J, Verlinden H. Analysis of Peptide Ligand Specificity of Different Insect Adipokinetic Hormone Receptors. International Journal of Molecular Sciences. 2018; 19(2):542. https://doi.org/10.3390/ijms19020542
Chicago/Turabian StyleMarchal, Elisabeth, Sam Schellens, Emilie Monjon, Evert Bruyninckx, Heather G. Marco, Gerd Gäde, Jozef Vanden Broeck, and Heleen Verlinden. 2018. "Analysis of Peptide Ligand Specificity of Different Insect Adipokinetic Hormone Receptors" International Journal of Molecular Sciences 19, no. 2: 542. https://doi.org/10.3390/ijms19020542
APA StyleMarchal, E., Schellens, S., Monjon, E., Bruyninckx, E., Marco, H. G., Gäde, G., Vanden Broeck, J., & Verlinden, H. (2018). Analysis of Peptide Ligand Specificity of Different Insect Adipokinetic Hormone Receptors. International Journal of Molecular Sciences, 19(2), 542. https://doi.org/10.3390/ijms19020542





