Efficacy and Safety of Platelet-Rich Plasma Injections for the Treatment of Female Sexual Dysfunction and Stress Urinary Incontinence: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Acquisition and Risk of Bias
2.4. Data Synthesis and Statistical Analysis
3. Results
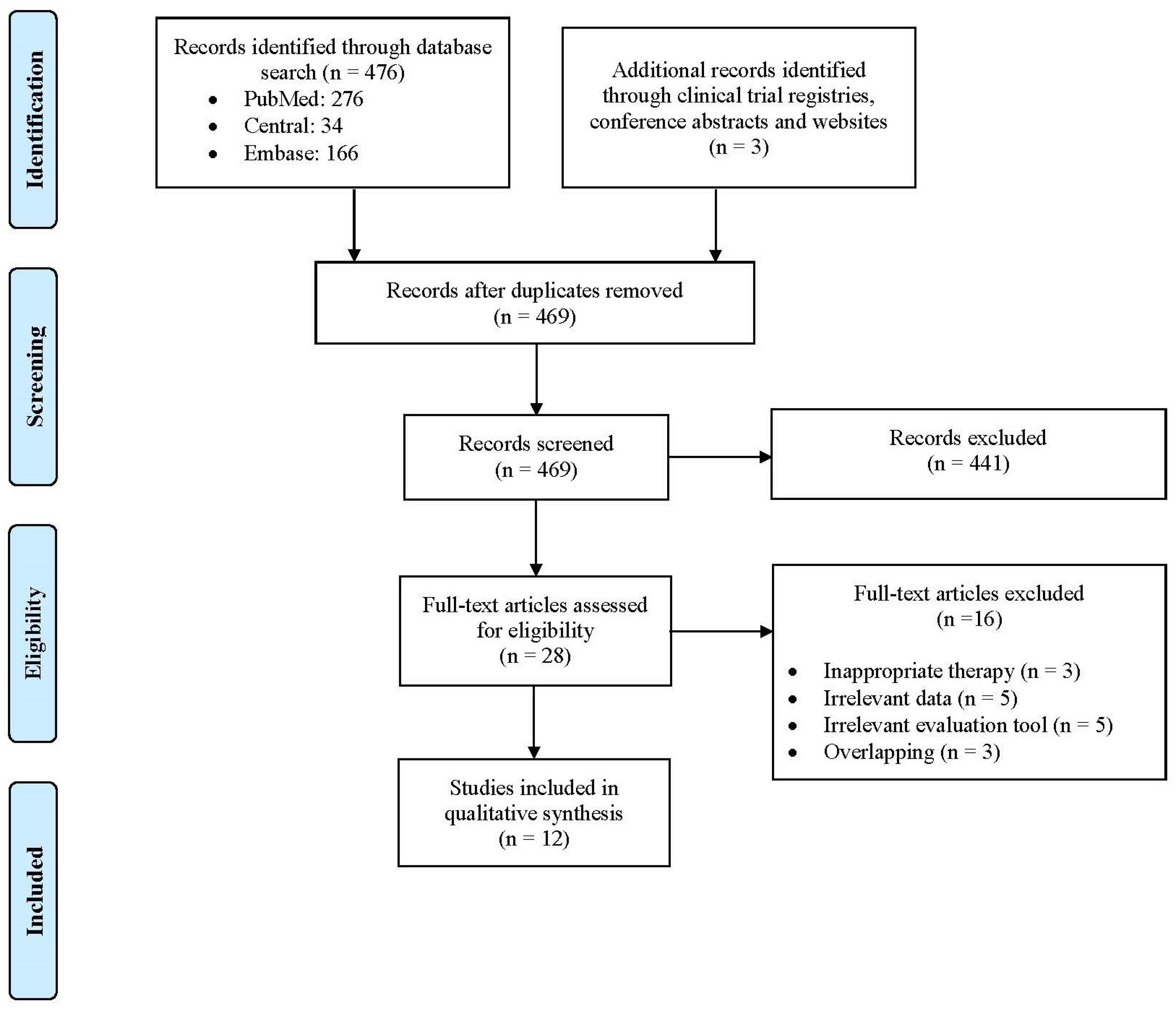
3.1. Study Selection, Study Characteristics and Quality Assessment
3.2. PRP Preparation and Application Technique
3.3. PRP Injections Effect Estimate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chow, P.-M.; Chuang, Y.-C.; Hsu, K.C.P.; Shen, Y.-C.; Liu, S.-P. Impact of Female Stress Urinary Incontinence on Quality of Life, Mental Health, Work Limitation, and Healthcare Seeking in China, Taiwan, and South Korea (LUTS Asia): Results from a Cross-Sectional, Population-Based Study. Int. J. Women’s Health 2022, 14, 1871–1880. [Google Scholar] [CrossRef]
- Alidost, F.; Pakzad, R.; Dolatian, M.; Abdi, F. Sexual Dysfunction among Women of Reproductive Age: A Systematic Review and Meta-Analysis. Int. J. Reprod. Biomed. 2021. [Google Scholar] [CrossRef]
- Basson, R.; Berman, J.; Burnett, A.; Derogatis, L.; Ferguson, D.; Fourcroy, J.; Goldstein, I.; Graziottin, A.; Heiman, J.; Laan, E.; et al. Report of the International Consensus Development Conference on Female Sexual Dysfunction: Definitions and Classifications. J. Urol. 2000, 163, 888–893. [Google Scholar] [CrossRef]
- Levine, K.B.; Williams, R.E.; Hartmann, K.E. Vulvovaginal Atrophy Is Strongly Associated with Female Sexual Dysfunction among Sexually Active Postmenopausal Women. Menopause 2008, 15, 661–666. [Google Scholar] [CrossRef]
- Vitale, S.G.; La Rosa, V.L.; Rapisarda, A.M.C.; Laganà, A.S. Sexual Life in Women with Stress Urinary Incontinence. Oman Med. J. 2017, 32, 174–175. [Google Scholar] [CrossRef]
- Pinheiro Sobreira Bezerra, L.R.; Britto, D.F.; Ribeiro Frota, I.P.; Lira Do Nascimento, S.; Morais Brilhante, A.V.; Lucena, S.V.; Moura Brasil, D.M. The Impact of Urinary Incontinence on Sexual Function: A Systematic Review. Sex. Med. Rev. 2020, 8, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Eppley, B.L.; Pietrzak, W.S.; Blanton, M. Platelet-Rich Plasma: A Review of Biology and Applications in Plastic Surgery: Plast. Reconstr. Surg. 2006, 118, 147e–159e. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Cheng, L.; Cui, X.; Lei, X.; Tang, J.; Cheng, B. Application of Standardized Platelet-rich Plasma in Elderly Patients with Complex Wounds. Wound Rep. Reg. 2019, 27, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Streit-Ciećkiewicz, D.; Kołodyńska, A.; Futyma-Gąbka, K.; Grzybowska, M.; Gołacki, J.; Futyma, K. Platelet Rich Plasma in Gynecology—Discovering Undiscovered—Review. Int. J. Environ. Res. Public Health 2022, 19, 5284. [Google Scholar] [CrossRef]
- Dawood, A.S.; Salem, H.A. Current Clinical Applications of Platelet-Rich Plasma in Various Gynecological Disorders: An Appraisal of Theory and Practice. Clin. Exp. Reprod. Med. 2018, 45, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Prodromidou, A.; Zacharakis, D.; Athanasiou, S.; Protopapas, A.; Michala, L.; Kathopoulis, N.; Grigoriadis, T. The Emerging Role on the Use of Platelet-Rich Plasma Products in the Management of Urogynaecological Disorders. Surg. Innov. 2022, 29, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Sukgen, G.; Ellibeş Kaya, A.; Karagün, E.; Çalışkan, E. Platelet-Rich Plasma Administration to the Lower Anterior Vaginal Wall to Improve Female Sexuality Satisfaction. J. Turk. Soc. Obstet. Gynecol. 2020, 16, 228–234. [Google Scholar] [CrossRef]
- Jiang, Y.-H.; Lee, P.-J.; Kuo, H.-C. Therapeutic Efficacy of Urethral Sphincter Injections of Platelet-Rich Plasma for the Treatment of Stress Urinary Incontinence Due to Intrinsic Sphincter Deficiency: A Proof-of-Concept Clinical Trial. Int. Neurourol. J. 2021, 25, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2020. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Herbenick, D.; Reece, M. Original research—outcomes assessment: Development and Validation of the Female Genital Self-Image Scale. J. Sex. Med. 2010, 7, 1822–1830. [Google Scholar] [CrossRef]
- DeRogatis, L.; Clayton, A.; Lewis-D’Agostino, D.; Wunderlich, G.; Fu, Y. Validation of the Female Sexual Distress Scale-Revised for Assessing Distress in Women with Hypoactive Sexual Desire Disorder. J. Sex. Med. 2008, 5, 357–364. [Google Scholar] [CrossRef]
- Giraldi, A.; Rellini, A.; Pfaus, J.G.; Bitzer, J.; Laan, E.; Jannini, E.A.; Fugl-Meyer, A.R. Questionnaires for Assessment of Female Sexual Dysfunction: A Review and Proposal for a Standardized Screener. J. Sex. Med. 2011, 8, 2681–2706. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M. Society and the Adolescent Self-Image; Princeton University Press: Princeton, NJ, USA, 1965. [Google Scholar] [CrossRef]
- Bachmann, G. Urogenital Ageing: An Old Problem Newly Recognized. Maturitas 1995, 22, S1–S5. [Google Scholar] [CrossRef]
- Fritel, X.; Van Den Heuvel, E.; Wagg, A.; Ragot, S.; Tannenbaum, C. Predicting Response to a Community-based Educational Workshop on Incontinence among Community-dwelling Older Women: Post Hoc Analysis of the CACTUS-D Trial. Neurourol. Urodyn. 2021, 40, 705–713. [Google Scholar] [CrossRef]
- Klovning, A.; Avery, K.; Sandvik, H.; Hunskaar, S. Comparison of Two Questionnaires for Assessing the Severity of Urinary Incontinence: The ICIQ-UI SF versus the Incontinence Severity Index. Neurourol. Urodyn. 2009, 28, 411–415. [Google Scholar] [CrossRef]
- Shumaker, S.A.; Wyman, J.F.; Uebersax, J.S.; McClish, D.; Fantl, J.A.; The Continence Program in Women (CPW) Research Group. Health-Related Quality of Life Measures for Women with Urinary Incontinence: The Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Qual. Life Res. 1994, 3, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Swift, S. Test-Retest Reliability of the Cough Stress Test in the Evaluation of Urinary Incontinence. Obstet. Gynecol. 1999, 94, 99–102. [Google Scholar] [CrossRef]
- Krhut, J.; Zachoval, R.; Smith, P.P.; Rosier, P.F.W.M.; Valanský, L.; Martan, A.; Zvara, P. Pad Weight Testing in the Evaluation of Urinary Incontinence: Pad Weight Testing. Neurourol. Urodynam. 2014, 33, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Lo, C.K.-L.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing Reviewers’ to Authors’ Assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef]
- Atallah, Á.N. Efficacy and Effectiveness of Treatment. Sao Paulo Med. J. 1996, 114, 1195. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stephenson, K.R.; Meston, C.M. When Are Sexual Difficulties Distressing for Women? The Selective Protective Value of Intimate Relationships. J. Sex. Med. 2010, 7, 3683–3694. [Google Scholar] [CrossRef] [PubMed]
- Erekson, E.A.; Li, F.-Y.; Martin, D.K.; Fried, T.R. Vulvovaginal Symptoms Prevalence in Postmenopausal Women and Relationship to Other Menopausal Symptoms and Pelvic Floor Disorders. Menopause 2016, 23, 368–375. [Google Scholar] [CrossRef]
- Nandy, S.; Ranganathan, S. Urge Incontinence. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Wrenn, K. Dysuria, Frequency, and Urgency. In Clinical Methods: The History, Physical, and Laboratory Examinations; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990. [Google Scholar]
- Shermadou, E.S.; Rahman, S.; Leslie, S.W. Anatomy, Abdomen and Pelvis: Bladder. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Apolikhina, I.A.; Sokolova, A.V.; Saidova, A.S.; Gorbunova, E.A. Autologous Platelet-Rich Plasma Combined with Hyaluronic Acid Is a New Method of Minimally Invasive Treatment of Stress Urinary Incontinence in Women. Med. Sov. 2018, 16–20. [Google Scholar] [CrossRef]
- Athanasiou, S.; Kalantzis, C.; Zacharakis, D.; Kathopoulis, N.; Pontikaki, A.; Grigoriadis, T. The Use of Platelet-Rich Plasma as a Novel Nonsurgical Treatment of the Female Stress Urinary Incontinence: A Prospective Pilot Study. Female Pelvic Med. Reconstr. Surg. 2021, 27, e668–e672. [Google Scholar] [CrossRef]
- Amirzargar, M.A.; Jafari, M.; Mohamadi, B.; Moradi, A. Comparison of Platelet Rich Plasma in Combination with Autologous Fat Injection Versus Injection of Autologous Fat in Bladder Neck for Treatment of Stress Urinary Incontinence of Women. J. Res. Urol. 2016, 1, 12–17. [Google Scholar]
- Long, C.-Y.; Lin, K.-L.; Shen, C.-R.; Ker, C.-R.; Liu, Y.-Y.; Loo, Z.-X.; Hsiao, H.-H.; Lee, Y.-C. A Pilot Study: Effectiveness of Local Injection of Autologous Platelet-Rich Plasma in Treating Women with Stress Urinary Incontinence. Sci. Rep. 2021, 11, 1584. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-H.; Kuo, H.-C. The Efficacy and Mid-Term Durability of Urethral Sphincter Injections of Platelet-Rich Plasma in Treatment of Female Stress Urinary Incontinence. Front. Pharmacol. 2022, 13, 847520. [Google Scholar] [CrossRef]
- Samy Tahoon, A.; El-Din Hussein Salem, H.; Anwar Abdo Mousa, A. The Role of Platelet Rich Plasma Injections in Cases of Stress Incontinence. Qeios 2022, preprint. [Google Scholar] [CrossRef]
- Daneshpajooh, A.; Mirzaei, M.; Farsinejad, A.; NaghibzadehTahami, A.; Eslami, A. The Effect of Periurethral Injection of Pure Platelet-Rich Plasma in the Treatment of Urinary Incontinence in Female Patients: A Randomized Clinical Trial. J. Kerman Univ. Med. Sci. 2021, 28, 330–337. [Google Scholar] [CrossRef]
- Runels, C. A Pilot Study of the Effect of Localized Injections of Autologous Platelet Rich Plasma (PRP) for the Treatment of Female Sexual Dysfunction. J. Women’s Health Care 2014, 3, 169. [Google Scholar] [CrossRef]
- Romashchenko, O.; Grygorenko, V.; Biloholovska, V.; Babych, O.; Melnykov, S.; Yakovenko, L. Use of Platelet-Rich Plasma for the Treatment of Dyspareunia in Postmenopausal Women. J. Sex. Med. 2022, 19, S34. [Google Scholar] [CrossRef]
- Saleh, D.M.; Abdelghani, R. Clinical Evaluation of Autologous Platelet Rich Plasma Injection in Postmenopausal Vulvovaginal Atrophy: A Pilot Study. J. Cosmet. Dermatol. 2022, 21, 4269–4275. [Google Scholar] [CrossRef]
- Hersant, B.; SidAhmed-Mezi, M.; Belkacemi, Y.; Darmon, F.; Bastuji-Garin, S.; Werkoff, G.; Bosc, R.; Niddam, J.; Hermeziu, O.; La Padula, S.; et al. Efficacy of Injecting Platelet Concentrate Combined with Hyaluronic Acid for the Treatment of Vulvovaginal Atrophy in Postmenopausal Women with History of Breast Cancer: A Phase 2 Pilot Study. Menopause 2018, 25, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Einarsson, J.I.; Jonsdottir, K.; Mandle, R. Use of Autologous Platelet Gel in Female Pelvic Organ Prolapse Surgery: A Feasibility Study. J. Minim. Invasive Gynecol. 2009, 16, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Shirvan, M.K.; Alamdari, D.H.; Ghoreifi, A. A Novel Method for Iatrogenic Vesicovaginal Fistula Treatment: Autologous Platelet Rich Plasma Injection and Platelet Rich Fibrin Glue Interposition. J. Urol. 2013, 189, 2125–2129. [Google Scholar] [CrossRef]
- Castellani, D.; Valloni, A.; Piccirilli, A.; Paradiso Galatioto, G.; Vicentini, C. An Innovative Approach to Treating Vaginal Mesh Exposure after Abdominal Sacral Colpopexy: Endoscopic Resection of Mesh and Platelet-Rich Plasma; Initial Experience in Three Women. Int. Urogynecol. J. 2017, 28, 325–327. [Google Scholar] [CrossRef]
- Lee, P.-J.; Jiang, Y.-H.; Kuo, H.-C. A Novel Management for Postprostatectomy Urinary Incontinence: Platelet-Rich Plasma Urethral Sphincter Injection. Sci. Rep. 2021, 11, 5371. [Google Scholar] [CrossRef] [PubMed]
- Poulios, E.; Mykoniatis, I.; Pyrgidis, N.; Zilotis, F.; Kapoteli, P.; Kotsiris, D.; Kalyvianakis, D.; Hatzichristou, D. Platelet-Rich Plasma (PRP) Improves Erectile Function: A Double-Blind, Randomized, Placebo-Controlled Clinical Trial. J. Sex. Med. 2021, 18, 926–935. [Google Scholar] [CrossRef]
- Alkandari, M.H.; Touma, N.; Carrier, S. Platelet-Rich Plasma Injections for Erectile Dysfunction and Peyronie’s Disease: A Systematic Review of Evidence. Sex. Med. Rev. 2022, 10, 341–352. [Google Scholar] [CrossRef]
- Hilliges, M.; Falconer, C.; Ekman-Ordeberg, G.; Johansson, O. Innervation of the Human Vaginal Mucosa as Revealed by PGP 9.5 Immunohistochemistry. Cells Tissues Organs 1995, 153, 119–126. [Google Scholar] [CrossRef]
- Li, T.; Liao, Q.; Zhang, H.; Gao, X.; Li, X.; Zhang, M. Anatomic Distribution of Nerves and Microvascular Density in the Human Anterior Vaginal Wall: Prospective Study. PLoS ONE 2014, 9, e110239. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.L.; Izeta, A.; Herrera-Imbroda, B.; Amend, B.; Brinchmann, J.E. Cell Therapy for Stress Urinary Incontinence. Tissue Eng. Part B Rev. 2015, 21, 365–376. [Google Scholar] [CrossRef]
- Poulios, E.; Mykoniatis, I.; Pyrgidis, N.; Kalyvianakis, D.; Hatzichristou, D. Platelet-Rich Plasma for the Treatment of Erectile Dysfunction: A Systematic Review of Preclinical and Clinical Studies. Sex. Med. Rev. 2023, 11, 359–368. [Google Scholar] [CrossRef] [PubMed]
- National Clinical Practice Guideline Assessment and Management of Stress Urinary Incontinence in Women. Available online: https://www.hse.ie/eng/about/who/acute-hospitals-division/woman-infants/clinical-guidelines/assessment-and-management-of-stress-urinary-incontinence.pdf (accessed on 25 October 2023).
- Krakowsky, Y.; Grober, E.D. A Practical Guide to Female Sexual Dysfunction: An Evidence-Based Review for Physicians in Canada. Can. Urol. Assoc. J. 2018, 12, 211–216. [Google Scholar] [CrossRef]
- Apolikhina, I.; Gorbunova, E.; Saidova, A.; Sukhikh, G. Prp injections in the treatment for stress urinary incontinence. Int. Urogynecol. J. 2017, 28, S233–S234. [Google Scholar]
- Kalantzis, C.; Athanasiou, S.; Zacharakis, D.; Petrakis, V.; Pontikaki, A.; Grigoriadis, T. The role of platelet rich plasma (PRP) for treatment of stress urinary incontinence. Female Pelvic Med. Reconstr. Surg. 2021, 27, E664. [Google Scholar]
- Manin, E.; Taraschi, G.; Berndt, S.; Martinez de Tejada, B.; Abdulcadir, J. Autologous Platelet-Rich Plasma for Clitoral Reconstruction: A Case Study. Arch. Sex. Behav. 2022, 51, 673–678. [Google Scholar] [CrossRef]
- Al-Hamadani, I.T.; Shehab E mahmod, N.A. A. Comparative Changes in Sexual Dysfunction of Married Women after Colpoperineorrhaphy Versus Colpoperineorrhaphy with Additional Platelet Rich Plasma Injection. Indian J. Foren. Med. Toxicol. 2019, 13, 314. [Google Scholar] [CrossRef]
- Luksenburg, A.; Barcia, J.J.; Sergio, R.; Fernandez, S.; Pelosi, M.A.; Pelosi, M.A. Stress Urinary Incontinence: Treatment With Platelet-Rich-Plasma Injection and Placement of Polydioxanone Threads—A Pilot Study. Am. J. Cosmet. Surg 2022, 39, 76–84. [Google Scholar] [CrossRef]
- Medel, S.; Alarab, M.; Kufaishi, H.; Drutz, H.; Shynlova, O. Attachment of Primary Vaginal Fibroblasts to Absorbable and Nonabsorbable Implant Materials Coated With Platelet-Rich Plasma: Potential Application in Pelvic Organ Prolapse Surgery. Female Pelvic Med. Reconstr. Surg. 2015, 21, 190–197. [Google Scholar] [CrossRef]
- Aguilar, P.; Hersant, B.; SidAhmed-Mezi, M.; Bosc, R.; Vidal, L.; Meningaud, J.P. Novel technique of vulvo-vaginal rejuvenation by lipofilling and injection of combined platelet-rich-plasma and hyaluronic acid: A case-report. SpringerPlus 2016, 5, 1184. [Google Scholar] [CrossRef]
- Jiang, Y.; Lee, P.; Lee, Y.; Kuo, H. Urethral sphincter injections of platelet-rich plasma (PRP) in treatment of urinary incontinence due to intrinsic sphincteric deficiency refractory to conventional treatment. Neurourol. Urodyn. 2019, 38, S262–S263. [Google Scholar]
- Khera, B. Efficacy of PRP in genitourinary syndrome of menopause: A prospective study. BJOG: Int. J. Obstet. Gynaecol. 2021, 128, 214–215. [Google Scholar]
- Willison, F.B.; Nguyen, T. Innovative treatment of uterovaginal prolapse with autologous membrane from platelet rich plasma. BJOG: Int. J. Obstet. Gynaecol. 2021, 126, 235. [Google Scholar]
- Behnia-Willison, F.; Pour, N.R.; Mohamadi, B.; Willison, N.; Rock, M.; Holten, I.W.; O’Shea, R.; Miller, J. Use of Platelet-rich Plasma for Vulvovaginal Autoimmune Conditions Like Lichen Sclerosus. Plast. Reconstr. Surg. Glob. Open. 2016, 4, e1124. [Google Scholar] [CrossRef]
- Goldstein, A.T.; King, M.; Runels, C.; Gloth, M.; Pfau, R. Intradermal injection of autologous platelet-rich plasma for the treatment of vulvar lichen sclerosus. J. Am. Acad. Dermatol. 2017, 76, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Park, E.S.; Kim, T.H. Rejuvenation Using Platelet-rich Plasma and Lipofilling for Vaginal Atrophy and Lichen Sclerosus. J. Menopausal. Med. 2017, 23, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Posey, L.K.; Runels, C. In office surgery and use of platelet rich plasma for treatment of vulvar lichen sclerosus to alleviate painful sexual intercourse. J. Low. Genit. Tract Dis. 2017, 21, S14. [Google Scholar]
| Study | Type of Study | Disorder | Participants/Participants Receiving PRP (n) | PRP Preparation Technique | Injected Area | PRP Injection Technique | Effect Estimate | Follow-Up |
|---|---|---|---|---|---|---|---|---|
| Amirzargar et al., 2016 [36] | Prospective single-arm | SUI | 30/30 | n/a | Vagina | 12 mL; once | Incontinence score based on questionnaire—17 ± 1.8 → 5.9 ± 2.5 | No |
| Apolikhina et al., 2018 [34] | Prospective single-arm | SUI | 19/19 | RegenKit® | Urethra | 6 mL. 12 patients—2 times in 6 months; 6 patients—once; 1 patient—3 times in 9 months every 3 months | The cough stress test was negative in 18 patients | 12 months |
| Athanasiou et al., 2021 [35] | Prospective single-arm | SUI | 20/20 | RegenKit® | Anterior vaginal wall, periurethral area | 5.5 mL; twice in 4–6 weeks | ICIQ-FLUTS—18 ± 9.5 → 12 ± 8.2; 1 h pad test—15 ± 7.9 g → 6.2 ± 3.8 g | No |
| Chiang et al., 2022 [38] | Prospective single-arm | SUI | 26/26 | n/a | Urethral sphincter | 5 mL; 4 times in 3 months monthly | UDI-6—5.1 ± 2.3 → 3.2 ± 2.5 | 12 months |
| Daneshpajooh et al., 2021 [40] | RCT (comparator: midurethral sling procedure) | SUI | 20/10 | n/a | Urethra | 3 mL. 7 patients—once; 2 patients—twice in 1 month; 1 patient—3 times in 2 months monthly | ICIQ-SF—18 ± 1.9 → 8.0 ± 6.8; UDI-6—12 ± 2.5 → 6.6 ± 5.7. The cough stress test—Positive: 100%, → Positive: 30% | 3 months |
| Hersant et al., 2018 [44] | Prospective single-arm | FSD | 20/20 | RegenKit® | Posterior wall of the vagina, posterior wall of the introitus | 2 mL, once | VHI—11 ± 2.1 → 19 ± 3.8; FSDS-R—36 ± 2.5 → 30 ± 2.5 | 6 months |
| Long et al., 2021 [37] | Prospective single-arm | SUI | 20/20 | RegenKit® | Anterior vaginal mucosa | 5 mL; 4 times in 3 months monthly | ICIQ-SF—12 ± 3 → 7.3 ± 4.3; UDI-6—39 ± 14 → 28 ± 17 | 6 months |
| Romashchenko et al., 2022 [42] | Prospective single-arm | FSD | 52/52 | n/a | Paraurethral zone, introitus vagina, vagina | 6 mL; twice in 21–22 days | Vsmax—1.6–2.3 cm/sec at rest and 3.1–4.1 cm/sec in 30 min after video-erotic stimulation → 3.7–5.8 cm/sec at rest and 5.3–8.5 cm/sex after the stimulation | No |
| Runels et al., 2014 [41] | Prospective single-arm | FSD | 11/11 | RegenKit®, TruPRP® | Anterior vaginal wall, clitoris | 5 mL once | FSFI—24 → 30; FSDS-R—17 → 7.3 | No |
| Saleh et al., 2022 [43] | Prospective single-arm | FSD | 37/37 | RegenKit® | Posterior vaginal wall, vulva (labia majora, labia minora and vestibular fossa) | 4 and 8 mL, twice in 1 month | VHI—12 ± 2.7 → 17 ± 3.9 | 1 month |
| Sukgen et al., 2019 [12] | Retrospective single-arm | FSD | 52/52 | n/a | Anterior vaginal wall, clitoris, paraurethral vaginal wall. | 2 mL, 5 times every 4 weeks for 4 months | FSFI—14 ± 3.8 → 28 ± 4.8; FGSIS—17 ± 5.6 → 24 ± 2.2; FSDS-R—19 ± 12 → 11 ± 1.9; RSES—21 ± 6.1 → 22 ± 5.7 | 6 months |
| Tahoon et al., 2022 [39] | Prospective single-arm | SUI | 20/20 | n/a | Anterior vaginal wall, paraurethral vaginal wall | 4 mL once | ICIQ-SF—12 ± 2.9 → 5 ± 1.4; UDI-6—35 ± 6.9 → 16 ± 4.6 | 3 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dankova, I.; Pyrgidis, N.; Tishukov, M.; Georgiadou, E.; Nigdelis, M.P.; Solomayer, E.-F.; Marcon, J.; Stief, C.G.; Hatzichristou, D. Efficacy and Safety of Platelet-Rich Plasma Injections for the Treatment of Female Sexual Dysfunction and Stress Urinary Incontinence: A Systematic Review. Biomedicines 2023, 11, 2919. https://doi.org/10.3390/biomedicines11112919
Dankova I, Pyrgidis N, Tishukov M, Georgiadou E, Nigdelis MP, Solomayer E-F, Marcon J, Stief CG, Hatzichristou D. Efficacy and Safety of Platelet-Rich Plasma Injections for the Treatment of Female Sexual Dysfunction and Stress Urinary Incontinence: A Systematic Review. Biomedicines. 2023; 11(11):2919. https://doi.org/10.3390/biomedicines11112919
Chicago/Turabian StyleDankova, Irina, Nikolaos Pyrgidis, Maksim Tishukov, Efstratia Georgiadou, Meletios P. Nigdelis, Erich-Franz Solomayer, Julian Marcon, Christian G. Stief, and Dimitrios Hatzichristou. 2023. "Efficacy and Safety of Platelet-Rich Plasma Injections for the Treatment of Female Sexual Dysfunction and Stress Urinary Incontinence: A Systematic Review" Biomedicines 11, no. 11: 2919. https://doi.org/10.3390/biomedicines11112919
APA StyleDankova, I., Pyrgidis, N., Tishukov, M., Georgiadou, E., Nigdelis, M. P., Solomayer, E.-F., Marcon, J., Stief, C. G., & Hatzichristou, D. (2023). Efficacy and Safety of Platelet-Rich Plasma Injections for the Treatment of Female Sexual Dysfunction and Stress Urinary Incontinence: A Systematic Review. Biomedicines, 11(11), 2919. https://doi.org/10.3390/biomedicines11112919







