Respiratory and Peripheral Muscle Weakness and Body Composition Abnormalities in Non-Cystic Fibrosis Bronchiectasis Patients: Gender Differences
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Ethics
2.3. Bronchiectasis Severity Scores
2.4. Radiological Extension of Bronchiectasis
2.5. Nutritional and Body Assessment
2.5.1. Lung Function Assessment
2.5.2. Limb Muscle Function
2.6. Respiratory Muscle Evaluation
Exercise Capacity
2.7. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Study Subjects
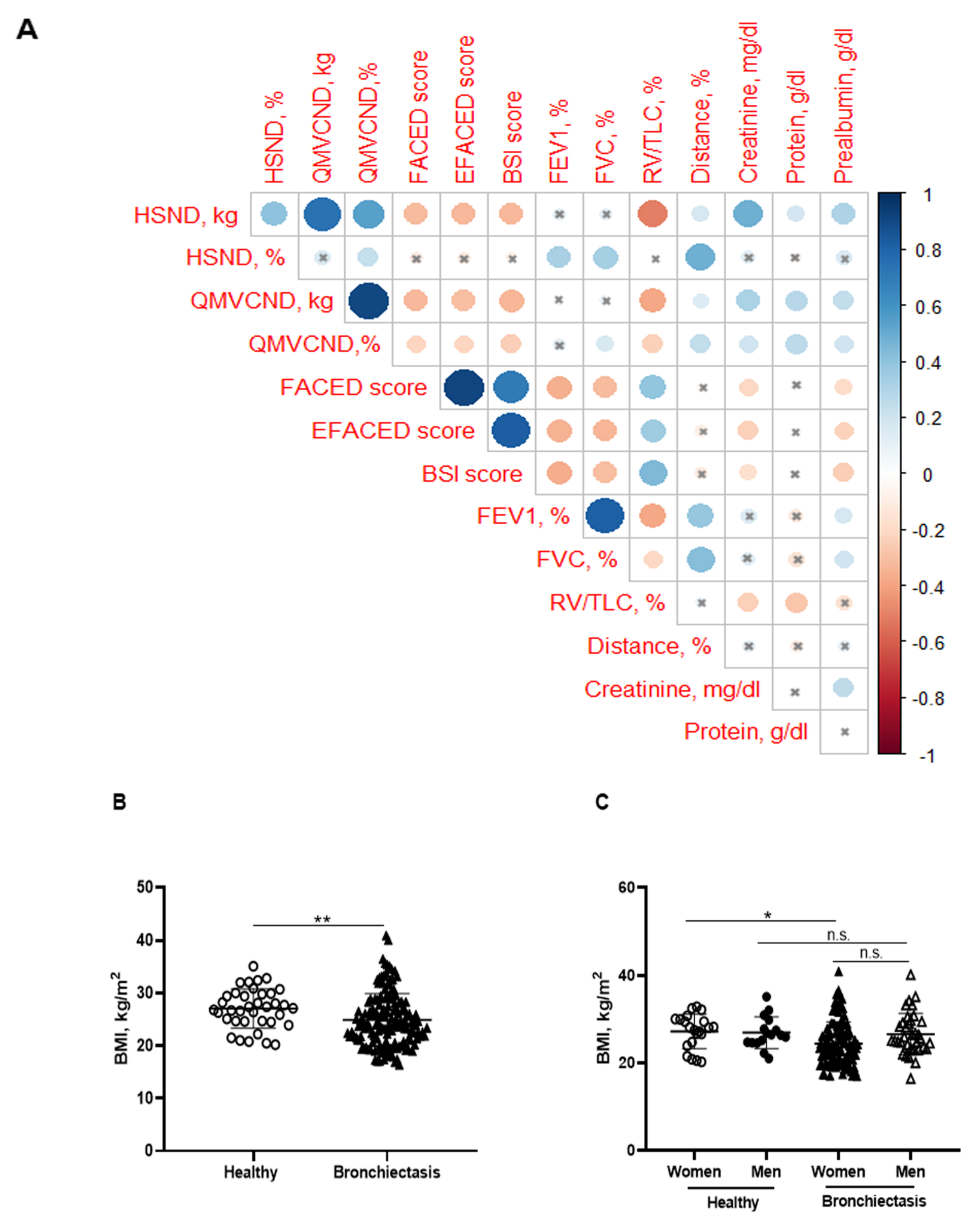
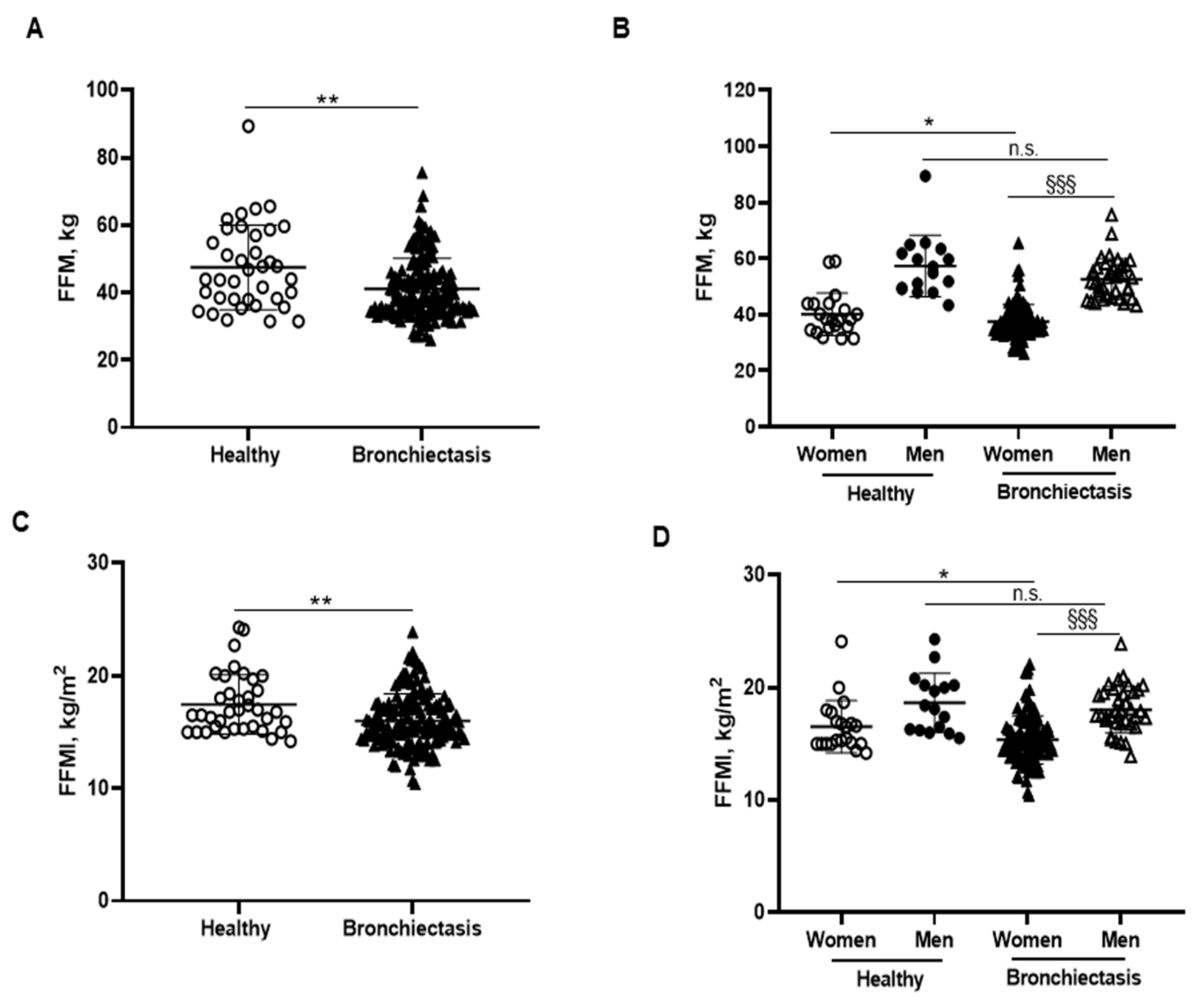
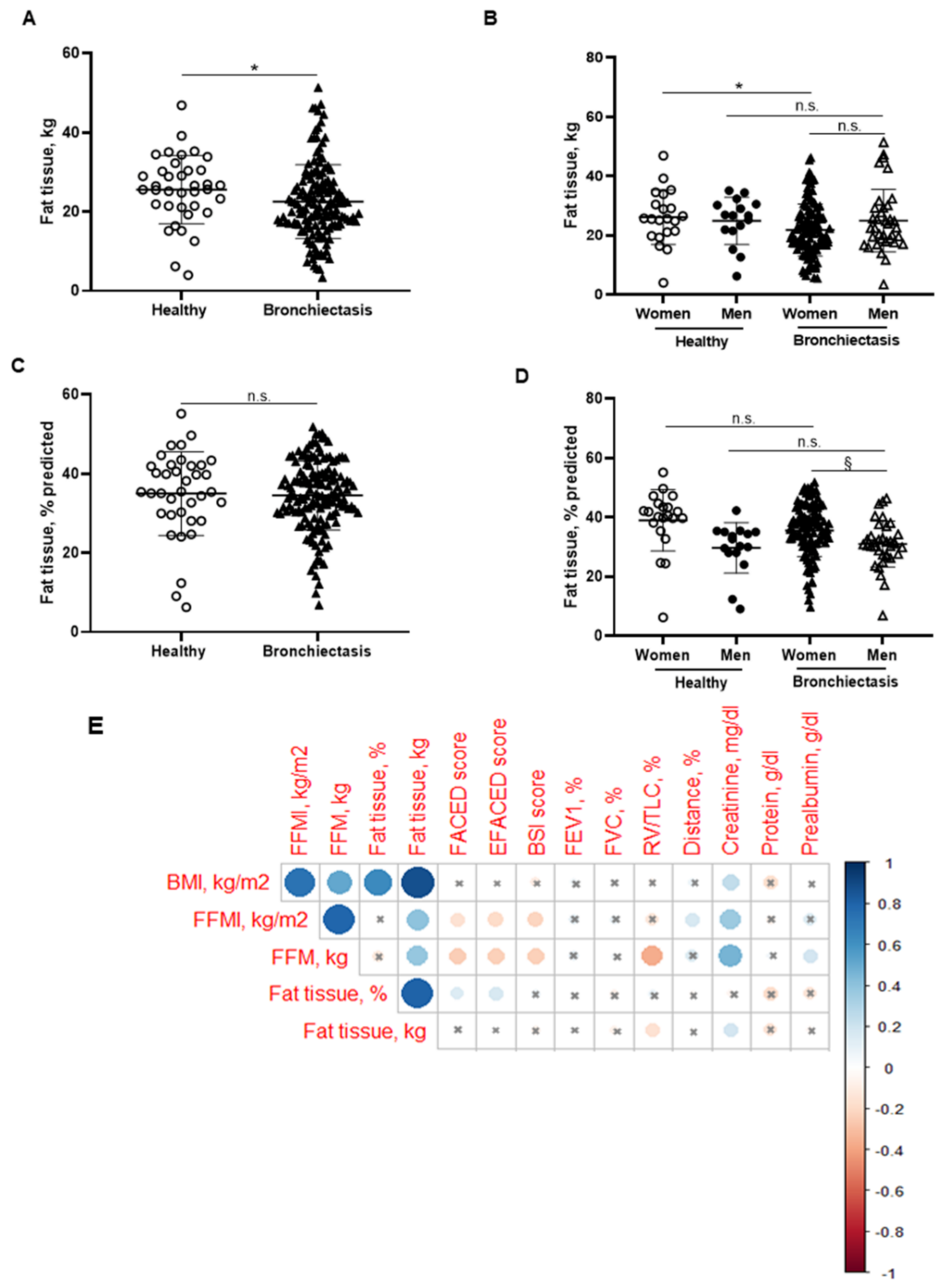
3.2. Body Composition
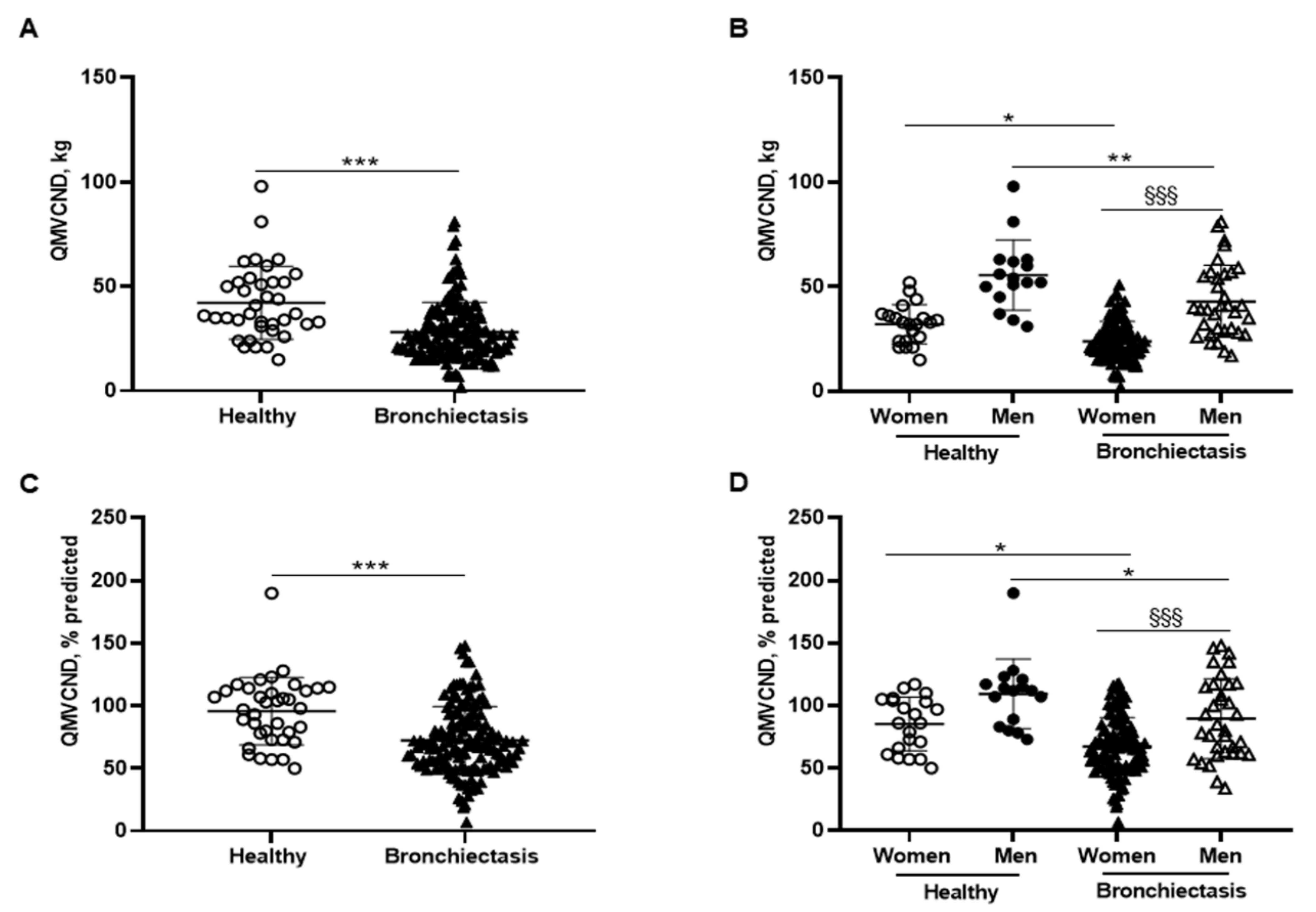
3.3. Upper Limb Muscle Strength
3.4. Lower Limb Muscle Strength
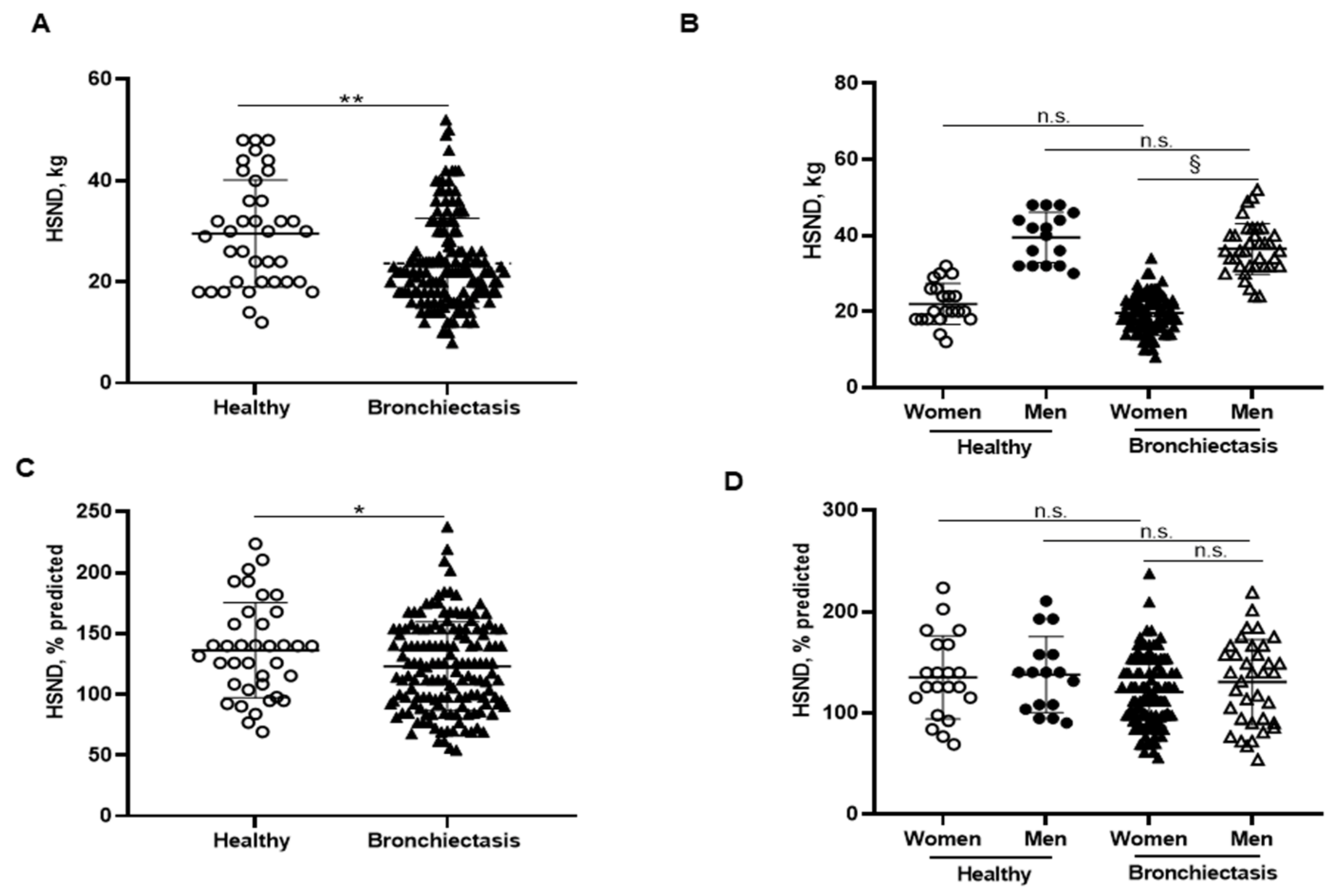
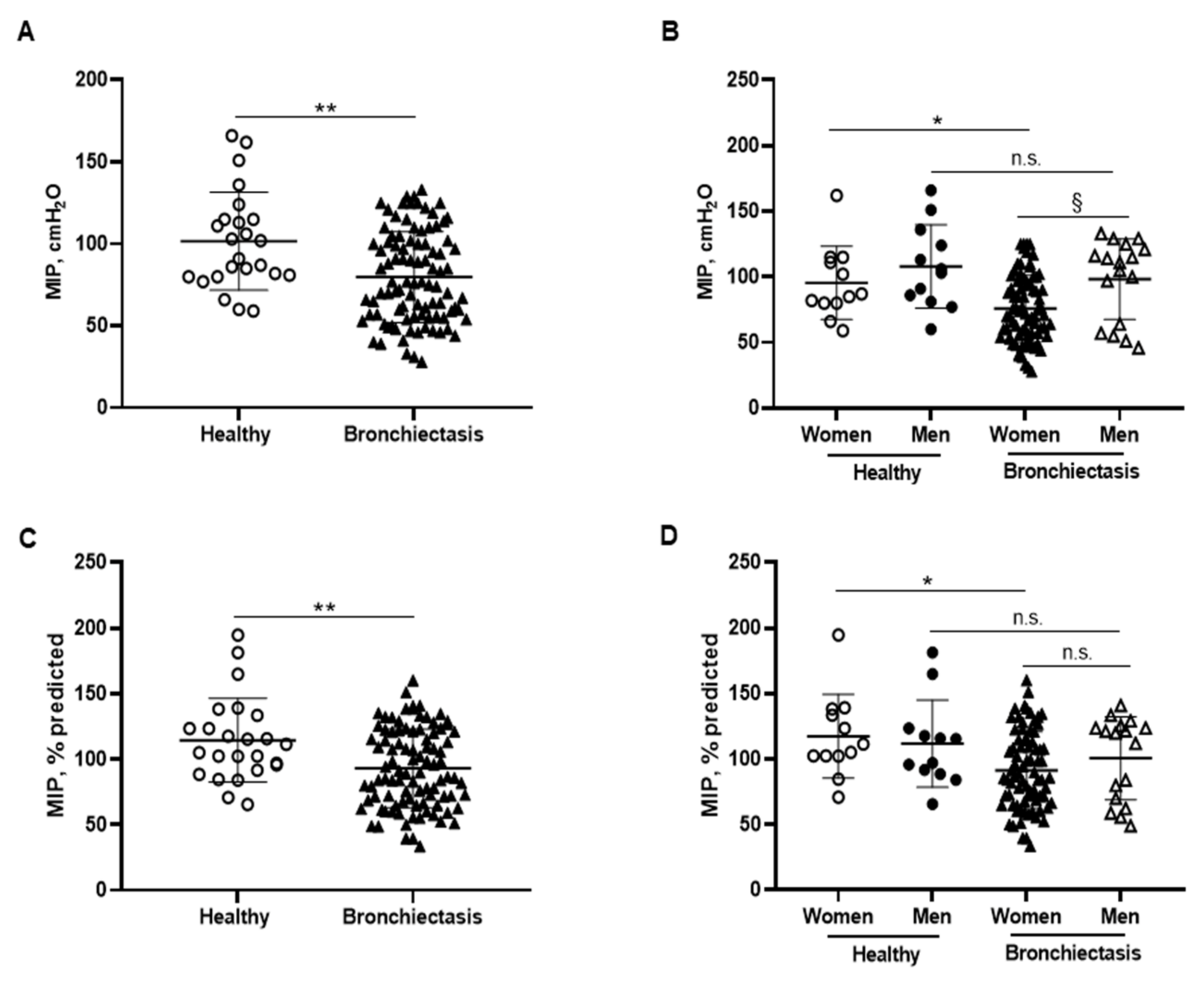
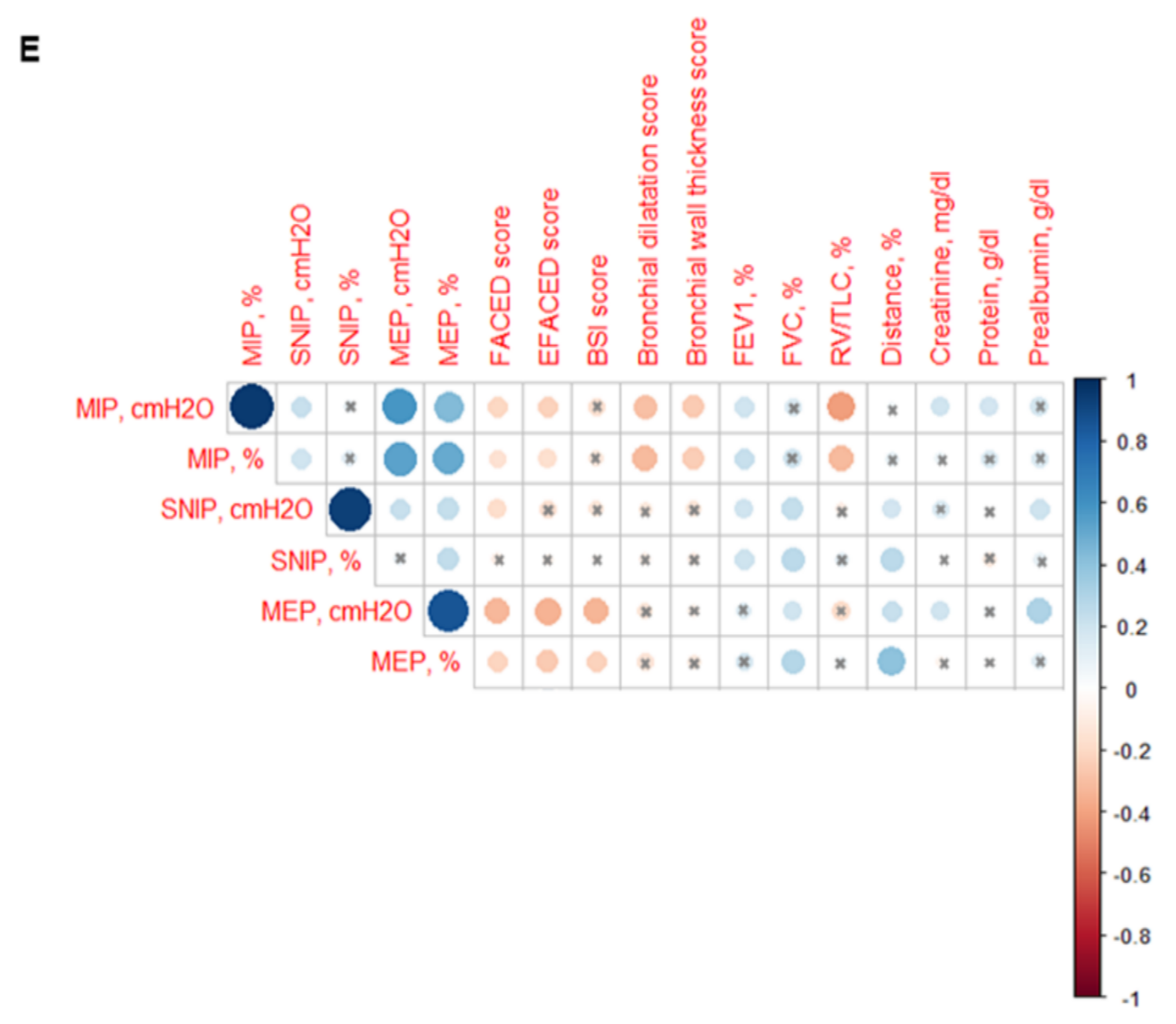
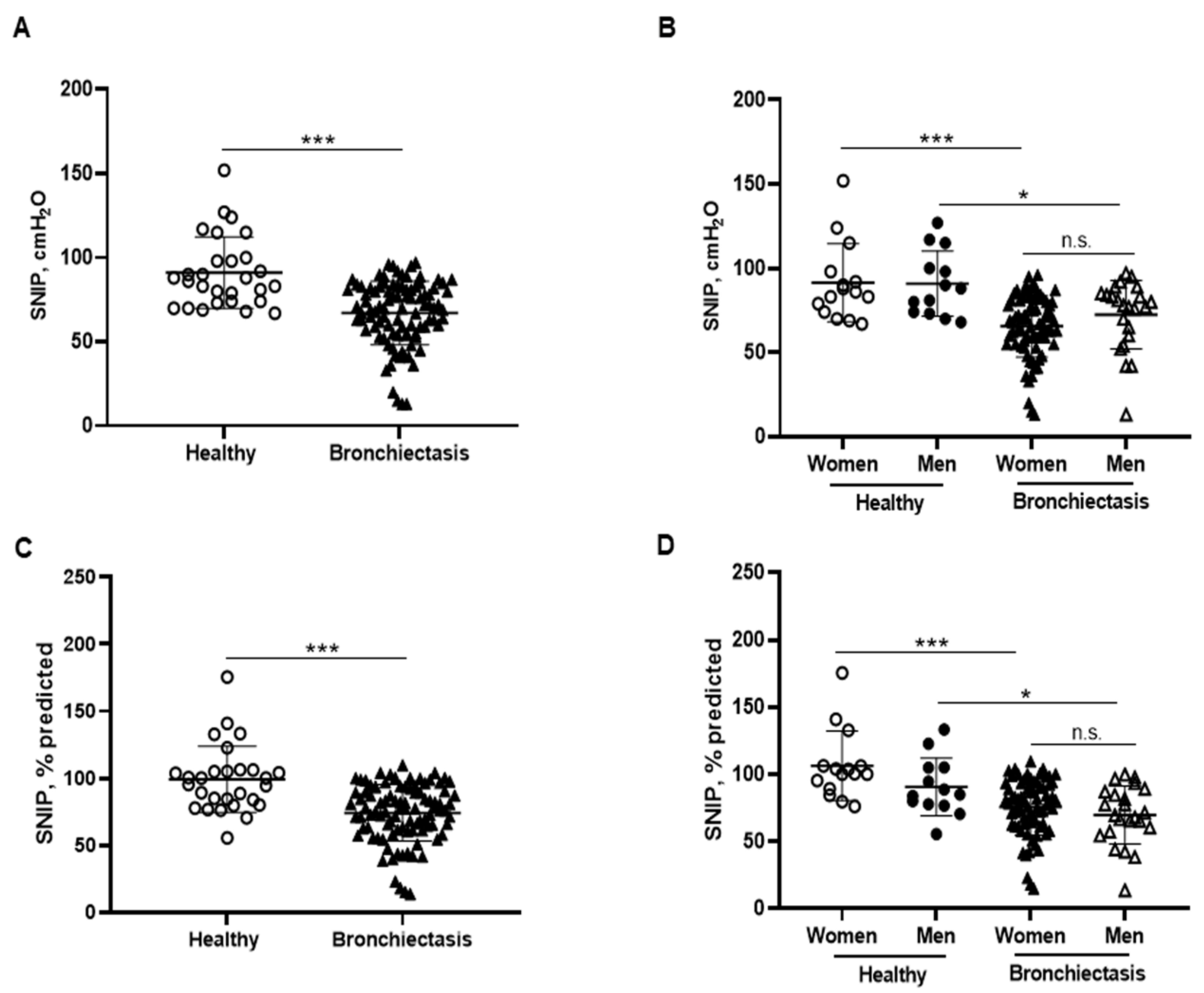
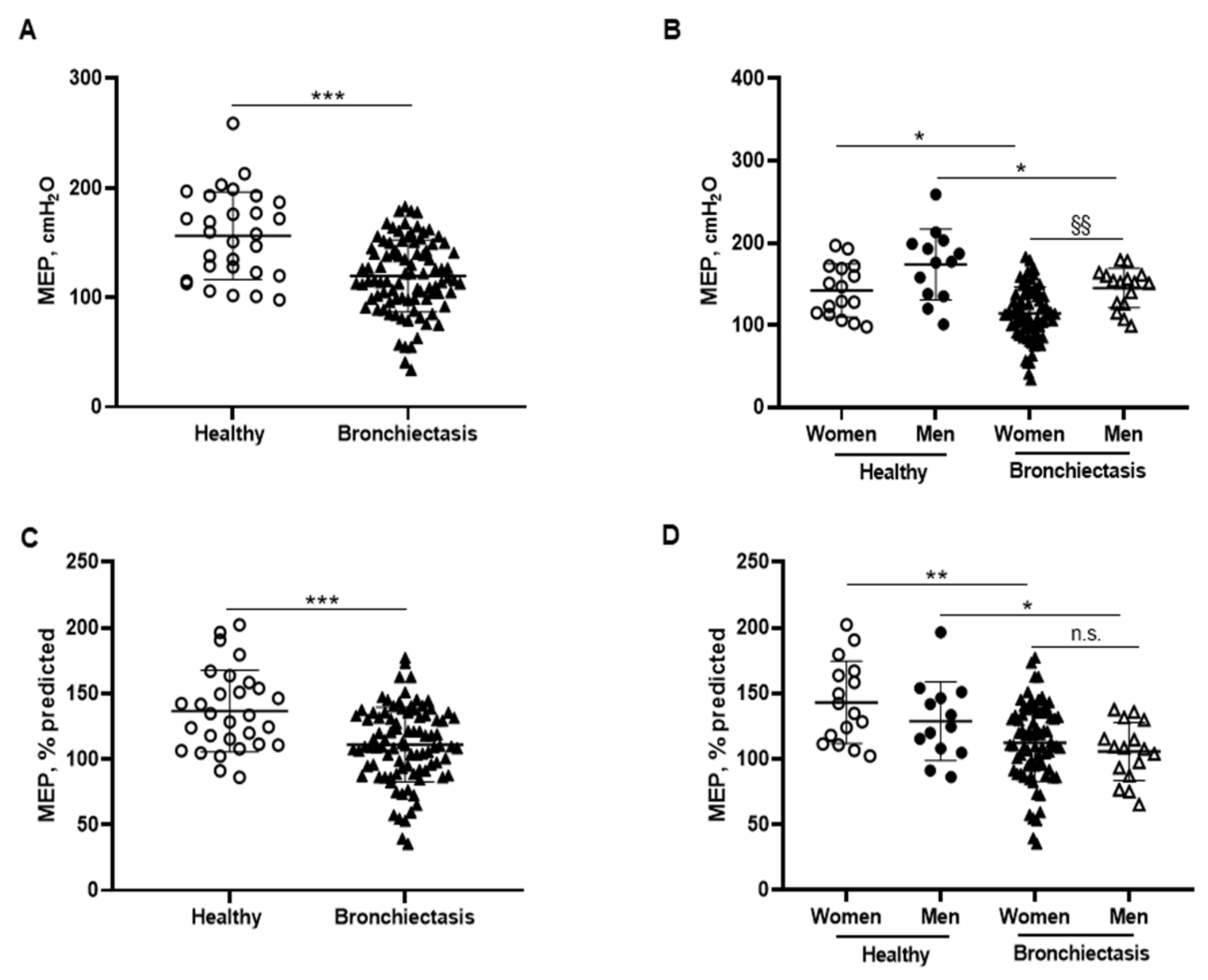
3.5. Inspiratory and Expiratory Muscle Strength
4. Discussion
5. Study Critique
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saleh, A.D.; Chalmers, J.D.; De Soyza, A.; Fardon, T.C.; Koustas, S.O.; Scott, J.; Simpson, A.J.; Brown, J.S.; Hurst, J.R. The heterogeneity of systemic inflammation in bronchiectasis. Respir. Med. 2017, 127, 33–39. [Google Scholar] [CrossRef] [Green Version]
- King, P. The pathophysiology of bronchiectasis. Int. J. Chron. Obstruct. Pulmon. Dis. 2009, 4, 411. [Google Scholar] [CrossRef] [PubMed]
- Seitz, A.E.; Olivier, K.N.; Steiner, C.A.; Montes de Oca, R.; Holland, S.M.; Prevots, D.R. Trends and Burden of Bronchiectasis-Associated Hospitalizations in the United States, 1993–2006. Chest 2010, 138, 944–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-García, M.Á.; Máiz, L.; Olveira, C.; Girón, R.M.; de la Rosa, D.; Blanco, M.; Cantón, R.; Vendrell, M.; Polverino, E.; de Gracia, J.; et al. Spanish Guidelines on the Evaluation and Diagnosis of Bronchiectasis in Adults. Arch. Bronconeumol. 2018, 54, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Villa, C.; Dobarganes, Y.; Olveira, C.; Girón, R.; García-Clemente, M.; Máiz, L.; Sibila, O.; Golpe, R.; Menéndez, R.; et al. Phenotypic Clustering in Non-Cystic Fibrosis Bronchiectasis Patients: The Role of Eosinophils in Disease Severity. Int. J. Environ. Res. Public Health 2021, 18, 8431. [Google Scholar] [CrossRef]
- Wang, X.; Villa, C.; Dobarganes, Y.; Olveira, C.; Girón, R.; García-Clemente, M.; Maíz, L.; Sibila, O.; Golpe, R.; Menéndez, R.; et al. Differences in Nutritional Status and Inflammatory Biomarkers between Female and Male Patients with Bronchiectasis: A Large-Cohort Study. Biomedicines 2021, 9, 905. [Google Scholar] [CrossRef]
- Qin, L.; Guitart, M.; Admetlló, M.; Esteban-Cucó, S.; Maiques, J.M.; Xia, Y.; Zha, J.; Carbullanca, S.; Duran, X.; Wang, X.; et al. Do Redox Balance and Inflammatory Events Take Place in Mild Bronchiectasis? A Hint to Clinical Implications. J. Clin. Med. 2021, 10, 4534. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Guitart, M.; Curull, V.; Sánchez-Font, A.; Duran, X.; Tang, J.; Admetlló, M.; Barreiro, E. Systemic Profiles of microRNAs, Redox Balance, and Inflammation in Lung Cancer Patients: Influence of COPD. Biomedicines 2021, 9, 1347. [Google Scholar] [CrossRef]
- van Bakel, S.I.J.; Gosker, H.R.; Langen, R.C.; Schols, A.M.W.J. Towards Personalized Management of Sarcopenia in COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2021, 16, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Maltais, F.; Decramer, M.; Casaburi, R.; Barreiro, E.; Burelle, Y.; Debigaŕe, R.; Richard Dekhuijzen, P.N.; Franssen, F.; Gayan-Ramirez, G.; Gea, J.; et al. An official American thoracic society/european respiratory society statement: Update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2014, 189, 1121–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaitovich, A.; Barreiro, E. Skeletal Muscle Dysfunction in Chronic Obstructive Pulmonary Disease. What We Know and Can Do for Our Patients. Am. J. Respir. Crit. Care Med. 2018, 198, 175–186. [Google Scholar] [CrossRef]
- Puig-Vilanova, E.; Rodriguez, D.A.; Lloreta, J.; Ausin, P.; Pascual-Guardia, S.; Broquetas, J.; Roca, J.; Gea, J.; Barreiro, E. Oxidative stress, redox signaling pathways, and autophagy in cachectic muscles of male patients with advanced COPD and lung cancer. Free Radic. Biol. Med. 2015, 79, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Puig-Vilanova, E.; Martínez-Llorens, J.; Ausin, P.; Roca, J.; Gea, J.; Barreiro, E. Quadriceps muscle weakness and atrophy are associated with a differential epigenetic profile in advanced COPD. Clin. Sci. 2015, 128, 905–921. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, E.; Schols, A.M.W.J.; Polkey, M.I.; Galdiz, J.B.; Gosker, H.R.; Swallow, E.B.; Coronell, C.; Gea, J. Cytokine profile in quadriceps muscles of patients with severe COPD. Thorax 2007, 63, 100–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seymour, J.M.; Spruit, M.A.; Hopkinson, N.S.; Natanek, S.A.; Man, W.D.C.D.-C.D.C.; Jackson, A.; Gosker, H.R.; Schols, A.M.W.J.; Moxham, J.; Polkey, M.I.; et al. The prevalence of quadriceps weakness in COPD and the relationship with disease severity. Eur. Respir. J. 2010, 36, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Marquis, K.; Debigaré, R.; Lacasse, Y.; LeBlanc, P.; Jobin, J.; Carrier, G.; Maltais, F. Midthigh Muscle Cross-Sectional Area Is a Better Predictor of Mortality than Body Mass Index in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2002, 166, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Swallow, E.B.; Reyes, D.; Hopkinson, N.S.; Man, W.D.C.; Porcher, R.; Cetti, E.J.; Moore, A.J.; Moxham, J.; Polkey, M.I. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax 2007, 62, 115–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barreiro, E.; Peinado, V.I.; Galdiz, J.B.; Ferrer, E.; Marin-Corral, J.; Sánchez, F.; Gea, J.; Barberà, J.A. Cigarette smoke-induced oxidative stress: A role in chronic obstructive pulmonary disease skeletal muscle dysfunction. Am. J. Respir. Crit. Care Med. 2010, 182, 477–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barreiro, E.; Salazar-Degracia, A.; Sancho-Muñoz, A.; Aguiló, R.; Rodríguez-Fuster, A.; Gea, J. Endoplasmic reticulum stress and unfolded protein response in diaphragm muscle dysfunction of patients with stable chronic obstructive pulmonary disease. J. Appl. Physiol. 2019, 126, 1572–1586. [Google Scholar] [CrossRef]
- Barreiro, E.; de la Puente, B.; Minguella, J.; Corominas, J.M.; Serrano, S.; Hussain, S.N.A.; Gea, J. Oxidative Stress and Respiratory Muscle Dysfunction in Severe Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2005, 171, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Marin-Corral, J.; Minguella, J.; Ramirez-Sarmiento, A.L.; Hussain, S.N.A.; Gea, J.; Barreiro, E. Oxidised proteins and superoxide anion production in the diaphragm of severe COPD patients. Eur. Respir. J. 2009, 33, 1309–1319. [Google Scholar] [CrossRef] [Green Version]
- Crimi, C.; Heffler, E.; Augelletti, T.; Campisi, R.; Noto, A.; Vancheri, C.; Crimi, N. Utility of ultrasound assessment of diaphragmatic function before and after pulmonary rehabilitation in COPD patients. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 3131–3139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferri, S.; Crimi, C.; Heffler, E.; Campisi, R.; Noto, A.; Crimi, N. Vitamin D and disease severity in bronchiectasis. Respir. Med. 2019, 148, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Muñoz, G.; Lopez-de-Andrés, A.; Hernández-Barrera, V.; Jiménez-García, R.; Pedraza-Serrano, F.; Puente-Maestu, L.; de Miguel-Díez, J. Bronchiectasis in patients hospitalized with acute exacerbation of COPD in Spain: Influence on mortality, hospital stay, and hospital costs (2006-2014) according to gender. PLoS ONE 2019, 14, e0211222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidaillac, C.; Yong, V.F.L.; Jaggi, T.K.; Soh, M.M.-M.; Chotirmall, S.H. Gender differences in bronchiectasis: A real issue? Breathe 2018, 14, 108–121. [Google Scholar] [CrossRef]
- Pinkerton, K.E.; Harbaugh, M.; Han, M.L.K.; Le Saux, C.J.; Van Winkle, L.S.; Martin, W.J.; Kosgei, R.J.; Carter, E.J.; Sitkin, N.; Smiley-Jewell, S.M.; et al. Women and lung disease: Sex differences and global health disparities. Am. J. Respir. Crit. Care Med. 2015, 192, 11–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ausín, P.; Martínez-Llorens, J.; Sabaté-Bresco, M.; Casadevall, C.; Barreiro, E.; Gea, J. Sex differences in function and structure of the quadriceps muscle in chronic obstructive pulmonary disease patients. Chron. Respir. Dis. 2017, 14, 127–139. [Google Scholar] [CrossRef]
- Martínez-García, M.Á.; Máiz, L.; Olveira, C.; Girón, R.M.; de la Rosa, D.; Blanco, M.; Cantón, R.; Vendrell, M.; Polverino, E.; de Gracia, J.; et al. Spanish Guidelines on Treatment of Bronchiectasis in Adults. Arch. Bronconeumol. 2018, 54, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Polverino, E.; Goeminne, P.C.; McDonnell, M.J.; Aliberti, S.; Marshall, S.E.; Loebinger, M.R.; Murris, M.; Cantón, R.; Torres, A.; Dimakou, K.; et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur. Respir. J. 2017, 50, 1700629. [Google Scholar] [CrossRef] [PubMed]
- Mechanisms of acute respiratory failure. Am. Rev. Respir. Dis. 1977, 115, 1071–1078. [CrossRef]
- Soler-Cataluña, J.J.; Piñera, P.; Trigueros, J.A.; Calle, M.; Casanova, C.; Cosío, B.G.; López-Campos, J.L.; Molina, J.; Almagro, P.; Gómez, J.T.; et al. Spanish COPD Guidelines (GesEPOC) 2021 Update Diagnosis and Treatment af COPD Exacerbation Syndrome. Arch. Bronconeumol. 2021, 21. [Google Scholar] [CrossRef]
- Shrestha, B.; Dunn, L. The Declaration of Helsinki on Medical Research involving Human Subjects: A Review of Seventh Revision. J. Nepal Health Res. Counc. 2020, 17, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, M.A.; de Gracia, J.; Vendrell Relat, M.; Giron, R.-M.; Maiz Carro, L.; de la Rosa Carrillo, D.; Olveira, C. Multidimensional approach to non-cystic fibrosis bronchiectasis: The FACED score. Eur. Respir. J. 2014, 43, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, M.A.; Athanazio, R.A.; Girón, R.M.; Máiz-Carro, L.; de la Rosa, D.; Olveira, C.; de Gracia, J.; Vendrell, M.; Prados-Sánchez, C.; Gramblicka, G.; et al. Predicting high risk of exacerbations in bronchiectasis: The E-FACED score. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 275–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalmers, J.D.; Goeminne, P.; Aliberti, S.; McDonnell, M.J.; Lonni, S.; Davidson, J.; Poppelwell, L.; Salih, W.; Pesci, A.; Dupont, L.J.; et al. The bronchiectasis severity index an international derivation and validation study. Am. J. Respir. Crit. Care Med. 2014, 189, 576–585. [Google Scholar] [CrossRef]
- Bhalla, M.; Turcios, N.; Aponte, V.; Jenkins, M.; Leitman, B.S.; McCauley, D.I.; Naidich, D.P. Cystic Fibrosis: Scoring System with Thin-Section CT. Radiology 1991, 179, 783–788. [Google Scholar] [CrossRef]
- Roberts, H.R. Airflow obstruction in bronchiectasis: Correlation between computed tomography features and pulmonary function tests. Thorax 2000, 55, 198–204. [Google Scholar] [CrossRef] [Green Version]
- Francisco García-Río, C.; Calle, M.; Felip Burgos, A.; Gáldiz, J.B.; Giner, J.; González-Mangado, N.; Ortega, F.; Puente Maestu, L. Normativa Sobre la Espirometría. Arch. Bronconeumol. 2013, 49, 388–401. [Google Scholar] [CrossRef]
- Roca, J.; Burgos, F.; Barberà, J.A.; Sunyer, J.; Rodriguez-Roisin, R.; Castellsagué, J.; Sanchis, J.; Antóo, J.M.; Casan, P.; Clausen, J.L. Prediction equations for plethysmographic lung volumes. Respir. Med. 1998, 92, 454–460. [Google Scholar] [CrossRef] [Green Version]
- Roca, J.; Rodriguez-Roisin, R.; Cobo, E.; Burgos, F.; Perez, J.; Clausen, J.L. Single-breath carbon monoxide diffusing capacity prediction equations from a Mediterranean population. Am. Rev. Respir. Dis. 1990, 141, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Roca, J.; Sanchis, J.; Agusti-Vidal, A.; Segarra, F.; Navajas, D.; Rodriguez-Roisin, R.; Casan, P.; Sans, S. Spirometric reference values from a Mediterranean population. Bull. Eur. Physiopathol. Respir. 1986, 22, 217–224. [Google Scholar]
- Luna-Heredia, E.; Martín-Peña, G.; Ruiz-Galiana, J. Handgrip dynamometry in healthy adults. Clin. Nutr. 2005, 24, 250–258. [Google Scholar] [CrossRef]
- Mathiowetz, V.; Kashman, N.; Volland, G.; Weber, K.; Dowe, M.; Rogers, S. Grip and Pinch Strength: Normative Data for Adults. Arch. Phys. Med. Rehabil. 1985, 66, 69–74. [Google Scholar]
- Laveneziana, P.; Albuquerque, A.; Aliverti, A.; Babb, T.; Barreiro, E.; Dres, M.; Dubé, B.P.; Fauroux, B.; Gea, J.; Guenette, J.A.; et al. ERS statement on respiratory muscle testing at rest and during exercise. Eur. Respir. J. 2019, 53, 1801214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araújo, P.R.S.; Resqueti, V.R.; Nascimento, J., Jr.; Carvalho, L.D.A.; Cavalcanti, A.G.L.; Silva, V.C.; Silva, E.; Moreno, M.A.; de Andrade, A.d.F.D.; Fregonezi, G.A.D.F. Reference values for sniff nasal inspiratory pressure in healthy subjects in Brazil: A multicenter study. J. Bras. Pneumol. 2012, 38, 700–707. [Google Scholar] [CrossRef] [Green Version]
- Maillard, J.O.; Burdet, L.; Van Melle, G.; Fitting, J.W. Reproducibility of twitch mouth pressure, sniff nasal inspiratory pressure, and maximal inspiratory pressure. Eur. Respir. J. 1998, 11, 901–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Issues, S.; Test, M.W.; Equipment, R.; Preparation, P. American Thoracic Society ATS Statement: Guidelines for the Six-Minute Walk Test. Am. J. Respir. Crit. Care 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Barreiro, E.; Bustamante, V.; Cejudo, P.; Gáldiz, J.B.; Gea, J.; de Lucas, P.; Martínez-Llorens, J.; Ortega, F.; Puente-Maestu, L.; Roca, J.; et al. Guidelines for the Evaluation and Treatment of Muscle Dysfunction in Patients with Chronic Obstructive Pulmonary Disease. Arch. Bronconeumol. 2015, 51, 384–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enright, P.L.; Sherrill, D.L. Reference Equations for the Six-Minute Walk.pdf. Am. J. Respir. Crit. Care Med. 1998, 158, 1384–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, S.J.; Nyulasi, I.B.; Strauss, B.J.G.; Kotsimbos, T.; Bailey, M.; Wilson, J.W. Fat-free mass depletion in cystic fibrosis: Associated with lung disease severity but poorly detected by body mass index. Nutrition 2010, 26, 753–759. [Google Scholar] [CrossRef]
- Despotes, K.A.; Choate, R.; Addrizzo-Harris, D.; Aksamit, T.R.; Barker, A.; Basavaraj, A.; Daley, C.L.; Eden, E.; DiMango, A.; Fennelly, K.; et al. Nutrition and Markers of Disease Severity in Patients With Bronchiectasis. Chronic Obstr. Pulm. Dis. J. COPD Found. 2020, 7, 390–403. [Google Scholar] [CrossRef]
- Alcaraz-Serrano, V.; Gimeno-Santos, E.; Gimeno-Santos, E.; Scioscia, G.; Gabarrús, A.; Navarro, A.; Herrero-Cortina, B.; Herrero-Cortina, B.; Amaro, R.; Fernández-Barat, L.; et al. Association between physical activity and risk of hospitalisation in bronchiectasis. Eur. Respir. J. 2020, 55, 1902138. [Google Scholar] [CrossRef]
- Yildiz, S.; Inal-Ince, D.; Calik-Kutukcu, E.; Vardar-Yagli, N.; Saglam, M.; Arikan, H.; Coplu, L. Clinical Determinants of Incremental Shuttle Walk Test in Adults with Bronchiectasis. Lung 2018, 196, 343–349. [Google Scholar] [CrossRef]
- Milani, R.V.; Ries, A.L.; Myers, J. Peripheral Muscle Weakness Contributes to Exercise Limitation in COPD. J. Cardiopulm. Rehabil. 1996, 16, 420–421. [Google Scholar] [CrossRef]
- de Camargo, A.A.; Boldorini, J.C.; Holland, A.E.; de Castro, R.A.S.; Lanza, F.d.C.; Athanazio, R.A.; Rached, S.Z.; Carvalho-Pinto, R.; Cukier, A.; Stelmach, R.; et al. Determinants of Peripheral Muscle Strength and Activity in Daily Life in People with Bronchiectasis. Phys. Ther. 2018, 98, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Decramer, M.; Gosselink, R.; Troosters, T.; Verschueren, M.; Evers, G. Muscle weakness is related to utilization of health care resources in COPD patients. Eur. Respir. J. 1997, 10, 417–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holland, A.E.; Cox, N.S.; Houchen-Wolloff, L.; Rochester, C.L.; Garvey, C.; ZuWallack, R.; Nici, L.; Limberg, T.; Lareau, S.C.; Yawn, B.P.; et al. Defining Modern Pulmonary Rehabilitation. An Official American Thoracic Society Workshop Report. Ann. Am. Thorac. Soc. 2021, 18, e12–e29. [Google Scholar] [CrossRef]
- Gielen, S.; Adams, V.; Linke, A.; Erbs, S.; Möbius-Winkler, S.; Schubert, A.; Schuler, G.; Hambrecht, R. Exercise training in chronic heart failure: Correlation between reduced local inflammation and improved oxidative capacity in the skeletal muscle. Eur. J. Cardiovasc. Prev. Rehabil. 2005, 12, 393–400. [Google Scholar] [CrossRef]
- Hambrecht, R.; Schulze, P.C.; Gielen, S.; Linke, A.; Möbius-Winkler, S.; Erbs, S.; Kratzsch, J.; Schubert, A.; Adams, V.; Schuler, G. Effects of exercise training on insulin-like growth factor-I expression in the skeletal muscle of non-cachectic patients with chronic heart failure. Eur. J. Cardiovasc. Prev. Rehabil. 2005, 12, 401–406. [Google Scholar] [CrossRef]
- Tzanis, G.; Philippou, A.; Karatzanos, E.; Dimopoulos, S.; Kaldara, E.; Nana, E.; Pitsolis, T.; Rontogianni, D.; Koutsilieris, M.; Nanas, S. Effects of High-Intensity Interval Exercise Training on Skeletal Myopathy of Chronic Heart Failure. J. Card. Fail. 2017, 23, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; ZuWallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.C.; et al. An Official American Thoracic Society/European Respiratory Society Statement: Key Concepts and Advances in Pulmonary Rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, E.; Sznajder, J.I.; Nader, G.A.; Budinger, G.R.S. Muscle dysfunction in patients with lung diseases a growing epidemic. Am. J. Respir. Crit. Care Med. 2015, 191, 616–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, D.A.; Kalko, S.; Puig-Vilanova, Ø.; Perez-Olabarría, M.; Falciani, F.; Gea, J.; Cascante, M.; Barreiro, E.; Roca, J.; Puig-Vilanova, E.; et al. Muscle and blood redox status after exercise training in severe COPD patients. Free Radic. Biol. Med. 2012, 52, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Vogiatzis, I.; Simoes, D.C.M.; Stratakos, G.; Kourepini, E.; Terzis, G.; Manta, P.; Athanasopoulos, D.; Roussos, C.; Wagner, P.D.; Zakynthinos, S. Effect of pulmonary rehabilitation on muscle remodelling in cachectic patients with COPD. Eur. Respir. J. 2010, 36, 301–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maltais, F.; LeBlanc, P.; Simard, C.; Jobin, J.; Bérubé, C.; Bruneau, J.; Carrier, L.; Belleau, R. Skeletal muscle adaptation to endurance training in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1996, 154, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Maltais, F.; LeBlanc, P.; Jobin, J.; Bérubé, C.; Bruneau, J.; Carrier, L.; Breton, M.J.; Falardeau, G.; Belleau, R. Intensity of training and physiologic adaptation in patients with chronic obstructive pulmonary disease. Pneumologie 1997, 51, 972–973. [Google Scholar] [CrossRef] [PubMed]
- Casaburi, R.; Porszasz, J.; Burns, M.R.; Carithers, E.R.; Chang, R.S.Y.; Cooper, C.B. Physiologic benefits of exercise training in rehabilitation of patients with severe chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1997, 155, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- Sala, E.; Roca, J.; Marrades, R.M.; Alonso, J.; Gonzalez de Suso, J.M.; Moreno, A.; Barberá, J.A.; Nadal, J.; de Jover, L.; Rodriguez-Roisin, R.; et al. Effects of Endurance Training on Skeletal Muscle Bioenergetics in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 1999, 159, 1726–1734. [Google Scholar] [CrossRef] [Green Version]
- Casaburi, R.; Patessio, A.; Ioli, F.; Zanaboni, S.; Donner, C.F.; Wasserman, K. Reductions in Exercise Lactic Acidosis and Ventilation as a Result of Exercise Training in Patients with Obstructive Lung Disease. Am. Rev. Respir. Dis. 1991, 143, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Ozalp, O.; Inal-Ince, D.; Calik, E.; Vardar-Yagli, N.; Saglam, M.; Savci, S.; Arikan, H.; Bosnak-Guclu, M.; Coplu, L. Extrapulmonary features of bronchiectasis: Muscle function, exercise capacity, fatigue, and health status. Multidiscip. Respir. Med. 2012, 7, 3. [Google Scholar] [CrossRef] [Green Version]
- Héritier, F.; Rahm, F.; Pasche, P.; Fitting, J.W. Sniff nasal inspiratory pressure. A noninvasive assessment of inspiratory muscle strength. Am. J. Respir. Crit. Care Med. 1994, 150, 1678–1683. [Google Scholar] [CrossRef] [PubMed]
- Crimi, C.; Ferri, S.; Campisi, R.; Crimi, N. The Link between Asthma and Bronchiectasis: State of the Art. Respiration 2020, 99, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Ferri, S.; Crimi, C.; Campisi, R.; Cacopardo, G.; Paoletti, G.; Puggioni, F.; Crimi, N.; Heffler, E. Impact of asthma on bronchiectasis severity and risk of exacerbations. J. Asthma 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
| Healthy Controls | Bronchiectasis Patients | |
|---|---|---|
| N = 37 | N = 150 | |
| Age, years, (SD) | 62.4 (9.8) | 64.6 (13.2) |
| Disease severity, (SD) | ||
| FACED score | NA | 1.67 (1.40) |
| EFACED score | NA | 1.94 (1.64) |
| BSI score | NA | 5.74 (3.42) |
| Exacerbations in previous year | NA | 0.91 (1.11) |
| Hospitalizations for exacerbations | NA | 0.21 (0.66) |
| Chronic colonization by PA, N (%) | NA | 19 (12.7) |
| Radiological extension, (SD) | ||
| Total extent bronchiectasis score | NA | 7.6 (3.6) |
| Bronchial dilatation score | NA | 1.2 (0.3) |
| Bronchial wall thickness score | NA | 1.2 (0.3) |
| Global severity score | NA | 10 (3.5) |
| Smoking history | ||
| Current smokers, N (%) | 0 | 7 (5) |
| Ex-smokers, N (%) | 0 | 51 (34) |
| Never smokers, N (%) | 37 | 92 (61) |
| Packs-year, (SD) | 0 | 20.5 (16.7) |
| Lung function, (SD) | ||
| FEV1, % predicted | 99 (12) | 74 (22) *** |
| FVC, % predicted | 100 (12) | 83 (20) *** |
| FEV1/FVC, % | 78 (6) | 68 (13) *** |
| DLCO, % predicted | 92 (6) | 75 (16) *** |
| KCO, % predicted | 88 (13) | 78 (14) * |
| RV, % predicted | 107 (8) | 147 (35) *** |
| TLC, % predicted | 97 (7) | 101 (16) |
| RV/TLC, % | 38 (3) | 54 (10) *** |
| Exercise capacity, (SD) | ||
| 6-min walking distance, meters | 533 (68) | 463 (99) *** |
| Distance, % predicted | 106 (13) | 95 (19) *** |
| Initial oxygen saturation, % | 98 (1) | 96 (2) *** |
| Medium oxygen saturation, % | 97 (2) | 94 (3) *** |
| Minimum oxygen saturation, % | 96 (2) | 93 (4) *** |
| Final oxygen saturation, % | 96 (2) | 93 (4) *** |
| Blood parameters, (SD) | ||
| CRP, mg/dL | 0.2 (0.1) | 0.6 (0.9) *** |
| ESR, mm/h | 5.7 (3.9) | 11.0 (9.8) ** |
| Fibrinogen, mg/dL | 333.6 (63.7) | 393.8 (97.2) *** |
| Alpha-1 antitrypsin | 118.9 (16.9) | 135.3 (27.2) ** |
| Hemoglobin, g/dL | 14.4 (1.3) | 13.9 (1.9) * |
| Hematocrit, % | 43.1 (3.8) | 42.2 (4.8) |
| Creatinine, mg/dL | 0.8 (0.2) | 0.8 (0.2) |
| Total proteins, g/dL | 7.2 (0.3) | 7.1 (0.5) |
| Albumin, g/dL | 4.6 (0.2) | 4.4 (0.3) *** |
| Prealbumin, g/dL | 26.3 (4.5) | 22.7 (5.2) ** |
| Healthy Controls | Bronchiectasis Patients | |||
|---|---|---|---|---|
| Women | Men | Women | Men | |
| N = 21 | N = 16 | N = 114 | N = 36 | |
| Age, years | 63.4 (9.7) | 61 (10.1) | 65.4 (12.4) | 62.0 (15.3) |
| Disease severity | ||||
| BSI | NA | NA | 5.98 (3.57) | 4.97 (2.80) § |
| EFACED | NA | NA | 2.08 (1.75) | 1.5 (1.16) § |
| FACED | NA | NA | 1.76 (1.47) | 1.39 (1.10) |
| Exacerbations in previous year | NA | NA | 0.94 (1.17) | 0.83 (0.88) |
| Hospitalization for exacerbations | NA | NA | 0.25 (0.73) | 0.08 (0.37) |
| Chronic colonization by PA | NA | NA | 14 (12.3) | 5 (13.9) |
| Radiological extension, (SD) | ||||
| Total extent of bronchiectasis score | NA | NA | 7.3 (3.5) | 8.7 (3.5) § |
| Bronchial dilatation score | NA | NA | 1.2 (0.4) | 1.1 (0.2) |
| Bronchial wall thickness score | NA | NA | 1.2 (0.3) | 1.2 (0.2) |
| Global severity score | NA | NA | 9.7 (3.5) | 11 (3.6) § |
| Smoking history | ||||
| Current smokers, N | 0 | 0 | 6 (5) | 1 (3) |
| Ex-smokers, N | 0 | 0 | 35 (31) | 16 (44) |
| Never smokers, N | 21 (100) | 16 (100) | 73 (64) | 19 (53) |
| Packs-year, (SD) | 0 | 0 | 21.3 (15.2) | 18.5 (20.6) |
| Lung function, (SD) | ||||
| FEV1, % predicted | 101 (12) | 96 (13) | 74 (22) *** | 74 (23) ** |
| FVC, % predicted | 100 (12) | 100 (12) | 84 (20) ** | 83 (21) * |
| FEV1/FVC, % | 80 (5) | 75 (7) | 67 (13) *** | 68 (11) |
| DLCO, % predicted | 88 (8) | 94 (4) | 74 (16) * | 77 (16) * |
| KCO, % predicted | 82 (3) | 92 (15) | 76 (15) | 84 (11) * |
| RV, % predicted | 107 (9) | 106 (8) | 152 (36) * | 132 (28) *§ |
| TLC, % predicted | 98 (6) | 97 (8) | 103 (15) | 93 (15) * |
| RV/TLC, % | 39 (1) | 38 (4) | 57 (10) * | 47 (7) ** §§§ |
| Exercise capacity, (SD) | ||||
| 6-min walking distance, meters | 496 (60) | 579 (44) | 447 (94) * | 512 (99) * §§ |
| Distance, % predicted | 105 (10) | 108 (15) | 94 (18) * | 97 (23) * |
| Initial oxygen saturation, % | 98 (1) | 98 (1) | 96 (2) ** | 96 (2) ** |
| Medium oxygen saturation, % | 97 (2) | 97 (1) | 94 (3) ** | 94 (3) ** |
| Minimum oxygen saturation, % | 96 (2) | 97 (2) | 92 (4) *** | 93 (3) ** |
| Final oxygen saturation, % | 96 (2) | 97 (2) | 92 (4) *** | 93 (3) ** |
| Blood parameters, (SD) | ||||
| CRP, mg/dL | 0.2 (0.1) | 0.2 (0.1) | 0.6 (0.9) * | 0.6 (0.6) * |
| ESR, mm/h | 6.6 (3.6) | 4.7 (4.0) | 11.3 (9.9) * | 10 (9.6) * |
| Fibrinogen, mg/dL | 349.5 (64) | 313.8 (59.3) | 397.4 (88.6) * | 381.8 (122.1) * |
| Alpha-1 antitrypsin, mg/dL | 117.5 (19.0) | 121.2 (13.4) | 134.3 (24.5) ** | 138.7 (35.0) * |
| Hemoglobin, g/dL | 13.7 (1.0) | 15.2 (1.0) | 13.7 (2.1) | 14.5 (1.0) * § |
| Hematocrit, % | 41.3 (3.4) | 45.6 (2.9) | 41.7 (5) | 43.9 (3.6) § |
| Creatinine, mg/dL | 0.7 (0.1) | 1.0 (0.1) | 0.7 (0.1) | 1.0 (0.2) §§§ |
| Total proteins, g/dL | 7.2 (0.3) | 7.2 (0.3) | 7.1 (0.4) | 7.2 (0.5) |
| Albumin, g/dL | 4.6 (0.2) | 4.6 (0.2) | 4.4 (0.3) ** | 4.4 (0.4) * |
| Prealbumin, g/dL | 25.5 (4.7) | 27.6 (3.9) | 21.8 (4.7) ** | 25.3 (5.9) §§ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Balaña-Corberó, A.; Martínez-Llorens, J.; Qin, L.; Xia, Y.; Zha, J.; Maiques, J.M.; Barreiro, E. Respiratory and Peripheral Muscle Weakness and Body Composition Abnormalities in Non-Cystic Fibrosis Bronchiectasis Patients: Gender Differences. Biomedicines 2022, 10, 334. https://doi.org/10.3390/biomedicines10020334
Wang X, Balaña-Corberó A, Martínez-Llorens J, Qin L, Xia Y, Zha J, Maiques JM, Barreiro E. Respiratory and Peripheral Muscle Weakness and Body Composition Abnormalities in Non-Cystic Fibrosis Bronchiectasis Patients: Gender Differences. Biomedicines. 2022; 10(2):334. https://doi.org/10.3390/biomedicines10020334
Chicago/Turabian StyleWang, Xuejie, Ana Balaña-Corberó, Juana Martínez-Llorens, Liyun Qin, Yingchen Xia, Jianhua Zha, José María Maiques, and Esther Barreiro. 2022. "Respiratory and Peripheral Muscle Weakness and Body Composition Abnormalities in Non-Cystic Fibrosis Bronchiectasis Patients: Gender Differences" Biomedicines 10, no. 2: 334. https://doi.org/10.3390/biomedicines10020334
APA StyleWang, X., Balaña-Corberó, A., Martínez-Llorens, J., Qin, L., Xia, Y., Zha, J., Maiques, J. M., & Barreiro, E. (2022). Respiratory and Peripheral Muscle Weakness and Body Composition Abnormalities in Non-Cystic Fibrosis Bronchiectasis Patients: Gender Differences. Biomedicines, 10(2), 334. https://doi.org/10.3390/biomedicines10020334







