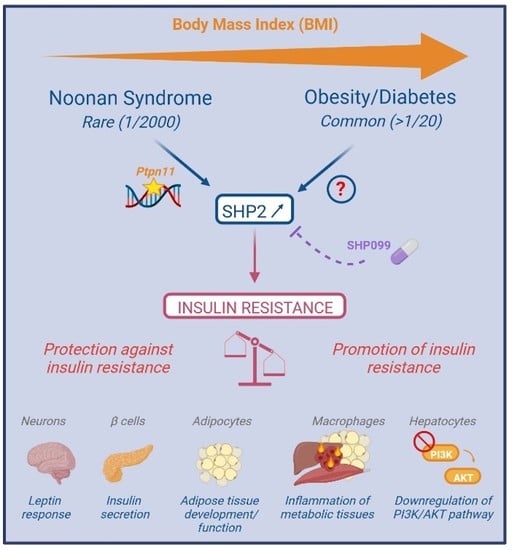
The Tyrosine Phosphatase SHP2: A New Target for Insulin Resistance?
Abstract
1. The Tyrosine Phosphatase SHP2
1.1. Structure, Function and Regulation
1.2. Physiological Roles of SHP2 during Development and Homeostasis, and Pathological Consequences of Its Dysfunction
2. SHP2, in Insulin Resistance: A Fragmented and Confusing View from Cellular and Tissue Specific Models
2.1. General Considerations about Insulin Signaling and Insulin Resistance
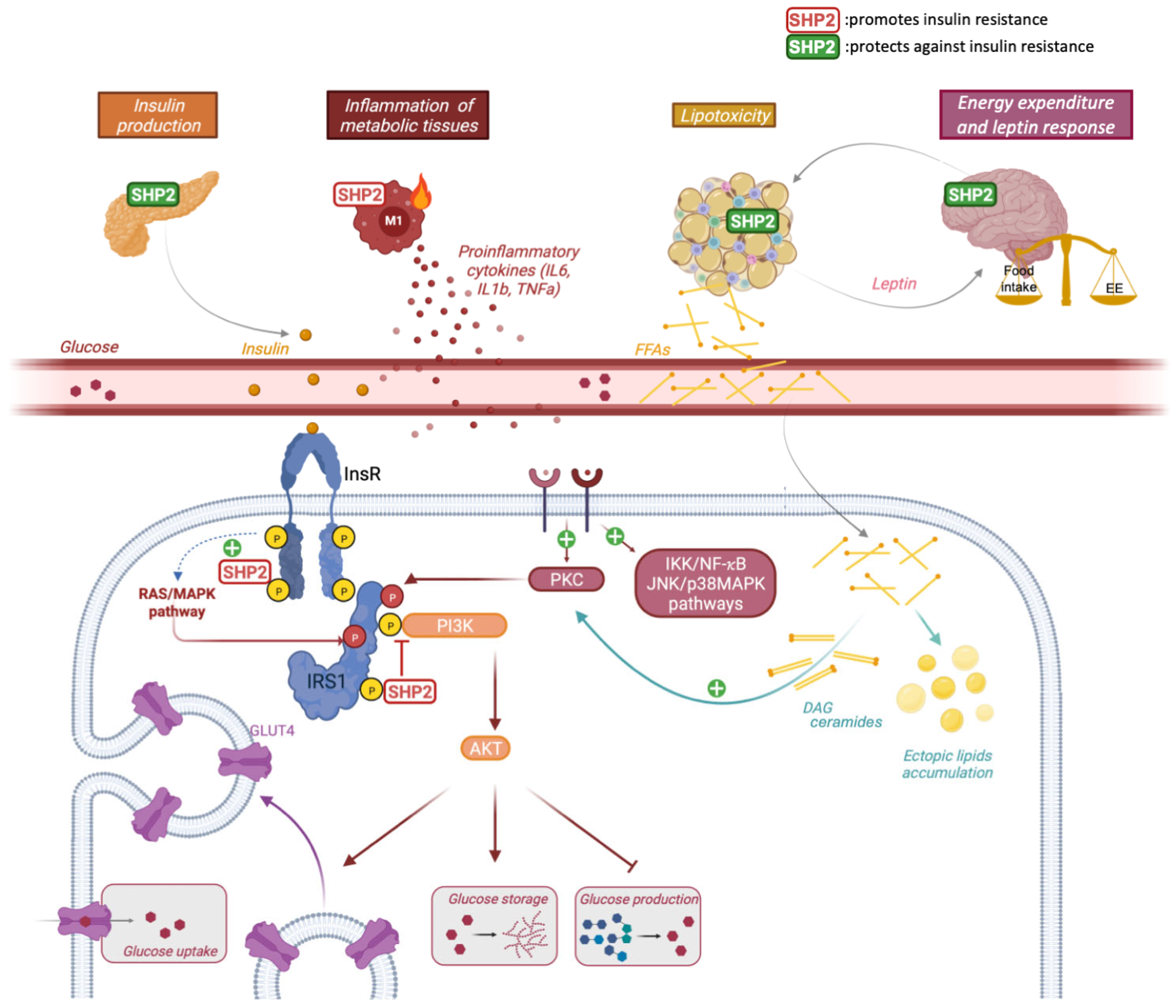
2.2. A Direct and Dual Role of SHP2 in Insulin Signaling
2.3. A protective or Causal Role of SHP2 in Insulin Resistance In Vivo?
3. SHP2 in Insulin Resistance: Lessons from Integrated Models
3.1. Genetic Diseases, Susceptibility Gene and SHP2 Dysregulation in Obesity/Diabetes
3.2. SHP2 Targeting in Obesity/Diabetes
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Tajan, M.; Serra, D.A.; Valet, P.; Edouard, T.; Yart, A. SHP2 sails from physiology to pathology. Eur. J. Med. Genet. 2015, 58, 509–525. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, S.; Zhao, M.; Yang, X.; Yu, B. Strategies Targeting Protein Tyrosine Phosphatase SHP2 for Cancer Therapy. J. Med. Chem. 2022, 65, 3066–3079. [Google Scholar] [CrossRef] [PubMed]
- Hartman, Z.; Geldenhuys, W.J.; Agazie, Y.M. A specific amino acid context in EGFR and HER2 phosphorylation sites enables selective binding to the active site of Src homology phosphatase 2 (SHP2). J. Biol. Chem. 2020, 295, 3563–3575. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yan, R.; Song, J. DephosSite: A machine learning approach for discovering phosphotase-specific dephosphorylation sites. Sci. Rep. 2016, 6, 23510. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Xie, J.; Zhong, Q.; Wang, Y.; Zhang, S.; Luo, F.; Wen, F.; Xie, J.; Zhao, J.; Sun, X.; et al. A novel partially open state of SHP2 points to a “multiple gear” regulation mechanism. J. Biol. Chem. 2021, 296, 100538. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Xie, J.; Kong, W.; Xie, J.; Li, Y.; Du, L.; Zheng, Q.; Sun, L.; Guan, M.; Li, H.; et al. Phase Separation of Disease-Associated SHP2 Mutants Underlies MAPK Hyperactivation. Cell 2020, 183, 490–502.e18. [Google Scholar] [CrossRef]
- Lin, C.-C.; Suen, K.M.; Jeffrey, P.-A.; Wieteska, L.; Lidster, J.A.; Bao, P.; Curd, A.P.; Stainthorp, A.; Seiler, C.; Koss, H.; et al. Receptor tyrosine kinases regulate signal transduction through a liquid-liquid phase separated state. Mol. Cell 2022, 82, 1089–1106.e12. [Google Scholar] [CrossRef]
- Burmeister, B.T.; Wang, L.; Gold, M.G.; Skidgel, R.A.; O’Bryan, J.P.; Carnegie, G.K. Protein Kinase A (PKA) Phosphorylation of Shp2 Protein Inhibits Its Phosphatase Activity and Modulates Ligand Specificity. J. Biol. Chem. 2015, 290, 12058–12067. [Google Scholar] [CrossRef]
- Sun, J.; Lu, S.; Ouyang, M.; Lin, L.J.; Zhuo, Y.; Liu, B.; Chien, S.; Neel, B.G.; Wang, Y. Antagonism between binding site affinity and conformational dynamics tunes alternative ci.is-interactions within Shp2. Nat. Commun. 2013, 4, 2037. [Google Scholar] [CrossRef]
- Tsutsumi, R.; Masoudi, M.; Takahashi, A.; Fujii, Y.; Hayashi, T.; Kikuchi, I.; Satou, Y.; Taira, M.; Hatakeyama, M. YAP and TAZ, Hippo signaling targets, act as a rheostat for nuclear SHP2 function. Dev. Cell 2013, 26, 658–665. [Google Scholar] [CrossRef]
- Tsutsumi, R.; Harizanova, J.; Stockert, R.; Schröder, K.; Bastiaens, P.I.H.; Neel, B.G. Assay to visualize specific protein oxidation reveals spatio-temporal regulation of SHP2. Nat. Commun. 2017, 8, 466. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Hale, A.J.; Lemeer, S.; den Hertog, J. Differential oxidation of protein-tyrosine phosphatases during zebrafish caudal fin regeneration. Sci. Rep. 2017, 7, 8460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Chen, D.; Fan, H.; Wu, R.; Tu, J.; Zhang, F.Q.; Wang, M.; Zheng, H.; Qu, C.-K.; Elf, S.E.; et al. Cellular signals converge at the NOX2-SHP-2 axis to induce reductive carboxylation in cancer cells. Cell Chem. Biol. 2022, 29, 1200–1208.E6. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Chen, W.; Zhu, J.; Wu, G.; Shen, R.; Xi, M.; Sun, H. Therapeutic potential of targeting SHP2 in human developmental disorders and cancers. Eur. J. Med. Chem. 2020, 190, 112117. [Google Scholar] [CrossRef]
- Dance, M.; Montagner, A.; Salles, J.P.; Yart, A.; Raynal, P. The molecular functions of Shp2 in the Ras/Mitogen-activated protein kinase (ERK1/2) pathway. Cell. Signal. 2008, 20, 453–459. [Google Scholar] [CrossRef]
- Buday, L.; Vas, V. Novel regulation of Ras proteins by direct tyrosine phosphorylation and dephosphorylation. Cancer Metastasis Rev. 2020, 39, 1067–1073. [Google Scholar] [CrossRef]
- Bunda, S.; Burrell, K.; Heir, P.; Zeng, L.; Alamsahebpour, A.; Kano, Y.; Raught, B.; Zhang, Z.Y.; Zadeh, G.; Ohh, M. Inhibition of SHP2-mediated dephosphorylation of Ras suppresses oncogenesis. Nat. Commun. 2015, 6, 8859. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Tsiaras, W.G.; Araki, T.; Wen, G.; Minichiello, L.; Klein, R.; Neel, B.G. Receptor-specific regulation of phosphatidylinositol 3’-kinase activation by the protein tyrosine phosphatase Shp2. Mol. Cell. Biol. 2002, 22, 4062–4072. [Google Scholar] [CrossRef]
- Guo, W.; Liu, W.; Chen, Z.; Gu, Y.; Peng, S.; Shen, L.; Shen, Y.; Wang, X.; Feng, G.-S.; Sun, Y.; et al. Tyrosine phosphatase SHP2 negatively regulates NLRP3 inflammasome activation via ANT1-dependent mitochondrial homeostasis. Nat. Commun. 2017, 8, 2168. [Google Scholar] [CrossRef]
- Lee, I.; Pecinova, A.; Pecina, P.; Neel, B.G.; Araki, T.; Kucherlapati, R.; Roberts, A.E.; Hüttemann, M. A suggested role for mitochondria in Noonan syndrome. Biochim. Biophys. Acta 2010, 1802, 275–283. [Google Scholar] [CrossRef]
- Zang, Q.S.; Martinez, B.; Yao, X.; Maass, D.L.; Ma, L.; Wolf, S.E.; Minei, J.P. Sepsis-induced cardiac mitochondrial dysfunction involves altered mitochondrial-localization of tyrosine kinase Src and tyrosine phosphatase SHP2. PLoS ONE 2012, 7, e43424. [Google Scholar] [CrossRef] [PubMed]
- Chughtai, N.; Schimchowitsch, S.; Lebrun, J.-J.; Ali, S. Prolactin induces SHP-2 association with Stat5, nuclear translocation, and binding to the beta-casein gene promoter in mammary cells. J. Biol. Chem. 2002, 277, 31107–31114. [Google Scholar] [CrossRef] [PubMed]
- Jakob, S.; Schroeder, P.; Lukosz, M.; Buchner, N.; Spyridopoulos, I.; Altschmied, J.; Haendeler, J. Nuclear protein tyrosine phosphatase Shp-2 is one important negative regulator of nuclear export of telomerase reverse transcriptase. J. Biol. Chem. 2008, 283, 33155–33161. [Google Scholar] [CrossRef] [PubMed]
- Jakob, S.; Altschmied, J.; Haendeler, J. “Shping 2” different cellular localizations—A potential new player in aging processes. Aging 2009, 1, 664–668. [Google Scholar] [CrossRef][Green Version]
- He, Z.; Zhang, S.S.; Meng, Q.; Li, S.; Zhu, H.H.; Raquil, M.A.; Alderson, N.; Zhang, H.; Wu, J.; Rui, L.; et al. Shp2 controls female body weight and energy balance by integrating leptin and estrogen signals. Mol. Cell. Biol. 2012, 32, 1867–1878. [Google Scholar] [CrossRef]
- Ran, H.; Kong, S.; Zhang, S.; Cheng, J.; Zhou, C.; He, B.; Xin, Q.; Lydon, J.P.; De Mayo, F.J.; Feng, G.-S.; et al. Nuclear Shp2 directs normal embryo implantation via facilitating the ERα tyrosine phosphorylation by the Src kinase. Proc. Natl. Acad. Sci. USA 2017, 114, 4816–4821. [Google Scholar] [CrossRef]
- Batth, T.S.; Papetti, M.; Pfeiffer, A.; Tollenaere, M.A.X.; Francavilla, C.; Olsen, J.V. Large-Scale Phosphoproteomics Reveals Shp-2 Phosphatase-Dependent Regulators of Pdgf Receptor Signaling. Cell Rep. 2018, 22, 2784–2796. [Google Scholar] [CrossRef]
- Corallino, S.; Iwai, L.K.; Payne, L.S.; Huang, P.H.; Sacco, F.; Cesareni, G.; Castagnoli, L. Alterations in the phosphoproteomic profile of cells expressing a non-functional form of the SHP2 phosphatase. N. Biotechnol. 2016, 33, 524–536. [Google Scholar] [CrossRef]
- Vemulapalli, V.; Chylek, L.A.; Erickson, A.; Pfeiffer, A.; Gabriel, K.-H.; LaRochelle, J.; Subramanian, K.; Cao, R.; Stegmaier, K.; Mohseni, M.; et al. Time-resolved phosphoproteomics reveals scaffolding and catalysis-responsive patterns of SHP2-dependent signaling. eLife 2021, 10, e64251. [Google Scholar] [CrossRef]
- Chaudhari, M.; Thapa, N.; Ismail, H.; Chopade, S.; Caragea, D.; Köhn, M.; Newman, R.H.; Kc, D.B. DTL-DephosSite: Deep Transfer Learning Based Approach to Predict Dephosphorylation Sites. Front. Cell Dev. Biol. 2021, 9, 662983. [Google Scholar] [CrossRef]
- Kan, C.; Yang, F.; Wang, S. SHP2-Mediated Signal Networks in Stem Cell Homeostasis and Dysfunction. Stem Cells Int. 2018, 2018, 8351374. [Google Scholar] [CrossRef] [PubMed]
- Vainonen, J.P.; Momeny, M.; Westermarck, J. Druggable cancer phosphatases. Sci. Transl. Med. 2021, 13, eabe2967. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Ramdas, B.; Wan, C.; Sandusky, G.; Mohseni, M.; Zhang, C.; Kapur, R. SHP2 inhibition reduces leukemogenesis in models of combined genetic and epigenetic mutations. J. Clin. Investig. 2019, 129, 5468–5473. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Everingham, S.; Ramdas, B.; Kapur, R.; Craig, A.W. SHP2 phosphatase promotes mast cell chemotaxis toward stem cell factor via enhancing activation of the Lyn/Vav/Rac signaling axis. J. Immunol. 2014, 192, 4859–4866. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Han, T.; Tang, H.; Huang, K.; Min, J.; Li, J.; Ding, X.; Xu, Z. Shp2 Inhibits Proliferation of Esophageal Squamous Cell Cancer via Dephosphorylation of Stat3. Int. J. Mol. Sci. 2017, 18, 134. [Google Scholar] [CrossRef] [PubMed]
- Bard-Chapeau, E.A.; Li, S.; Ding, J.; Zhang, S.S.; Zhu, H.H.; Princen, F.; Fang, D.D.; Han, T.; Bailly-Maitre, B.; Poli, V.; et al. Ptpn11/Shp2 acts as a tumor suppressor in hepatocellular carcinogenesis. Cancer Cell 2011, 19, 629–639. [Google Scholar] [CrossRef]
- Liu, J.J.; Xin, B.; Du, L.; Chen, L.; Long, Y.; Feng, G.-S. Pharmaceutical Shp2 inhibition suppresses primary and metastasized liver tumors by provoking hepatic innate immunity. Hepatology 2022. Online Version of Record before inclusion in an issue. [Google Scholar] [CrossRef]
- Chen, W.S.; Liang, Y.; Zong, M.; Liu, J.J.; Kaneko, K.; Hanley, K.L.; Zhang, K.; Feng, G.-S. Single-cell transcriptomics reveals opposing roles of Shp2 in Myc-driven liver tumor cells and microenvironment. Cell Rep. 2021, 37, 109974. [Google Scholar] [CrossRef]
- Tajan, M.; Paccoud, R.; Branka, S.; Edouard, T.; Yart, A. The RASopathy Family: Consequences of Germline Activation of the RAS/MAPK Pathway. Endocr. Rev. 2018, 39, 676–700. [Google Scholar] [CrossRef]
- Kim, H.K.; Feng, G.S.; Chen, D.; King, P.D.; Kamiya, N. Targeted disruption of Shp2 in chondrocytes leads to metachondromatosis with multiple cartilaginous protrusions. J. Bone Miner. Res. 2014, 29, 761–769. [Google Scholar] [CrossRef]
- Do Carmo, J.M.; da Silva, A.A.; Ebaady, S.E.; Sessums, P.O.; Abraham, R.S.; Elmquist, J.K.; Lowell, B.B.; Hall, J.E. Shp2 signaling in POMC neurons is important for leptin’s actions on blood pressure, energy balance, and glucose regulation. Am. J. Physiol. 2014, 307, R1438–R1447. [Google Scholar] [CrossRef]
- Zhang, E.E.; Chapeau, E.; Hagihara, K.; Feng, G.S. Neuronal Shp2 tyrosine phosphatase controls energy balance and metabolism. Proc. Natl. Acad. Sci. USA 2004, 101, 16064–16069. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhu, H.H.; Bauler, T.J.; Wang, J.; Ciaraldi, T.; Alderson, N.; Li, S.; Raquil, M.A.; Ji, K.; Wang, S.; et al. Nonreceptor tyrosine phosphatase Shp2 promotes adipogenesis through inhibition of p38 MAP kinase. Proc. Natl. Acad. Sci. USA 2012, 110, E79–E88. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Delibegovic, M.; Matsuo, I.; Nagata, N.; Liu, S.; Bettaieb, A.; Xi, Y.; Araki, K.; Yang, W.; Kahn, B.B.; et al. Altered glucose homeostasis in mice with liver-specific deletion of Src homology phosphatase 2. J. Biol. Chem. 2010, 285, 39750–39758. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S.; Hao, E.; Yu, J.; Liu, W.; Wang, J.; Levine, F.; Feng, G.S. Coordinated regulation by Shp2 tyrosine phosphatase of signaling events controlling insulin biosynthesis in pancreatic beta-cells. Proc. Natl. Acad. Sci. USA 2009, 106, 7531–7536. [Google Scholar] [CrossRef] [PubMed]
- Princen, F.; Bard, E.; Sheikh, F.; Zhang, S.S.; Wang, J.; Zago, W.M.; Wu, D.; Diaz Trelles, R.; Bailly-Maitre, B.; Kahn, C.R.; et al. Deletion of Shp2 Tyrosine Phosphatase in Muscle Leads to Dilated Cardiomyopathy, Insulin Resistance and Premature Death. Mol. Cell. Biol. 2009, 29, 378–388. [Google Scholar] [CrossRef]
- Nagata, N.; Matsuo, K.; Bettaieb, A.; Bakke, J.; Matsuo, I.; Graham, J.; Xi, Y.; Liu, S.; Tomilov, A.; Tomilova, N.; et al. Hepatic SRC homology phosphatase 2 regulates energy balance in mice. Endocrinology 2012, 153, 3158–3169. [Google Scholar] [CrossRef][Green Version]
- De Rocca Serra-Nedelec, A.; Edouard, T.; Treguer, K.; Tajan, M.; Araki, T.; Dance, M.; Mus, M.; Montagner, A.; Tauber, M.; Salles, J.P.; et al. Noonan syndrome-causing SHP2 mutants inhibit insulin-like growth factor 1 release via growth hormone-induced ERK hyperactivation, which contributes to short stature. Proc. Natl. Acad. Sci. USA 2012, 109, 4257–4262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, W.; Zhao, Q.; Chen, J.; Wang, T.; Ji, J. The role of the protein tyrosine phosphatase SHP2 in ossification. Dev. Dyn. 2022, 251, 748–758. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, X.; Wang, Y.; Yang, Y.; Sun, D.; Li, H.; Chen, L. Targeting SHP2 as a therapeutic strategy for inflammatory diseases. Eur. J. Med. Chem. 2021, 214, 113264. [Google Scholar] [CrossRef]
- Mélique, S.; Yang, C.; Lesourne, R. Negative times negative equals positive, THEMIS sets the rule on thymic selection and peripheral T cell responses. Biomed. J. 2022, 45, 334–346. [Google Scholar] [CrossRef]
- Niogret, C.; Birchmeier, W.; Guarda, G. SHP-2 in Lymphocytes’ Cytokine and Inhibitory Receptor Signaling. Front. Immunol. 2019, 10, 2468. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-J.; Wang, C.-W.; Tao, L.; Yan, Y.-H.; Zhang, M.-J.; Liu, Z.-X.; Li, Y.-X.; Zhao, H.-Q.; Li, X.-M.; He, X.-D.; et al. Insulin signaling regulates longevity through protein phosphorylation in Caenorhabditis elegans. Nat. Commun. 2021, 12, 4568. [Google Scholar] [CrossRef] [PubMed]
- Séité, S.; Harrison, M.C.; Sillam-Dussès, D.; Lupoli, R.; Van Dooren, T.J.M.; Robert, A.; Poissonnier, L.-A.; Lemainque, A.; Renault, D.; Acket, S.; et al. Lifespan prolonging mechanisms and insulin upregulation without fat accumulation in long-lived reproductives of a higher termite. Commun. Biol. 2022, 5, 44. [Google Scholar] [CrossRef]
- Ruzzi, L.R.; Schilman, P.E.; San Martin, A.; Lew, S.E.; Gelb, B.D.; Pagani, M.R. The Phosphatase CSW Controls Life Span by Insulin Signaling and Metabolism Throughout Adult Life in Drosophila. Front. Genet. 2020, 11, 364. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2019, 10, 1607. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ballantyne, C.M. Metabolic Inflammation and Insulin Resistance in Obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef]
- Vinayagam, A.; Kulkarni, M.M.; Sopko, R.; Sun, X.; Hu, Y.; Nand, A.; Villalta, C.; Moghimi, A.; Yang, X.; Mohr, S.E.; et al. An Integrative Analysis of the InR/PI3K/Akt Network Identifies the Dynamic Response to Insulin Signaling. Cell Rep. 2016, 16, 3062–3074. [Google Scholar] [CrossRef]
- Tajan, M.; Batut, A.; Cadoudal, T.; Deleruyelle, S.; Le Gonidec, S.; Saint Laurent, C.; Vomscheid, M.; Wanecq, E.; Treguer, K.; De Rocca Serra-Nédélec, A.; et al. LEOPARD syndrome-associated SHP2 mutation confers leanness and protection from diet-induced obesity. Proc. Natl. Acad. Sci. USA 2014, 11, E4494–E4503. [Google Scholar] [CrossRef]
- Myers, M.G., Jr.; Mendez, R.; Shi, P.; Pierce, J.H.; Rhoads, R.; White, M.F. The COOH-terminal tyrosine phosphorylation sites on IRS-1 bind SHP-2 and negatively regulate insulin signaling. J. Biol. Chem. 1998, 273, 26908–26914. [Google Scholar] [CrossRef]
- Yue, X.; Han, T.; Hao, W.; Wang, M.; Fu, Y. SHP2 knockdown ameliorates liver insulin resistance by activating IRS-2 phosphorylation through the AKT and ERK1/2 signaling pathways. FEBS Open Bio 2020, 10, 2578–2587. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Wang, X.; Wu, Y.; Xu, Y.; Zhuo, S.; Qi, M.; Ji, W.; Zhan, L. Polarity Protein AF6 Controls Hepatic Glucose Homeostasis and Insulin Sensitivity by Modulating IRS1/AKT Insulin Pathway in an SHP2-Dependent Manner. Diabetes 2019, 68, 1577–1590. [Google Scholar] [CrossRef]
- Choi, E.; Kikuchi, S.; Gao, H.; Brodzik, K.; Nassour, I.; Yopp, A.; Singal, A.G.; Zhu, H.; Yu, H. Mitotic regulators and the SHP2-MAPK pathway promote IR endocytosis and feedback regulation of insulin signaling. Nat. Commun. 2019, 10, 1473. [Google Scholar] [CrossRef]
- Hall, C.; Yu, H.; Choi, E. Insulin receptor endocytosis in the pathophysiology of insulin resistance. Exp. Mol. Med. 2020, 52, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Saxton, T.M.; Henkemeyer, M.; Gasca, S.; Shen, R.; Rossi, D.J.; Shalaby, F.; Feng, G.S.; Pawson, T. Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase Shp-2. EMBO J. 1997, 16, 2352–2364. [Google Scholar] [CrossRef] [PubMed]
- Maegawa, H.; Hasegawa, M.; Sugai, S.; Obata, T.; Ugi, S.; Morino, K.; Egawa, K.; Fujita, T.; Sakamoto, T.; Nishio, Y.; et al. Expression of a dominant negative SHP-2 in transgenic mice induces insulin resistance. J. Biol. Chem. 1999, 274, 30236–30243. [Google Scholar] [CrossRef]
- Krajewska, M.; Banares, S.; Zhang, E.E.; Huang, X.; Scadeng, M.; Jhala, U.S.; Feng, G.S.; Krajewski, S. Development of diabesity in mice with neuronal deletion of Shp2 tyrosine phosphatase. Am. J. Pathol. 2008, 172, 1312–1324. [Google Scholar] [CrossRef]
- Bettaieb, A.; Matsuo, K.; Matsuo, I.; Nagata, N.; Chahed, S.; Liu, S.; Haj, F.G. Adipose-specific deletion of Src homology phosphatase 2 does not significantly alter systemic glucose homeostasis. Metabolism 2011, 286, 9225–9235. [Google Scholar] [CrossRef][Green Version]
- Qi, X.; Sun, Z.; Li, X.; Jiao, Y.; Chen, S.; Song, P.; Qian, Z.; Qian, J.; Qiu, X.; Tang, L. Shp2 suppresses fat accumulation in white adipose tissue by activating Wnt/β-catenin signaling following vertical sleeve gastrectomy in obese rats with type-2 diabetes. Exp. Ther. Med. 2022, 23, 302. [Google Scholar] [CrossRef]
- Liu, W.; Yin, Y.; Wang, M.; Fan, T.; Zhu, Y.; Shen, L.; Peng, S.; Gao, J.; Deng, G.; Meng, X.; et al. Disrupting phosphatase SHP2 in macrophages protects mice from high-fat diet-induced hepatic steatosis and insulin resistance by elevating IL-18 levels. J. Biol. Chem. 2020, 31, 10842–10856. [Google Scholar] [CrossRef]
- Ranza, E.; Guimier, A.; Verloes, A.; Capri, Y.; Marques, C.; Auclair, M.; Mathieu-Dramard, M.; Morin, G.; Thevenon, J.; Faivre, L.; et al. Overlapping phenotypes between SHORT and Noonan syndromes in patients with PTPN11 pathogenic variants. Clin. Genet. 2020, 98, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Noronha, R.M.; Villares, S.M.F.; Torres, N.; Quedas, E.P.S.; Homma, T.K.; Albuquerque, E.V.A.; Moraes, M.B.; Funari, M.F.A.; Bertola, D.R.; Jorge, A.A.L.; et al. Noonan syndrome patients beyond the obvious phenotype: A potential unfavorable metabolic profile. Am. J. Med. Genet. A 2021, 185, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Paccoud, R.; Saint-Laurent, C.; Piccolo, E.; Tajan, M.; Dortignac, A.; Pereira, O.; Le Gonidec, S.; Baba, I.; Gélineau, A.; Askia, H.; et al. SHP2 drives inflammation-triggered insulin resistance by reshaping tissue macrophage populations. Sci. Transl. Med. 2021, 13, 591. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Zhang, H.; Zhang, Y.; Zheng, M.; Liu, R.; Zhao, Y.; Zhang, X.; Cheng, H.; Cao, Q.; Ke, Y. Phosphatase Shp2 exacerbates intestinal inflammation by disrupting macrophage responsiveness to interleukin-10. J. Exp. Med. 2019, 216, 337–349. [Google Scholar] [CrossRef]
- Hsu, A.Y.; Gurol, T.; Sobreira, T.J.P.; Zhang, S.; Moore, N.; Cai, C.; Zhang, Z.Y.; Deng, Q. Development and Characterization of an Endotoxemia Model in Zebra Fish. Front. Immunol. 2018, 9, 607. [Google Scholar] [CrossRef]
- Wang, J.; Mizui, M.; Zeng, L.F.; Bronson, R.; Finnell, M.; Terhorst, C.; Kyttaris, V.C.; Tsokos, G.C.; Zhang, Z.Y.; Kontaridis, M.I. Inhibition of SHP2 ameliorates the pathogenesis of systemic lupus erythematosus. J. Clin. Investig. 2016, 126, 2077–2092. [Google Scholar] [CrossRef]
- Tao, B.; Jin, W.; Xu, J.; Liang, Z.; Yao, J.; Zhang, Y.; Wang, K.; Cheng, H.; Zhang, X.; Ke, Y. Myeloid-specific disruption of tyrosine phosphatase Shp2 promotes alternative activation of macrophages and predisposes mice to pulmonary fibrosis. J. Immunol. 2014, 193, 2801–2811. [Google Scholar] [CrossRef]
- Xu, J.; Tao, B.; Guo, X.; Zhou, S.; Li, Y.; Zhang, Y.; Zhou, Z.; Cheng, H.; Zhang, X.; Ke, Y. Macrophage-Restricted Shp2 Tyrosine Phosphatase Acts as a Rheostat for MMP12 through TGF-beta Activation in the Prevention of Age-Related Emphysema in Mice. J. Immunol. 2017, 199, 2323–2332. [Google Scholar] [CrossRef]
- Zhao, L.; Xia, J.; Li, T.; Zhou, H.; Ouyang, W.; Hong, Z.; Ke, Y.; Qian, J.; Xu, F. Shp2 Deficiency Impairs the Inflammatory Response Against Haemophilus influenzae by Regulating Macrophage Polarization. J. Infect. Dis. 2016, 214, 625–633. [Google Scholar] [CrossRef]
- Kraja, A.T.; Chasman, D.I.; North, K.E.; Reiner, A.P.P.; Yanek, L.R.; Kilpelainen, T.O.; Smith, J.A.; Dehghan, A.; Dupuis, J.; Johnson, A.D.; et al. Pleiotropic genes for metabolic syndrome and inflammation. Mol. Genet. Metab. 2014, 112, 317–338. [Google Scholar] [CrossRef]
- Ahmad, F.; Goldstein, B.J. Increased abundance of specific skeletal muscle protein-tyrosine phosphatases in a genetic model of insulin-resistant obesity and diabetes mellitus. Metab. Clin. Exp. 1995, 44, 1175–1184. [Google Scholar] [CrossRef]
- Bonini, J.A.; Colca, J.R.; Dailey, C.; White, M.; Hofmann, C. Compensatory alterations for insulin signal transduction and glucose transport in insulin-resistant diabetes. Am. J. Physiol. 1995, 269, E759–E765. [Google Scholar] [CrossRef] [PubMed]
- Banno, R.; Zimmer, D.; De Jonghe, B.C.; Atienza, M.; Rak, K.; Yang, W.; Bence, K.K. PTP1B and SHP2 in POMC neurons reciprocally regulate energy balance in mice. J. Clin. Investig. 2010, 120, 720–734. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Hou, Y.; Cheng, R.; Zheng, L.; Alvarez, A.A.; Hu, B.; Cheng, S.-Y.; Zhang, W.; Li, Y.; Feng, H. Targeting PDGFRα-activated glioblastoma through specific inhibition of SHP-2-mediated signaling. Neuro-Oncology 2019, 21, 1423–1435. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh Grewal, A.; Grover, R.; Sharma, N.; Chopra, B.; Kumar Dhingra, A.; Arora, S.; Redhu, S.; Lather, V. Recent updates on development of protein-tyrosine phosphatase 1B inhibitors for treatment of diabetes, obesity and related disorders. Bioorg. Chem. 2022, 121, 105626. [Google Scholar] [CrossRef] [PubMed]
| Targeted Organism/Tissue/Cell | Approach | Impact on Glucose Metabolism and Major Associated Phenotype | References |
|---|---|---|---|
| Cultured hepatocytes | SHP2 knockdown or inhibition | Improved insulin signaling | [59,63,64] |
| C. elegans | Ptp2−/− | Modulation of insulin signaling Increased lifespan | [53] |
| Drosophila | Csw−/− | Modulation of insulin signaling Increased lifespan | [55] |
| Mouse/ubiquitous | Ptpn11−/− Ptpn11+/− | Undetermined (embryonic lethality) No obvious phenotype | [66] |
| Mouse/ubiquitous | Ptpn11T468M/+ | Insulin hypersensitivity Improved glucose tolerance Resistance to HFD-induced insulin resistance | [61] |
| Mouse/ubiquitous | Ptpn11D61G/+ | Insulin resistance Glucose intolerance Inflammation | [74] |
| Mouse/transgenic (liver, muscle, adipose tissue) | SHP2∆PTP | Insulin resistance | [67] |
| HFD-fed mouse/systemic | SHP099 treatment | Improved glucose tolerance | [71,74] |
| Mouse/muscle specific | Ptpn11fl/fl × MHC-cre or MCK-cre | Insulin resistance Glucose intolerance Altered myofibers (number and size) Dilated cardiomyopathy | [46] |
| Mouse/neuron specific | Ptpn11fl/fl × CRE3 | Insulin resistance Diabetes Obesity Hyperphagia Leptin resistance Nephropathy | [41,68] |
| Mouse/transgenic (forebrain neuron, CamKII-driven) | SHP2D61A | Improved insulin sensitivity Improved glucose homeostasis | [25] |
| Mouse/POMC neuron specific | Ptpn11fl/fl × POMC-cre | Altered glucose metabolism Obesity Leptin resistance Reduced energy expenditure | [41,84] |
| Mouse/liver specific | Ptpn11fl/fl × Alb-cre | Improved insulin sensitivity Improved glucose tolerance Resistance to obesity Increased energy expenditure | [44,47] |
| Mouse/adipose tissue specific | Ptpn11fl/fl × Adipoq-cre | No phenotype | [69] |
| Ptpn11fl/fl × aP2-cre | Not assessed Severe lipodystrophy Altered adipogenesis Premature death | [43] | |
| Mouse/pancreas specific | Ptpn11fl/fl × Pdx1-cre | Glucose intolerance Reduced insulin secretion | [45] |
| Mouse/macrophage specific | Ptpn11fl/fl × Lyz2-cre | Resistance to HFD-induced insulin resistance | [71] |
| Human/ubiquitous | NS-Ptpn11 | Glucose intolerance Noonan syndrome | [72,73,74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saint-Laurent, C.; Mazeyrie, L.; Tajan, M.; Paccoud, R.; Castan-Laurell, I.; Valet, P.; Edouard, T.; Pradère, J.-P.; Dray, C.; Yart, A. The Tyrosine Phosphatase SHP2: A New Target for Insulin Resistance? Biomedicines 2022, 10, 2139. https://doi.org/10.3390/biomedicines10092139
Saint-Laurent C, Mazeyrie L, Tajan M, Paccoud R, Castan-Laurell I, Valet P, Edouard T, Pradère J-P, Dray C, Yart A. The Tyrosine Phosphatase SHP2: A New Target for Insulin Resistance? Biomedicines. 2022; 10(9):2139. https://doi.org/10.3390/biomedicines10092139
Chicago/Turabian StyleSaint-Laurent, Céline, Laurène Mazeyrie, Mylène Tajan, Romain Paccoud, Isabelle Castan-Laurell, Philippe Valet, Thomas Edouard, Jean-Philippe Pradère, Cédric Dray, and Armelle Yart. 2022. "The Tyrosine Phosphatase SHP2: A New Target for Insulin Resistance?" Biomedicines 10, no. 9: 2139. https://doi.org/10.3390/biomedicines10092139
APA StyleSaint-Laurent, C., Mazeyrie, L., Tajan, M., Paccoud, R., Castan-Laurell, I., Valet, P., Edouard, T., Pradère, J.-P., Dray, C., & Yart, A. (2022). The Tyrosine Phosphatase SHP2: A New Target for Insulin Resistance? Biomedicines, 10(9), 2139. https://doi.org/10.3390/biomedicines10092139