Differences in Tolerance to Hypoxia: Physiological, Biochemical, and Molecular-Biological Characteristics
Abstract
:1. Introduction
2. Cellular Response to Hypoxic Exposure
2.1. Structure and Functions of Hypoxia-Inducible Factor
2.2. HIF Expression Variability in Humans
3. Physiological, Biochemical, and Molecular-Biological Characteristics of People with Different Tolerance to Hypoxia and Methods of Its Evaluation
3.1. AMS Susceptibility and Methods of Its Determination
3.2. HAPE Susceptibility and Methods of Its Determination
4. Physiological, Biochemical, and Molecular-Biological Characteristics of Animals with Different Tolerances to Hypoxia and Methods of Its Evaluation
4.1. Methods for Determining Hypoxia Tolerance in Animals
4.2. Molecular-Biological, Pathophysiological and Biochemical Differences of Tolerant- and Susceptible-to-Hypoxia Animals
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hirota, K. Involvement of Hypoxia-Inducible Factors in the Dysregulation of Oxygen Homeostasis in Sepsis. Cardiovasc. Hematol. Disord. Drug Targets 2015, 15, 29–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiers, H.D.; Scheffer, G.-J.; van der Hoeven, J.G.; Eltzschig, H.K.; Pickkers, P.; Kox, M. Immunologic Consequences of Hypoxia during Critical Illness. Anesthesiology 2016, 125, 237–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schito, L.; Semenza, G.L. Hypoxia-Inducible Factors: Master Regulators of Cancer Progression. Trends Cancer 2016, 2, 758–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devraj, G.; Beerlage, C.; Brüne, B.; Kempf, V.A.J. Hypoxia and HIF-1 activation in bacterial infections. Microbes Infect. 2017, 19, 144–156. [Google Scholar] [CrossRef]
- Van Welden, S.; Selfridge, A.C.; Hindryckx, P. Intestinal hypoxia and hypoxia-induced signalling as therapeutic targets for IBD. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 596–611. [Google Scholar] [CrossRef]
- Watts, E.R.; Walmsley, S.R. Inflammation and Hypoxia: HIF and PHD Isoform Selectivity. Trends Mol. Med. 2019, 25, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Brooks, J.T.S.; Elvidge, G.P.; Glenny, L.; Gleadle, J.M.; Liu, C.; Ragoussis, J.; Smith, T.G.; Talbot, N.P.; Winchester, L.; Maxwell, P.H.; et al. Variations within oxygen-regulated gene expression in humans. J. Appl. Physiol. 2009, 106, 212–220. [Google Scholar] [CrossRef]
- Gorr, T.A.; Wichmann, D.; Hu, J.; Hermes-Lima, M.; Welker, A.F.; Terwilliger, N.; Wren, J.F.; Viney, M.; Morris, S.; Nilsson, G.E.; et al. Hypoxia Tolerance in Animals: Biology and Application. Physiol. Biochem. Zool. 2010, 83, 733–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mironova, G.D.; Shigaeva, M.I.; Gritsenko, E.N.; Murzaeva, S.V.; Gorbacheva, O.S.; Germanova, E.L.; Lukyanova, L.D. Functioning of the mitochondrial ATP-dependent potassium channel in rats varying in their resistance to hypoxia. Involvement of the channel in the process of animal’s adaptation to hypoxia. J. Bioenerg. Biomembr. 2010, 42, 473–481. [Google Scholar] [CrossRef]
- Serebrovskaya, T.V.; Xi, L. Individualized Intermittent Hypoxia Training: Principles and Practices. In Intermittent Hypoxia and Human Diseases; Springer: London, UK, 2012; pp. 281–289. [Google Scholar] [CrossRef]
- Jain, K.; Suryakumar, G.; Prasad, R.; Ganju, L. Upregulation of Cytoprotective Defense Mechanisms and Hypoxia-Responsive Proteins Imparts Tolerance to Acute Hypobaric Hypoxia. High Alt. Med. Biol. 2013, 14, 65–77. [Google Scholar] [CrossRef]
- Jain, K.; Suryakumar, G.; Prasad, R.; Ganju, L. Differential activation of myocardial ER stress response: A possible role in hypoxic tolerance. Int. J. Cardiol. 2013, 168, 4667–4677. [Google Scholar] [CrossRef] [PubMed]
- Kirova, Y.I.; Germanova, E.L.; Lukyanova, L.D. Phenotypic Features of the Dynamics of HIF-1α Levels in Rat Neocortex in Different Hypoxia Regimens. Bull. Exp. Biol. Med. 2013, 154, 718–722. [Google Scholar] [CrossRef]
- Jain, K.; Suryakumar, G.; Ganju, L.; Singh, S.B. Differential hypoxic tolerance is mediated by activation of heat shock response and nitric oxide pathway. Cell Stress Chaperones. 2014, 19, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Lukyanova, L.D.; Kirova, Y.I. Mitochondria-controlled signaling mechanisms of brain protection in hypoxia. Front. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacInnis, M.J.; Koehle, M.S. Evidence for and Against Genetic Predispositions to Acute and Chronic Altitude Illnesses. High Alt. Med. Biol. 2016, 17, 281–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlik, L.L.; Mikheeva, I.B.; Al’-Mugkhrabi, Y.M.; Berest, V.P.; Kirova, Y.I.; Germanova, E.L.; Luk’yanova, L.D.; Mironova, G.D. Specific Features of Immediate Ultrastructural Changes in Brain Cortex Mitochondria of Rats with Different Tolerance to Hypoxia under Various Modes of Hypoxic Exposures. Bull. Exp. Biol. Med. 2018, 164, 376–381. [Google Scholar] [CrossRef]
- Dzhalilova, D.S.; Kosyreva, A.M.; Diatroptov, M.E.; Ponomarenko, E.A.; Tsvetkov, I.S.; Zolotova, N.A.; Mkhitarov, V.A.; Khochanskiy, D.N.; Makarova, O.V. Dependence of the severity of the systemic inflammatory response on resistance to hypoxia in male Wistar rats. J. Inflamm. Res. 2019, 12, 73–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dzhalilova, D.S.; Kosyreva, A.M.; Diatroptov, M.E.; Zolotova, N.A.; Tsvetkov, I.S.; Mkhitarov, V.A.; Makarova, O.V.; Khochanskiy, D.N. Morphological Characteristics of the Thymus and Spleen and the Subpopulation Composition of Lymphocytes in Peripheral Blood during Systemic Inflammatory Response in Male Rats with Different Resistance to Hypoxia. Int. J. Inflam. 2019, 2019, 7584685. [Google Scholar] [CrossRef] [PubMed]
- Kurhaluk, N.; Lukash, O.; Nosar, V.; Portnychenko, A.; Portnichenko, V.; Wszedybyl-Winklewska, M.; Winklewski, P.J. Liver mitochondrial respiratory plasticity and oxygen uptake evoked by cobalt chloride in rats with low and high resistance to extreme hypobaric hypoxia. Can. J. Physiol. Pharmacol. 2019, 97, 392–399. [Google Scholar] [CrossRef]
- Mironova, G.D.; Pavlik, L.L.; Kirova, Y.I.; Belosludtseva, N.V.; Mosentsov, A.A.; Khmil, N.V.; Germanova, E.L.; Lukyanova, L.D. Effect of hypoxia on mitochondrial enzymes and ultrastructure in the brain cortex of rats with different tolerance to oxygen shortage. J. Bioenerg. Biomembr. 2019, 51, 329–340. [Google Scholar] [CrossRef]
- Belosludtsev, K.N.; Dubinin, M.V.; Talanov, E.Y.; Starinets, V.S.; Tenkov, K.S.; Zakharova, N.M.; Belosludtseva, N.V. Transport of Ca2+ and Ca2+-Dependent Permeability Transition in the Liver and Heart Mitochondria of Rats with Different Tolerance to Acute Hypoxia. Biomolecules 2020, 10, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schultz, A.; Lavie, L.; Hochberg, I.; Beyar, R.; Stone, T.; Skorecki, K.; Lavie, P.; Roguin, A.; Levy, A.P. Interindividual Heterogeneity in the Hypoxic Regulation of VEGF: Significance for the Development of the Coronary Artery Collateral Circulation. Circulation 1999, 100, 547–552. [Google Scholar] [CrossRef] [Green Version]
- Semenza, G.L.; Wang, G.L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell. Biol. 1992, 12, 5447–5454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaelin, W.G.; Ratcliffe, P.J. Oxygen Sensing by Metazoans: The Central Role of the HIF Hydroxylase Pathway. Mol. Cell. 2008, 30, 393–402. [Google Scholar] [CrossRef]
- Semenza, G.L. Oxygen homeostasis. WIREs Syst. Biol. Med. 2010, 2, 336–361. [Google Scholar] [CrossRef]
- Ratcliffe, P.; Koivunen, P.; Myllyharju, J.; Ragoussis, J.; Bovée, J.V.; Batinic-Haberle, I.; Vinatier, C.; Trichet, V.; Robriquet, F.; Oliver, L.; et al. Update on hypoxia-inducible factors and hydroxylases in oxygen regulatory pathways: From physiology to therapeutics. Hypoxia (Auckl) 2017, 5, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Hirota, K. Basic Biology of Hypoxic Responses Mediated by the Transcription Factor HIFs and Its Implication for Medicine. Biomedicines 2020, 8, 32. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaakkola, P.; Mole, D.R.; Tian, Y.-M.; Wilson, M.I.; Gielbert, J.; Gaskell, S.J.; von Kriegsheim, A.; Hebestreit, H.F.; Mukherji, M.; Schofield, C.J.; et al. Targeting of HIF-α to the von Hippel-Lindau Ubiquitylation Complex by O2-Regulated Prolyl Hydroxylation. Science 2001, 292, 468–472. [Google Scholar] [CrossRef]
- Mahon, P.C.; Hirota, K.; Semenza, G.L. FIH-1: A novel protein that interacts with HIF-1α and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001, 15, 2675–2686. [Google Scholar] [CrossRef] [Green Version]
- Mole, D.R.; Maxwell, P.H.; Pugh, C.W.; Ratcliffe, P.J. Regulation of HIF by the von Hippel-Lindau tumour suppressor: Implications for cellular oxygen sensing. IUBMB Life 2001, 52, 43–47. [Google Scholar] [CrossRef]
- Wong, B.W.; Kuchnio, A.; Bruning, U.; Carmeliet, P. Emerging novel functions of the oxygen-sensing prolyl hydroxylase domain enzymes. Trends Biochem. Sci. 2013, 38, 3–11. [Google Scholar] [CrossRef]
- Zurlo, G.; Guo, J.; Takada, M.; Wei, W.; Zhang, Q. New Insights into Protein Hydroxylation and Its Important Role in Human Diseases. Biochim. Biophys. Acta 2016, 1866, 208–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieb, M.E.; Menzies, K.; Moschella, M.C.; Ni, R.; Taubman, M.B. Mammalian EGLN genes have distinct patterns of mRNA expression and regulation. Biochem. Cell Biol. 2002, 80, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Elkins, J.M.; Hewitson, K.S.; McNeill, L.A.; Seibel, J.F.; Schlemminger, I.; Pugh, C.W.; Ratcliffe, P.J.; Schofield, C.J. Structure of factor-inhibiting hypoxia-inducible factor (HIF) reveals mechanism of oxidative modification of HIF-1 alpha. J. Biol. Chem. 2003, 278, 1802–1806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Fu, Z.; Linke, S.; Chicher, J.; Gorman, J.J.; Visk, D.; Haddad, G.G.; Poellinger, L.; Peet, D.J.; Powell, F.; et al. The asparaginyl hydroxylase factor inhibiting HIF-1alpha is an essential regulator of metabolism. Cell Metab. 2010, 11, 364–378. [Google Scholar] [CrossRef] [Green Version]
- Hewitson, K.S.; McNeill, L.A.; Riordan, M.V.; Tian, Y.-M.; Bullock, A.N.; Welford, R.W.; Elkins, J.M.; Oldham, N.J.; Bhattacharya, S.; Gleadle, J.M.; et al. Hypoxia-inducible factor (HIF) asparagine hydroxylase is identical to factor inhibiting HIF (FIH) and is related to the cupin structural family. J. Biol. Chem. 2002, 277, 26351–26355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrismann, D.; Flashman, E.; Genn, D.N.; Mathioudakis, N.; Hewitson, K.S.; Ratcliffe, P.J.; Schofield, C.J. Studies on the activity of the hypoxia-inducible-factor hydroxylases using an oxygen consumption assay. Biochem. J. 2007, 401, 227–234. [Google Scholar] [CrossRef] [Green Version]
- Tarhonskaya, H.; Hardy, A.P.; Howe, E.A.; Loik, N.D.; Kramer, H.B.; McCullagh, J.S.O.; Schofield, C.J.; Flashman, E. Kinetic Investigations of the Role of Factor Inhibiting Hypoxia-inducible Factor (FIH) as an Oxygen Sensor. J. Biol. Chem. 2015, 290, 19726–19742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koivunen, P.; Hirsilä, M.; Günzler, V.; Kivirikko, K.I.; Myllyharju, J. Catalytic properties of the asparaginyl hydroxylase (FIH) in the oxygen sensing pathway are distinct from those of its prolyl 4-hydroxylases. J. Biol. Chem. 2004, 279, 9899–9904. [Google Scholar] [CrossRef] [Green Version]
- Bracken, C.P.; Fedele, A.O.; Linke, S.; Balrak, W.; Lisy, K.; Whitelaw, M.L.; Peet, D.J. Cell-specific regulation of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha stabilization and transactivation in a graded oxygen environment. J. Biol. Chem. 2006, 281, 22575–22585. [Google Scholar] [CrossRef] [Green Version]
- Keith, B.; Johnson, R.S.; Simon, M.C. HIF1α and HIF2α: Sibling rivalry in hypoxic tumour growth and progression. Nat. Rev. Cancer 2011, 12, 9–22. [Google Scholar] [CrossRef] [Green Version]
- Manalo, D.J.; Rowan, A.; Lavoie, T.; Natarajan, L.; Kelly, B.D.; Ye, S.Q.; Garcia, J.G.N.; Semenza, G.L. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 2005, 105, 659–669. [Google Scholar] [CrossRef]
- Wenger, R.H.; Stiehl, D.P.; Camenisch, G. Integration of oxygen signaling at the consensus HRE. Sci. STKE 2005, 2005, re12. [Google Scholar] [CrossRef] [Green Version]
- Schödel, J.; Oikonomopoulos, S.; Ragoussis, J.; Pugh, C.W.; Ratcliffe, P.J.; Mole, D.R. High-resolution genome-wide mapping of HIF-binding sites by ChIP-seq. Blood. 2011, 117, e207–e217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semenza, G.L. Hypoxia-Inducible Factors in Physiology and Medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.; McKnight, S.L.; Russell, D.W. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997, 11, 72–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyer, N.V.; Leung, S.W.; Semenza, G.L. The human hypoxia-inducible factor 1alpha gene: HIF1A structure and evolutionary conservation. Genomics 1998, 52, 159–165. [Google Scholar] [CrossRef]
- Patel, S.A.; Simon, M.C. Biology of Hypoxia-Inducible Factor-2α in Development and Disease. Cell Death Differ. 2008, 15, 628–634. [Google Scholar] [CrossRef]
- Wiener, C.M.; Booth, G.; Semenza, G.L. In vivo expression of mRNAs encoding hypoxia-inducible factor 1. Biochem. Biophys. Res. Commun. 1996, 225, 485–488. [Google Scholar] [CrossRef]
- Wiesener, M.S.; Jürgensen, J.S.; Rosenberger, C.; Scholze, C.K.; Hörstrup, J.H.; Warnecke, C.; Mandriota, S.; Bechmann, I.; Frei, U.A.; Pugh, C.W.; et al. Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. FASEB J. 2003, 17, 271–273. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.-J.; Wang, L.-Y.; Chodosh, L.A.; Keith, B.; Simon, M.C. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol. Cell Biol. 2003, 23, 9361–9374. [Google Scholar] [CrossRef] [Green Version]
- Koh, M.Y.; Powis, G. Passing the baton: The HIF switch. Trends Biochem. Sci. 2012, 37, 364–372. [Google Scholar] [CrossRef] [Green Version]
- Serocki, M.; Bartoszewska, S.; Janaszak-Jasiecka, A.; Ochocka, R.J.; Collawn, J.F.; Bartoszewski, R. miRNAs regulate the HIF switch during hypoxia: A novel therapeutic target. Angiogenesis 2018, 21, 183–202. [Google Scholar] [CrossRef] [Green Version]
- Bartoszewski, R.; Moszyńska, A.; Serocki, M.; Cabaj, A.; Polten, A.; Ochocka, R.; Dell’Italia, L.; Bartoszewska, S.; Króliczewski, J.; Dąbrowski, M.; et al. Primary endothelial cell–specific regulation of hypoxia-inducible factor (HIF)-1 and HIF-2 and their target gene expression profiles during hypoxia. FASEB J. 2019, 33, 7929–7941. [Google Scholar] [CrossRef]
- Bruning, U.; Cerone, L.; Neufeld, Z.; Fitzpatrick, S.F.; Cheong, A.; Scholz, C.C.; Simpson, D.A.; Leonard, M.O.; Tambuwala, M.M.; Cummins, E.P.; et al. MicroRNA-155 promotes resolution of hypoxia-inducible factor 1alpha activity during prolonged hypoxia. Mol. Cell Biol. 2011, 31, 4087–4096. [Google Scholar] [CrossRef] [Green Version]
- Bartoszewski, R.; Serocki, M.; Janaszak-Jasiecka, A.; Bartoszewska, S.; Kochan-Jamrozy, K.; Piotrowski, A.; Króliczewski, J.; Collawn, J.F. miR-200b downregulates Kruppel Like Factor 2 (KLF2) during acute hypoxia in human endothelial cells. Eur. J. Cell Biol. 2017, 96, 758–766. [Google Scholar] [CrossRef]
- Liu, Y.V.; Baek, J.H.; Zhang, H.; Diez, R.; Cole, R.N.; Semenza, G.L. RACK1 Competes with HSP90 for Binding to HIF-1α and is Required for O2-independent and HSP90 Inhibitor-induced Degradation of HIF-1α. Mol. Cell. 2007, 25, 207–217. [Google Scholar] [CrossRef] [Green Version]
- McMahon, S.; Charbonneau, M.; Grandmont, S.; Richard, D.E.; Dubois, C.M. Transforming growth factor beta1 induces hypoxia-inducible factor-1 stabilization through selective inhibition of PHD2 expression. J. Biol. Chem. 2006, 281, 24171–24181. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, H.; Makino, Y.; Okamoto, K.; Poellinger, L.; Ohnuma, K.; Morimoto, C.; Tanaka, H. TCR Engagement Increases Hypoxia-Inducible Factor-1α Protein Synthesis via Rapamycin-Sensitive Pathway under Hypoxic Conditions in Human Peripheral T Cells. J. Immunol. 2005, 174, 7592–7599. [Google Scholar] [CrossRef] [Green Version]
- Frede, S.; Stockmann, C.; Freitag, P.; Fandrey, J. Bacterial lipopolysaccharide induces HIF-1 activation in human monocytes via p44/42 MAPK and NF-κB. Biochem. J. 2006, 396, 517–527. [Google Scholar] [CrossRef]
- Belaiba, R.S.; Bonello, S.; Zähringer, C.; Schmidt, S.; Hess, J.; Kietzmann, T.; Görlach, A. Hypoxia up-regulates hypoxia-inducible factor-1alpha transcription by involving phosphatidylinositol 3-kinase and nuclear factor kappaB in pulmonary artery smooth muscle cells. Mol. Biol. Cell. 2007, 18, 4691–4697. [Google Scholar] [CrossRef] [Green Version]
- Bonello, S.; Zähringer, C.; BelAiba, R.S.; Djordjevic, T.; Hess, J.; Michiels, C.; Kietzmann, T.; Görlach, A. Reactive Oxygen Species Activate the HIF-1α Promoter Via a Functional NFκB Site. Arterioscler. Thromb, Vasc. Biol. 2007, 27, 755–761. [Google Scholar] [CrossRef] [Green Version]
- Rius, J.; Guma, M.; Schachtrup, C.; Akassoglou, K.; Zinkernagel, A.S.; Nizet, V.; Johnson, R.S.; Haddad, G.G.; Karin, M. NF-κB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1α. Nature 2008, 453, 807–811. [Google Scholar] [CrossRef] [Green Version]
- Knaup, K.X.; Jozefowski, K.; Schmidt, R.; Bernhardt, W.M.; Weidemann, A.; Juergensen, J.S.; Warnecke, C.; Eckardt, K.-U.; Wiesener, M.S. Mutual regulation of hypoxia-inducible factor and mammalian target of rapamycin as a function of oxygen availability. Mol. Cancer Res. 2009, 7, 88–98. [Google Scholar] [CrossRef] [Green Version]
- Koyasu, S.; Kobayashi, M.; Goto, Y.; Hiraoka, M.; Harada, H. Regulatory mechanisms of hypoxia-inducible factor 1 activity: Two decades of knowledge. Cancer Sci. 2018, 109, 560–571. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, R.; Senbanerjee, S.; Lin, Z.; Mir, S.; Hamik, A.; Wang, P.; Mukherjee, P.; Mukhopadhyay, D.; Jain, M.K. Inhibition of vascular permeability factor/vascular endothelial growth factor-mediated angiogenesis by the Kruppel-like factor KLF2. J Biol. Chem. 2005, 280, 28848–28851. [Google Scholar] [CrossRef] [Green Version]
- Kawanami, D.; Mahabeleshwar, G.H.; Lin, Z.; Atkins, G.B.; Hamik, A.; Haldar, S.M.; Maemura, K.; Lamanna, J.C.; Jain, M.K. Kruppel-like factor 2 inhibits hypoxia-inducible factor 1alpha expression and function in the endothelium. J. Biol. Chem. 2009, 284, 20522–20530. [Google Scholar] [CrossRef] [Green Version]
- Schaffer, K.; Taylor, C.T. The impact of hypoxia on bacterial infection. FEBS J. 2015, 282, 2260–2266. [Google Scholar] [CrossRef]
- Krzywinska, E.; Stockmann, C. Hypoxia, Metabolism and Immune Cell Function. Biomedicines 2018, 6, 56. [Google Scholar] [CrossRef] [Green Version]
- Dzhalilova, D.S.; Makarova, O.V. Molecular-biological mechanisms of interconnection between hypoxia, inflammatory and immune reactions. Immunologiya 2019, 40, 97–105. [Google Scholar] [CrossRef]
- Xue, X.; Ramakrishnan, S.; Anderson, E.; Taylor, M.; Zimmermann, E.M.; Spence, J.R.; Huang, S.; Greenson, J.K.; Shah, Y.M. Endothelial PAS Domain Protein 1 Activates the Inflammatory Response in the Intestinal Epithelium to Promote Colitis in Mice. Gastroenterology 2013, 145, 831–841. [Google Scholar] [CrossRef] [Green Version]
- Xue, X.; Ramakrishnan, S.K.; Shah, Y.M. Activation of HIF-1α does not increase intestinal tumorigenesis. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G187–G195. [Google Scholar] [CrossRef] [Green Version]
- Tissot van Patot, M.C.; Gassmann, M. Hypoxia: Adapting to High Altitude by Mutating EPAS-1, the Gene Encoding HIF-2α. High Alt. Med. Biol. 2011, 12, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Buroker, N.E.; Ning, X.-H.; Zhou, Z.-N.; Li, K.; Cen, W.-J.; Wu, X.-F.; Zhu, W.-Z.; Scott, C.R.; Chen, S.-H. EPAS1 and EGLN1 associations with high altitude sickness in Han and Tibetan Chinese at the Qinghai–Tibetan Plateau. Blood Cells Mol. Dis. 2012, 49, 67–73. [Google Scholar] [CrossRef]
- Song, D.; Li, L.; Arsenault, P.R.; Tan, Q.; Bigham, A.W.; Heaton-Johnson, K.J.; Master, S.R.; Lee, F.S. Defective Tibetan PHD2 Binding to p23 Links High Altitude Adaption to Altered Oxygen Sensing. J. Biol. Chem. 2014, 289, 14656–14665. [Google Scholar] [CrossRef] [Green Version]
- Zhang, E.; Zhang, J.; Jin, J.; Qin, J.; Li, H.; Huang, L. Variants of the Low Oxygen Sensors EGLN1 and HIF-1AN Associated with Acute Mountain Sickness. Int. J. Mol. Sci. 2014, 15, 21777–21787. [Google Scholar] [CrossRef] [Green Version]
- Wu, A.L.; Xiong, Y.S.; Li, Z.Q.; Liu, Y.G.; Quan, Q.; Wu, L.J. Correlation between single nucleotide polymorphisms in hypoxia-related genes and susceptibility to acute high-altitude pulmonary edema. Genet. Mol. Res. 2015, 14, 11562–11572. [Google Scholar] [CrossRef]
- Lorenzo, F.R.; Huff, C.; Myllymäki, M.; Olenchock, B.; Swierczek, S.; Tashi, T.; Gordeuk, V.; Wuren, T.; Ri-Li, G.; McClain, D.A.; et al. A genetic mechanism for Tibetan high-altitude adaptation. Nat. Genet. 2014, 46, 951–956. [Google Scholar] [CrossRef] [Green Version]
- Tanimoto, K.; Yoshiga, K.; Eguchi, H.; Kaneyasu, M.; Ukon, K.; Kumazaki, T.; Oue, N.; Yasui, W.; Imai, K.; Nakachi, K.; et al. Hypoxia-inducible factor-1alpha polymorphisms associated with enhanced transactivation capacity, implying clinical significance. Carcinogenesis 2003, 24, 1779–1783. [Google Scholar] [CrossRef]
- Ahmetov, I.I.; Hakimullina, A.M.; Lyubaeva, E.V.; Vinogradova, O.L.; Rogozkin, V.A. Effect of HIF1A gene polymorphism on human muscle performance. Bull. Exp. Biol. Med. 2008, 146, 351–353. [Google Scholar] [CrossRef] [PubMed]
- McPhee, J.S.; Perez-Schindler, J.; Degens, H.; Tomlinson, D.; Hennis, P.; Baar, K.; Williams, A.G. HIF1A P582S gene association with endurance training responses in young women. Eur. J. Appl. Physiol. 2011, 111, 2339–2347. [Google Scholar] [CrossRef]
- Gabbasov, R.T.; Arkhipova, A.A.; Borisova, A.V.; Hakimullina, A.M.; Kuznetsova, A.V.; Williams, A.G.; Day, S.H.; Ahmetov, I.I. The HIF1A gene Pro582Ser polymorphism in Russian strength athletes. J. Strength Cond. Res. 2013, 27, 2055–2058. [Google Scholar] [CrossRef]
- Cięszczyk, P.; Eider, J.; Arczewska, A.; Ostanek, M.; Leońska-Duniec, A.; Sawczyn, S.; Ficek, K.; Jascaniene, N.; Kotarska, K.; Sygit, K. The HIF1A gene Pro582Ser polymorphism in polish power-orientated athletes. Biol. Sport 2011, 28, 111–114. [Google Scholar] [CrossRef]
- De Carvalho Fraga, C.A.; Alves, L.R.; Marques-Silva, L.; de Sousa, A.A.; Jorge, A.S.B.; de Jesus, S.F.; Vilela, D.N.; Pinheiro, U.B.; Jones, K.M.; de Paula, A.M.B.; et al. High HIF-1α expression genotypes in oral lichen planus. Clin. Oral Investig. 2013, 17, 2011–2015. [Google Scholar] [CrossRef]
- Fraga, A.; Ribeiro, R.; Príncipe, P.; Lobato, C.; Pina, F.; Maurício, J.; Monteiro, C.; Sousa, H.; Calais da Silva, F.; Lopes, C.; et al. The HIF1A functional genetic polymorphism at locus +1772 associates with progression to metastatic prostate cancer and refractoriness to hormonal castration. Eur. J. Cancer. 2014, 50, 359–365. [Google Scholar] [CrossRef]
- Lessi, F.; Mazzanti, C.M.; Tomei, S.; Di Cristofano, C.; Minervini, A.; Menicagli, M.; Apollo, A.; Masieri, L.; Collecchi, P.; Minervini, R.; et al. VHL and HIF-1α: Gene variations and prognosis in early-stage clear cell renal cell carcinoma. Med. Oncol. 2014, 31, 840. [Google Scholar] [CrossRef]
- Strauss, E.; Waliszewski, K.; Oszkinis, G.; Staniszewski, R. Polymorphisms of genes involved in the hypoxia signaling pathway and the development of abdominal aortic aneurysms or large-artery atherosclerosis. J. Vasc. Surg. 2015, 61, 1105–1113.e3. [Google Scholar] [CrossRef] [Green Version]
- Beall, C.M.; Cavalleri, G.L.; Deng, L.; Elston, R.C.; Gao, Y.; Knight, J.; Li, C.; Li, J.C.; Liang, Y.; McCormack, M.; et al. Natural selection on EPAS1 (HIF2alpha) associated with low hemoglobin concentration in Tibetan highlanders. Proc. Natl. Acad. Sci. USA 2010, 107, 11459–11464. [Google Scholar] [CrossRef] [Green Version]
- Hanaoka, M.; Droma, Y.; Basnyat, B.; Ito, M.; Kobayashi, N.; Katsuyama, Y.; Kubo, K.; Ota, M. Genetic Variants in EPAS1 Contribute to Adaptation to High-Altitude Hypoxia in Sherpas. PLoS ONE 2012, 7, e50566. [Google Scholar] [CrossRef]
- Droma, Y.; Ota, M.; Hanaoka, M.; Katsuyama, Y.; Basnyat, B.; Neupane, P.; Arjyal, A.; Pandit, A.; Sharma, D.; Ito, M.; et al. Two hypoxia sensor genes and their association with symptoms of acute mountain sickness in Sherpas. Aviat. Space Environ. Med. 2008, 79, 1056–1060. [Google Scholar] [CrossRef]
- Simonson, T.S.; Yang, Y.; Huff, C.D.; Yun, H.; Qin, G.; Witherspoon, D.J.; Bai, Z.; Lorenzo, F.R.; Xing, J.; Jorde, L.B.; et al. Genetic evidence for high-altitude adaptation in Tibet. Science 2010, 329, 72–75. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Yang, Z.; Zhang, H.; Cui, C.; Qi, X.; Luo, X.; Tao, X.; Wu, T.; Ouzhuluobu; Basang; et al. Genetic variations in Tibetan populations and high-altitude adaptation at the Himalayas. Mol. Biol. Evol. 2011, 28, 1075–1081. [Google Scholar] [CrossRef] [Green Version]
- Beall, C.M. Adaptation to High Altitude: Phenotypes and Genotypes. Annu. Rev. Anthropol. 2014, 43, 251–272. [Google Scholar] [CrossRef]
- Villafuerte, F.C.; Corante, N. Chronic Mountain Sickness: Clinical Aspects, Etiology, Management, and Treatment. High. Alt. Med. Biol. 2016, 17, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Hackett, P.H. High Altitude Cerebral Edema and Acute Mountain Sickness: A pathophysiology update. In Hypoxia: Into the Next Millennium; Roach, R.C., Wagner, P.D., Hackett, P.H., Eds.; Plenum/Kluwer Academic Publishing: New York, NY, USA, 1999; pp. 23–46. [Google Scholar]
- Tang, X.-G.; Zhang, J.; Qin, J.; Gao, X.; Li, Q.; Yu, J.; Ding, X.; Huang, L. Age as a risk factor for acute mountain sickness upon rapid ascent to 3700 m among young adult Chinese men. Clin. Interv. Aging 2014, 9, 1287–1294. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Wang, R.; Li, W.; Xie, H.; Wang, C.; Hao, Y.; Sun, Y.; Jia, Z. Plasma cytokine profiling to predict susceptibility to acute mountain sickness. Eur. Cytokine Netw. 2016, 27, 90–96. [Google Scholar] [CrossRef]
- Soree, P.; Gupta, R.K.; Singh, K.; Desiraju, K.; Agrawal, A.; Vats, P.; Bharadwaj, A.; Baburaj, T.P.; Chaudhary, P.; Singh, V.K.; et al. Raised HIF1α during normoxia in high altitude pulmonary edema susceptible non-mountaineers. Sci. Rep. 2016, 6, 26468. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Wang, R.; Li, W.; Xie, H.; Wang, C.; Hao, Y.; Sun, Y.; Jia, Z. Plasma proteomic study of acute mountain sickness susceptible and resistant individuals. Sci. Rep. 2018, 8, 1265. [Google Scholar] [CrossRef] [Green Version]
- Hackett, P.H.; Roach, R.C. High-altitude illness. N. Engl. J. Med. 2001, 345, 107–114. [Google Scholar] [CrossRef]
- Bartsch, P.; Roach, R.C. Acute Mountain Sickness and High-altitude Pulmonary Edema. In High Altitude: An Exploration in Human Adaptation. Lung Biology in Health and Disease; Hornbein, T.F., Schoene, R.B., Eds.; Marcel Dekker: New York, NY, USA, 2001; pp. 731–776. [Google Scholar]
- MacInnis, M.J.; Koehle, M.S.; Rupert, J.L. Evidence for a Genetic Basis for Altitude Illness: 2010 Update. High Alt. Med. Biol. 2010, 11, 349–368. [Google Scholar] [CrossRef] [PubMed]
- Simonson, T.S. Altitude Adaptation: A Glimpse Through Various Lenses. High Alt. Med. Biol. 2015, 16, 125–137. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Sun, Z.-J.; Cao, F.; Zhao, H.; Li, C.-W.; Zhang, J. Obesity is a risk factor for acute mountain sickness: A prospective study in Tibet railway construction workers on Tibetan plateau. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 119–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, G.; Zhu, G.; Sun, W.; Yin, C.; Ren, X.; Wang, T.; Liu, M. Association of Arterial Oxygen Saturation and Acute Mountain Sickness Susceptibility: A Meta-analysis. Cell Biochem. Biophys. 2014, 70, 1427–1432. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhang, J.; Gao, X.; Li, Q.; Li, J.; Yu, J.; Qin, J.; Huang, L. Sleep quality changes in insomniacs and non-insomniacs after acute altitude exposure and its relationship with acute mountain sickness. Neuropsychiatr. Dis. Treat. 2014, 10, 1423–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziaee, V.; Yunesian, M.; Ahmadinejad, Z.; Halabchi, F.; Kordi, R.; Alizadeh, R.; Afsharjoo, H.R. Acute Mountain Sickness in Iranian Trekkers Around Mount Damavand (5671m) in Iran. Wild. Environ. Med. 2003, 14, 214–219. [Google Scholar] [CrossRef] [Green Version]
- Honigman, B.; Theis, M.K.; Koziol-McLain, J.; Roach, R.; Yip, R.; Houston, C.; Moore, L.G.; Pearce, P. Acute mountain sickness in a general tourist population at moderate altitudes. Ann. Intern. Med. 1993, 118, 587–592. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, C.; Chen, Y.; Luo, Y.-J. Association between acute mountain sickness (AMS) and age: A meta-analysis. Mil. Med. Res. 2018, 5, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behan, M.; Wenninger, J.M. Sex Steroidal Hormones and Respiratory Control. Respir. Physiol. Neurobiol. 2008, 164, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Muza, S.R.; Rock, P.B.; Fulco, C.S.; Zamudio, S.; Braun, B.; Cymerman, A.; Butterfield, G.E.; Moore, L.G. Women at altitude: Ventilatory acclimatization at 4,300 m. J. Appl. Physiol. 2001, 91, 1791–1799. [Google Scholar] [CrossRef]
- Gargaglioni, L.H.; Marques, D.A.; Patrone, L.G.A. Sex differences in breathing. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2019, 238, 110543. [Google Scholar] [CrossRef]
- Young, T.; Palta, M.; Dempsey, J.; Skatrud, J.; Weber, S.; Badr, S. The occurrence of sleep-disordered breathing among middle-aged adults. N. Engl. J. Med. 1993, 328, 1230–1235. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.-H.; Chen, Y.-C.; Kao, W.-F.; Lin, Y.-J.; Chen, J.-C.; Chiu, T.-F.; Hsu, T.-Y.; Chen, H.-C.; Liu, S.-W. Epidemiology of acute mountain sickness on Jade Mountain, Taiwan: An annual prospective observational study. High Alt. Med. Biol. 2010, 11, 43–49. [Google Scholar] [CrossRef]
- MacInnis, M.J.; Carter, E.A.; Freeman, M.G.; Pandit, B.P.; Siwakoti, A.; Subedi, A.; Timalsina, U.; Widmer, N.; Thapa, G.B.; Koehle, M.S.; et al. A Prospective Epidemiological Study of Acute Mountain Sickness in Nepalese Pilgrims Ascending to High Altitude (4380 m). PLoS ONE 2013, 8, e75644. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.-P.; Wu, J.-L.; Tan, C.; Chen, Y.; Guo, R.; Luo, Y.-J. Sex-based differences in the prevalence of acute mountain sickness: A meta-analysis. Military Med. Res. 2019, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.R.; D’Zatko, K.; Tatsugawa, K.; Murray, K.; Parker, D.; Streeper, T.; Willard, K. Mt. Whitney: Determinants of summit success and acute mountain sickness. Med. Sci. Sports Exerc. 2008, 40, 1820–1827. [Google Scholar] [CrossRef]
- Wu, T.-Y.; Ding, S.-Q.; Liu, J.-L.; Jia, J.-H.; Chai, Z.-C.; Dai, R.-C.; Zhao, J.-Z.; Tang, Q.D.; Kayser, B. Smoking, acute mountain sickness and altitude acclimatisation: A cohort study. Thorax 2012, 67, 914–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Lu, H.-X.; Wang, Y.-X.; Chen, Y.; Yang, S.; Luo, Y.-J. Association between smoking and the risk of acute mountain sickness: A meta-analysis of observational studies. Mil. Med. Res. 2016, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luks, A.M.; Swenson, E.R.; Bärtsch, P. Acute high-altitude sickness. Eur. Respir. Rev. 2017, 26, 160096. [Google Scholar] [CrossRef]
- Julian, C.G.; Subudhi, A.W.; Wilson, M.J.; Dimmen, A.C.; Pecha, T.; Roach, R.C. Acute mountain sickness, inflammation, and permeability: New insights from a blood biomarker study. J. Appl. Physiol. 2011, 111, 392–399. [Google Scholar] [CrossRef] [Green Version]
- Julian, C.G.; Subudhi, A.W.; Hill, R.C.; Wilson, M.J.; Dimmen, A.C.; Hansen, K.C.; Roach, R.C. Exploratory proteomic analysis of hypobaric hypoxia and acute mountain sickness in humans. J. Appl. Physiol. 2013, 116, 937–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatia, B.; Thomas, S.; Purkayastha, S.S. Seasonal variations in the survival index of rats at simulated high altitudes. Int. J. Biometeorol. 1966, 10, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Kumar, R.; Pal, K. Individual variation in response to simulated hypoxic stress of rats. Ind. J. Exp. Biol. 2012, 50, 744–748. [Google Scholar]
- Padhy, G.; Sethy, N.K.; Ganju, L.; Bhargava, K. Abundance of Plasma Antioxidant Proteins Confers Tolerance to Acute Hypobaric Hypoxia Exposure. High Alt. Med. Biol. 2013, 14, 289–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tregub, P.; Kulikov, V.; Bespalov, A. Tolerance to acute hypoxia maximally increases in case of joint effect of normobaric hypoxia and permissive hypercapnia in rats. Pathophysiology 2013, 20, 165–170. [Google Scholar] [CrossRef]
- Schaber, M.; Leichtfried, V.; Fries, D.; Wille, M.; Gatterer, H.; Faulhaber, M.; Würtinger, P.; Schobersberger, W. Influence of Acute Normobaric Hypoxia on Hemostasis in Volunteers with and without Acute Mountain Sickness. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Kammerer, T.; Faihs, V.; Hulde, N.; Bayer, A.; Hübner, M.; Brettner, F.; Karlen, W.; Kröpfl, J.M.; Rehm, M.; Spengler, C.; et al. Changes of hemodynamic and cerebral oxygenation after exercise in normobaric and hypobaric hypoxia: Associations with acute mountain sickness. Ann. Occup. Environ. Med. 2018, 30. [Google Scholar] [CrossRef]
- Roach, R.C.; Hackett, P.H.; Oelz, O.; Bärtsch, P.; Luks, A.M.; MacInnis, M.J.; Baillie, J.K. The 2018 Lake Louise Acute Mountain Sickness Score. High Alt. Med. Biol. 2018, 19, 4–6. [Google Scholar] [CrossRef]
- Gong, H.; Tashkin, D.P.; Lee, E.Y.; Simmons, M.S. Hypoxia-altitude simulation test. Evaluation of patients with chronic airway obstruction. Am. Rev. Respir. Dis. 1984, 130, 980–986. [Google Scholar] [CrossRef]
- Dine, C.J.; Kreider, M.E. Hypoxia altitude simulation test. Chest 2008, 133, 1002–1005. [Google Scholar] [CrossRef]
- Cramer, D.; Ward, S.; Geddes, D. Assessment of oxygen supplementation during air travel. Thorax 1996, 51, 202–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, S.E.; Bradley, J.M.; Buick, J.B.; Bradbury, I.; Elborn, J.S. Flight assessment in patients with respiratory disease: Hypoxic challenge testing vs. predictive equations. QJM 2007, 100, 361–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burtscher, M.; Flatz, M.; Faulhaber, M. Prediction of Susceptibility to Acute Mountain Sickness by SaO2 Values during Short-Term Exposure to Hypoxia. High Alt. Med. Biol. 2004, 5, 335–340. [Google Scholar] [CrossRef]
- Burtscher, M.; Pachinger, O.; Ehrenbourg, I.; Mitterbauer, G.; Faulhaber, M.; Pühringer, R.; Tkatchouk, E. Intermittent hypoxia increases exercise tolerance in elderly men with and without coronary artery disease. Int. J. Cardiol. 2004, 96, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, M.; Brandstätter, E.; Gatterer, H. Preacclimatization in simulated altitudes. Sleep Breath 2007, 12, 109. [Google Scholar] [CrossRef]
- Canouï-Poitrine, F.; Veerabudun, K.; Larmignat, P.; Letournel, M.; Bastuji-Garin, S.; Richalet, J.-P. Risk Prediction Score for Severe High Altitude Illness: A Cohort Study. PLoS ONE 2014, 9, e100642. [Google Scholar] [CrossRef] [Green Version]
- Bakker, J.; Nijsten, M.W.; Jansen, T.C. Clinical use of lactate monitoring in critically ill patients. Ann. Intensive Care 2013, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Kushimoto, S.; Akaishi, S.; Sato, T.; Nomura, R.; Fujita, M.; Kudo, D.; Kawazoe, Y.; Yoshida, Y.; Miyagawa, N. Lactate, a useful marker for disease mortality and severity but an unreliable marker of tissue hypoxia/hypoperfusion in critically ill patients. Acute Med. Surg. 2016, 3, 293–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraut, J.A.; Madias, N.E. Lactic acidosis. N. Engl. J. Med. 2014, 371, 2309–2319. [Google Scholar] [CrossRef]
- Arteel, G.E.; Thurman, R.G.; Yates, J.M.; Raleigh, J.A. Evidence that hypoxia markers detect oxygen gradients in liver: Pimonidazole and retrograde perfusion of rat liver. Br. J. Cancer 1995, 72, 889–895. [Google Scholar] [CrossRef] [Green Version]
- Varia, M.A.; Calkins-Adams, D.P.; Rinker, L.H.; Kennedy, A.S.; Novotny, D.B.; Fowler, W.C.; Raleigh, J.A. Pimonidazole: A novel hypoxia marker for complementary study of tumor hypoxia and cell proliferation in cervical carcinoma. Gynecol. Oncol. 1998, 71, 270–277. [Google Scholar] [CrossRef]
- Song, H.; Ke, T.; Luo, W.-J.; Chen, J.-Y. Non-high altitude methods for rapid screening of susceptibility to acute mountain sickness. BMC Public Health 2013, 13, 902. [Google Scholar] [CrossRef] [Green Version]
- Sutherland, A.; Freer, J.; Evans, L.; Dolci, A.; Crotti, M.; Macdonald, J.H. MEDEX 2015: Heart Rate Variability Predicts Development of Acute Mountain Sickness. High Alt. Med. Biol. 2017, 18, 199–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoon, R.S.; Sharma, S.C.; Balasubramanian, V.; Chadha, K.S.; Mathew, O.P. Urinary catecholamine excretion on acute induction to high altitide (3658 m). J. Appl. Physiol. 1976, 41, 631–633. [Google Scholar] [CrossRef]
- Mazzeo, R.S.; Child, A.; Butterfield, G.E.; Mawson, J.T.; Zamudio, S.; Moore, L.G. Catecholamine response during 12 days of high-altitude exposure (4300 m) in women. J. Appl. Physiol. 1998, 84, 1151–1157. [Google Scholar] [CrossRef] [Green Version]
- Duplain, H.; Vollenweider, L.; Delabays, A.; Nicod, P.; Bärtsch, P.; Scherrer, U. Augmented sympathetic activation during short-term hypoxia and high-altitude exposure in subjects susceptible to high-altitude pulmonary edema. Circulation 1999, 99, 1713–1718. [Google Scholar] [CrossRef] [Green Version]
- Lanfranchi, P.A.; Colombo, R.; Cremona, G.; Baderna, P.; Spagnolatti, L.; Mazzuero, G.; Wagner, P.; Perini, L.; Wagner, H.; Cavallaro, C.; et al. Autonomic cardiovascular regulation in subjects with acute mountain sickness. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2364–H2372. [Google Scholar] [CrossRef]
- Long, M.; Qin, J.; Huang, L. The role of autonomic nervous system in the pathogenesis of acute mountain sickness. MED J. Chin. PLA 2007, 32, 405–408. [Google Scholar]
- Boos, C.J.; Bass, M.; O’Hara, J.P.; Vincent, E.; Mellor, A.; Sevier, L.; Abdul-Razakq, H.; Cooke, M.; Barlow, M.; Woods, D.R. The relationship between anxiety and acute mountain sickness. PLoS ONE 2018, 13, e0197147. [Google Scholar] [CrossRef] [PubMed]
- Woods, D.R.; Begley, J.; Stacey, M.; Smith, C.; Boos, C.J.; Hooper, T.; Hawkins, A.; Hodkinson, P.; Green, N.; Mellor, A. Severe acute mountain sickness, brain natriuretic peptide and NT-proBNP in humans. Acta Physiol. 2012, 205, 349–355. [Google Scholar] [CrossRef]
- Wallén, T.; Landahl, S.; Hedner, T.; Nakao, K.; Saito, Y. Brain natriuretic peptide predicts mortality in the elderly. Heart 1997, 77, 264–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasue, H.; Yoshimura, M.; Sumida, H.; Kikuta, K.; Kugiyama, K.; Jougasaki, M.; Ogawa, H.; Okumura, K.; Mukoyama, M.; Nakao, K. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation 1994, 90, 195–203. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Schmid, T.; Frank, R.; Brüne, B. PI3K/Akt is required for heat shock proteins to protect hypoxia-inducible factor 1alpha from pVHL-independent degradation. J. Biol. Chem. 2004, 279, 13506–13513. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Wang, F.; Li, F.; Yuan, J.; Zeng, H.; Wei, Q.; Tanguay, R.M.; Wu, T. Association of hsp70-2 and hsp-hom gene polymorphisms with risk of acute high-altitude illness in a Chinese population. Cell Stress Chaper 2005, 10, 349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartsch, P.; Vock, P.; Maggiorini, M. Respiratory symptoms, radiographic and physiologic correlations at high altitude. In Hypoxia: The Adaptations; Sutton, J.R., Coates, G., Remmers, J.E., Eds.; B.C. Decker: Toronto, ON, Canada, 1990; pp. 241–245. [Google Scholar]
- Bärtsch, P.; Maggiorini, M.; Ritter, M.; Noti, C.; Vock, P.; Oelz, O. Prevention of high-altitude pulmonary edema by nifedipine. N. Engl. J. Med. 1991, 325, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Penaloza, D.; Sime, F. Circulatory dynamics during high altitude pulmonary edema. Am. J. Cardiol. 1969, 23, 369–378. [Google Scholar] [CrossRef]
- Hultgren, H.N.; Marticorena, E.A. High altitude pulmonary edema. Epidemiologic observations in Peru. Chest 1978, 74, 372–376. [Google Scholar] [CrossRef]
- Oelz, O.; Ritter, M.; Jenni, R.; Maggiorini, M.; Waber, U.; Vock, P.; Bärtsch, P. Nifedipine for high altitude pulmonary oedema. Lancet 1989, 334, 1241–1244. [Google Scholar] [CrossRef]
- Hultgren, H.N.; Grover, R.F.; Hartley, L.H. Abnormal circulatory responses to high altitude in subjects with a previous history of high-altitude pulmonary edema. Circulation 1971, 44, 759–770. [Google Scholar] [CrossRef] [Green Version]
- Kawashima, A.; Kubo, K.; Kobayashi, T.; Sekiguchi, M. Hemodynamic responses to acute hypoxia, hypobaria, and exercise in subjects susceptible to high-altitude pulmonary edema. J. Appl. Physiol. 1989, 67, 1982–1989. [Google Scholar] [CrossRef]
- Eldridge, M.W.; Podolsky, A.; Richardson, R.S.; Johnson, D.H.; Knight, D.R.; Johnson, E.C.; Hopkins, S.R.; Michimata, H.; Grassi, B.; Feiner, J.; et al. Pulmonary hemodynamic response to exercise in subjects with prior high-altitude pulmonary edema. J. Appl. Physiol. 1996, 81, 911–921. [Google Scholar] [CrossRef]
- Grünig, E.; Mereles, D.; Hildebrandt, W.; Swenson, E.R.; Kübler, W.; Kuecherer, H.; Bärtsch, P. Stress Doppler echocardiography for identification of susceptibility to high altitude pulmonary edema. J. Am. Coll. Cardiol. 2000, 35, 980–987. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.K.; Himashree, G.; Singh, K.; Soree, P.; Desiraju, K.; Agrawal, A.; Ghosh, D.; Dass, D.; Reddy, P.K.; Panjwani, U.; et al. Elevated pulmonary artery pressure and brain natriuretic peptide in high altitude pulmonary edema susceptible non-mountaineers. Sci. Rep. 2016, 6, 21357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woods, D.R.; Mellor, A.; Begley, J.; Stacey, M.; O’Hara, J.; Hawkins, A.; Yarker, J.; Foxen, S.; Smith, C.; Boos, C. Brain natriuretic peptide and NT-proBNP levels reflect pulmonary artery systolic pressure in trekkers at high altitude. Physiol. Res. 2013, 62, 597–603. [Google Scholar] [CrossRef]
- Mellor, A.; Boos, C.; Holdsworth, D.; Begley, J.; Hall, D.; Lumley, A.; Burnett, A.; Hawkins, A.; O’Hara, J.; Ball, S.; et al. Cardiac biomarkers at high altitude. High Alt. Med. Biol. 2014, 15, 452–458. [Google Scholar] [CrossRef]
- Busch, T.; Bärtsch, P.; Pappert, D.; Grünig, E.; Hildebrandt, W.; Elser, H.; Falke, K.J.; Swenson, E.R. Hypoxia decreases exhaled nitric oxide in mountaineers susceptible to high-altitude pulmonary edema. Am. J. Respir. Crit. Care Med. 2001, 163, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Hesse, C.; Dehnert, C.; Siedler, H.; Kleinbongard, P.; Bardenheuer, H.J.; Kelm, M.; Bärtsch, P.; Haefeli, W.E. Hypoxia Impairs Systemic Endothelial Function in Individuals Prone to High-Altitude Pulmonary Edema. Am. J. Respir. Crit. Care Med. 2005, 172, 763–767. [Google Scholar] [CrossRef] [Green Version]
- Versari, D.; Daghini, E.; Virdis, A.; Ghiadoni, L.; Taddei, S. Endothelial dysfunction as a target for prevention of cardiovascular disease. Diabetes Care 2009, 32 (Suppl. 2), S314–S321. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Chang, Y.; Wei, W. Endothelial dysfunction and inflammation: Immunity in rheumatoid arthritis. Mediat. Inflamm. 2016, 6813016. [Google Scholar] [CrossRef] [Green Version]
- Kawashima, S. The two faces of endothelial nitric oxide synthase in the pathophysiology of atherosclerosis. Endothelium 2004, 11, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Münzel, T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation 2006, 113, 1708–1714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.-M.; Huang, A.; Kaley, G.; Sun, D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1829–H1836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badran, M.; Abuyassin, B.; Golbidi, S.; Ayas, N.; Laher, I. Uncoupling of Vascular Nitric Oxide Synthase Caused by Intermittent Hypoxia. Oxid. Med. Cell Longev. 2016, 2016, 2354870. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, L.; Janaszak-Jasiecka, A.; Siekierzycka, A.; Bartoszewska, S.; Woźniak, M.; Lejnowski, D.; Collawn, J.F.; Bartoszewski, R. Posttranscriptional and transcriptional regulation of endothelial nitric-oxide synthase during hypoxia: The role of microRNAs. Cell Mol. Biol. Lett. 2016, 21. [Google Scholar] [CrossRef] [Green Version]
- Hochachka, P.W.; Somero, G.N. Biochemical Adaptation-Mechanism and Process in Physiological Evolution; Oxford University Press: New York, NY, USA, 2001; p. 248. [Google Scholar]
- Bickler, P.E. Clinical perspectives: Neuroprotection lessons from hypoxia-tolerant organisms. J. Exp. Biol. 2004, 207, 3243–3249. [Google Scholar] [CrossRef] [Green Version]
- Larson, J.; Drew, K.L.; Folkow, L.P.; Milton, S.L.; Park, T.J. No oxygen? No problem! Intrinsic brain tolerance to hypoxia in vertebrates. J. Exp. Biol. 2014, 217, 1024–1039. [Google Scholar] [CrossRef] [Green Version]
- Nambu, J.R.; Chen, W.; Hu, S.; Crews, S.T. The Drosophila melanogaster similar bHLH-PAS gene encodes a protein related to human hypoxia-inducible factor 1 alpha and Drosophila single-minded. Gene 1996, 172, 249–254. [Google Scholar] [CrossRef]
- Ma, E.; Xu, T.; Haddad, G.G. Gene regulation by O2 deprivation: An anoxia-regulated novel gene in Drosophila melanogaster. Brain Res. Mol. Brain Res. 1999, 63, 217–224. [Google Scholar] [CrossRef]
- Jiang, H.; Guo, R.; Powell-Coffman, J.A. The Caenorhabditis elegans hif-1 gene encodes a bHLH-PAS protein that is required for adaptation to hypoxia. PNAS 2001, 98, 7916–7921. [Google Scholar] [CrossRef] [Green Version]
- Gorr, T.A.; Cahn, J.D.; Yamagata, H.; Bunn, H.F. Hypoxia-induced synthesis of hemoglobin in the crustacean Daphnia magna is hypoxia-inducible factor-dependent. J. Biol. Chem. 2004, 279, 36038–36047. [Google Scholar] [CrossRef] [Green Version]
- Law, S.H.; Wu, R.S.; Ng, P.K.; Yu, R.M.; Kong, R.Y. Cloning and expression analysis of two distinct HIF-alpha isoforms—gcHIF-1alpha and gcHIF-4alpha—From the hypoxia-tolerant grass carp, Ctenopharyngodon idellus. BMC Mol. Biol. 2006, 7, 15. [Google Scholar] [CrossRef] [Green Version]
- Larson, J.; Park, T.J. Extreme hypoxia tolerance of naked mole-rat brain. NeuroReport 2009, 20, 1634–1637. [Google Scholar] [CrossRef] [PubMed]
- Baptista, R.B.; Souza-Castro, N.; Almeida-Val, V.M.F. Acute hypoxia up-regulates HIF-1α and VEGF mRNA levels in Amazon hypoxia-tolerant Oscar (Astronotus ocellatus). Fish Physiol. Biochem. 2016, 42, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhou, Y.; Zhao, T.; Han, X.; Qiao, M.; Ding, X.; Li, D.; Wu, L.; Wu, K.; Zhu, L.; et al. A method for establishing the high-altitude cerebral edema (HACE) model by acute hypobaric hypoxia in adult mice. J. Neurosci. Methods 2015, 245, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Duan, J.; Yan, M.; Chen, J. Reproduction of a rat model of acute high-altitude sickness and evaluation of its related indexes. Med. J. Chin. ese People’s Lib. Army 2015, 40, 716–721. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, X.; Chen, X.; Zhang, J.; Ai, X.; Liang, Y.; Yu, Y.; Zhang, Y.; Meng, X.; Kuang, T.; et al. Establishment and evaluation of a simulated high-altitude hypoxic brain injury model in SD rats. Mol. Med. Rep. 2019, 19, 2758–2766. [Google Scholar] [CrossRef]
- Lukyanova, L.D.; Kirova, Y.I. Effect of Hypoxic Preconditioning on Free Radical Processes in Tissues of Rats with Different Resistance to Hypoxia. Bull. Exp. Biol. Med. 2011, 151, 292–296. [Google Scholar] [CrossRef]
- Dzhalilova, D.S.; Kosyreva, A.M.; Diatroptov, M.E.; Makarova, O.V. Relationship between Hypoxic Resistance and the Phase of 4-Day Corticosterone Biorhythm in Adult Male Rats. Bull. Exp. Biol. Med. 2017, 163, 687–690. [Google Scholar] [CrossRef]
- Dzhalilova, D.S.; Diatroptov, M.E.; Tsvetkov, I.S.; Makarova, O.V.; Kuznetsov, S.L. Expression of Hif-1α, Nf-κb, and Vegf Genes in the Liver and Blood Serum Levels of HIF-1α, Erythropoietin, VEGF, TGF-β, 8-Isoprostane, and Corticosterone in Wistar Rats with High and Low Resistance to Hypoxia. Bull. Exp. Biol. Med. 2018, 165, 781–785. [Google Scholar] [CrossRef]
- Shustov, E.B.; Karkischenko, N.N.; Karkischenko, V.N.; Semenov, K.K. Analysis of individual tolerance parameters of laboratory animals to hypoxia in biological modeling neuroprotective and antihypoxant action of medicines. Biomedicine 2013, 4, 149–157. (In Russian) [Google Scholar]
- Sanotskaya, N.V.; Matsievskii, D.D.; Lebedeva, M.A. Changes in Hemodynamics and Respiration in Rats with Different Resistance to Acute Hypoxia. Bull. Exp. Biol. Med. 2004, 138, 18–22. [Google Scholar] [CrossRef]
- Lukyanova, L.; Kurlaev, S. The role of noradrenaline in regulating myocardial oxidative metabolism in rats with different resistances to hypoxia. Biulleten’ eksperimental’noĭ biologii i meditsiny 1993, 114, 586–588. (In Russian) [Google Scholar]
- Lukyanova, L.; Bogomolov, V. A comparative analysis of the cerebral cortical proteins in rats with different sensitivities to hypoxia. Biulleten’ eksperimental’noĭ biologii i meditsiny 1993, 114, 657–660. (In Russian) [Google Scholar]
- Kumar, S.; Sharma, P.; Bansal, A.; Sharma, P.C.; Aggarwal, K.K. Hypobaric hypoxia-mediated protein expression in plasma of susceptible & tolerant rats. Indian. J. Med. Res. 2014, 140, 756. [Google Scholar] [PubMed]
- Dzhalilova, D.S.; Kosyreva, A.M.; Tsvetkov, I.S.; Zolotova, N.A.; Mkhitarov, V.A.; Mikhailova, L.P.; Makarova, O.V. Morphofunctional features of the immune system of male and female rats with different tolerance to hypoxia. Bull. Exp. Biol. Med. 2020, 169, 773–778. [Google Scholar]
- Wenninger, J.M.; Olson, E.B.; Cotter, C.J.; Thomas, C.F.; Behan, M. Hypoxic and hypercapnic ventilatory responses in aging male vs. aging female rats. J. Appl. Physiol. 2009, 106, 1522–1528. [Google Scholar] [CrossRef] [Green Version]
- Holley, H.S.; Behan, M.; Wenninger, J.M. Age and sex differences in the ventilatory response to hypoxia and hypercapnia in awake neonatal, pre-pubertal and young adult rats. Respir. Physiol. Neurobiol. 2012, 180, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Garcia, A.J.; Rotem-Kohavi, N.; Doi, A.; Ramirez, J.-M. Post-Hypoxic Recovery of Respiratory Rhythm Generation Is Gender Dependent. PLoS ONE 2013, 8, e60695. [Google Scholar] [CrossRef] [Green Version]
- Kosyreva, A.M.; Dzhalilova, D.S.; Tsvetkov, I.S.; Diatroptov, M.E.; Makarova, O.V. Age-Specific Features of Hypoxia Tolerance and Intensity of Lipopolysaccharide-Induced Systemic Inflammatory Response in Wistar Rats. Bull. Exp. Biol. Med. 2019, 166, 699–703. [Google Scholar] [CrossRef]
- Kwarecki, K.; Debiec, H.; Wróblewski, S. Biological Time-Related Changes in Tolerance of Male Mice to Hypoxia—II. Orcadian Rhythm of Lysosomal Susceptibility to Hypoxia. Chronobiol. Int. 1984, 1, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Masukawa, T.; Tochino, Y. Circadian rhythm in the cerebral resistance to hypoxia in mice. Jpn. J. Pharmacol. 1993, 61, 197–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diatroptov, M.E.; Makarova, O.V.; Diatroptova, M.A. Regularities of infradian rhythms of the esophageal epithelium proliferation activity in Japanese quails (Coturnix japonica) and male Vistar rats. Geofizich. Protsessy Biosfera 2014, 13, 82–96. (In Russian) [Google Scholar]
- Hochachka, P.W.; Rupert, J.L. Fine tuning the HIF-1 ’global’ O2 sensor for hypobaric hypoxia in Andean high-altitude natives. Bioessays 2003, 25, 515–519. [Google Scholar] [CrossRef]
- Shen, C.; Powell-Coffman, J.A. Genetic Analysis of Hypoxia Signaling and Response in C. elegans. Ann. N. Y. Acad. Sci. 2003, 995, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, I.; Cairns, R.A.; Fontana, L.; Lim, A.L.; Denko, N.C. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006, 3, 187–197. [Google Scholar] [CrossRef] [Green Version]
- Mankovska, I.; Bakunovsky, O.; Vargatiy, C. Oxygen-transport systems in humans at rest and during physical work after a long-term wintering sojourn at Ukrainian Antarctic station “Academician Vernadsky”. In Proceeding of the 2nd Ukrainian Antarctic Conference, Kiev, Ukraine, 22–24 June 2004; p. 11. (In Ukrainian). [Google Scholar]
- Lukyanova, L.D.; Germanova, E.L.; Kopaladze, R.A. Development of Resistance of an Organism under Various Conditions of Hypoxic Preconditioning: Role of the Hypoxic Period and Reoxygenation. Bull. Exp. Biol. Med. 2009, 147, 400–404. [Google Scholar] [CrossRef]
- Gomes, L.C.; Scorrano, L. Mitochondrial morphology in mitophagy and macroautophagy. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2013, 1833, 205–212. [Google Scholar] [CrossRef]
- Dudchenko, A.; Chernobaeva, G.; Belousova, V.V.; Vlasova, I.; Lukyanova, L. Bioenergetic parameters of the brain in rats with different resistance to hypoxia. Bull. Exp. Biol. Med. 1993, 115, 263–267. [Google Scholar] [CrossRef]
- Luk’yanova, L.D.; Chernobaeva, G.N.; Romanova, V.E. Effects of adaptation to intermittent hypoxia on oxidative phosphorylation in brain mitochondria of rats with different sensitivities toward oxygen deficiency. Bull. Exp. Biol. Med. 1995, 120, 1189–1192. [Google Scholar] [CrossRef]
- Lukyanova, L.D. Mitochondrial Signaling in Hypoxia. OJEMD 2013, 3, 20–32. [Google Scholar] [CrossRef] [Green Version]
- Luk’ianova, L.D. Molekuliarnye mekhanizmy tkanevoĭ gipoksii i adaptatsii organizma [Molecular mechanisms of tissue hypoxia and organism adaptation]. Fiziol. Zh. 2003, 49, 17–35. (In Russian) [Google Scholar] [PubMed]
- Kurhalyuk, N.M.; Serebrovskaya, T.V.; Kolesnikova, E.E. Role of cholino- and adrenoreceptors in regulation of rat antioxidant defense system and lipid peroxidation during adaptation to intermittent hypoxia. Probl. Ecol. Med. Genet. Cell Immunol. 2001, 7, 126–137. (In Ukrainian) [Google Scholar]
- Bezrukov, V.V.; Paramononva, G.I.; Rushkevich, Y.E.; Sykalo, N.V.; Timchenko, A.N.; Utko, N.A.; Kholin, V.A. Some physiological indices and life expectancy in rats with different resistance to hypoxia. Probl. Stareniya Dolgoletiya 2012, 21, 431–443. (In Russian) [Google Scholar]
- Gala, R.R. The physiology and mechanisms of the stress-induced changes in prolactin secretion in the rat. Life Sci. 1990, 46, 1407–1420. [Google Scholar] [CrossRef]
- Richalet, J.-P.; Letournel, M.; Souberbielle, J.-C. Effects of high-altitude hypoxia on the hormonal response to hypothalamic factors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R1685–R1692. [Google Scholar] [CrossRef] [Green Version]
- Bupha-Intr, T.; Haizlip, K.M.; Janssen, P.M.L. Role of Endothelin in the Induction of Cardiac Hypertrophy In Vitro. PLoS ONE 2012, 7, e43179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fandrey, J. Oxygen-dependent and tissue-specific regulation of erythropoietin gene expression. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R977–R988. [Google Scholar] [CrossRef]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar]
- Robbins, R.A.; Grisham, M.B. Nitric oxide. Int. J. Biochem. Cell Biol. 1997, 29, 857–860. [Google Scholar] [CrossRef]
- Loscalzo, J. Nitric oxide insufficiency, platelet activation, and arterial thrombosis. Circ. Res. 2001, 88, 756–762. [Google Scholar] [CrossRef] [Green Version]
- Sinha, S.; Dutta, A.; Singh, S.N.; Ray, U.S. Protein nitration, lipid peroxidation and DNA damage at high altitude in acclimatized lowlanders and native highlanders: Relation with oxygen consumption. Respir. Physiol. Neurobiol. 2010, 171, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Y.; Sharma, N.K.; Ahmad, M.F.; Sharma, M.; Garg, I.; Bhargava, K. Proteomic Identification of Novel Differentiation Plasma Protein Markers in Hypobaric Hypoxia-Induced Rat Model. PLoS ONE 2014, 9, e98027. [Google Scholar] [CrossRef] [Green Version]
- Kuzina, O.V.; Tseilikman, O.B.; Lapshin, M.S.; Kozochkin, D.A.; Komelkova, M.V.; Tseilikman, V.E. Correlation between the level of behavioral activity, circulating concentrations of corticosterone in rats with different resistance to hypoxia. Vestn. Yuzh-Ural. Gos. Univer. Ser. Obraz. Zdravookhran. Fiz. Kultura. 2014, 14, 54–58. (In Russian) [Google Scholar] [CrossRef]
- Komel’kova, M.V. Determination of an Immune Response Levels and Oxygen-Dependent Processes in Rat Viscerals in Relation to Hypoxia Sensitivity. Ph.D. Thesis, South Ural State Medical University, Chelyabinsk, Russian, 2015. (In Russian). [Google Scholar]
- Saturskaya, A.S. Change of tumor necrosis factor-alpha concentration on modelling diffuse cardiosclerosis in rats with different resistance to hypoxia. Vestnik Vitebskogo Gosudarstvennogo Meditsinskogo Universiteta. 2015, 3, 32–37. (In Russian) [Google Scholar]
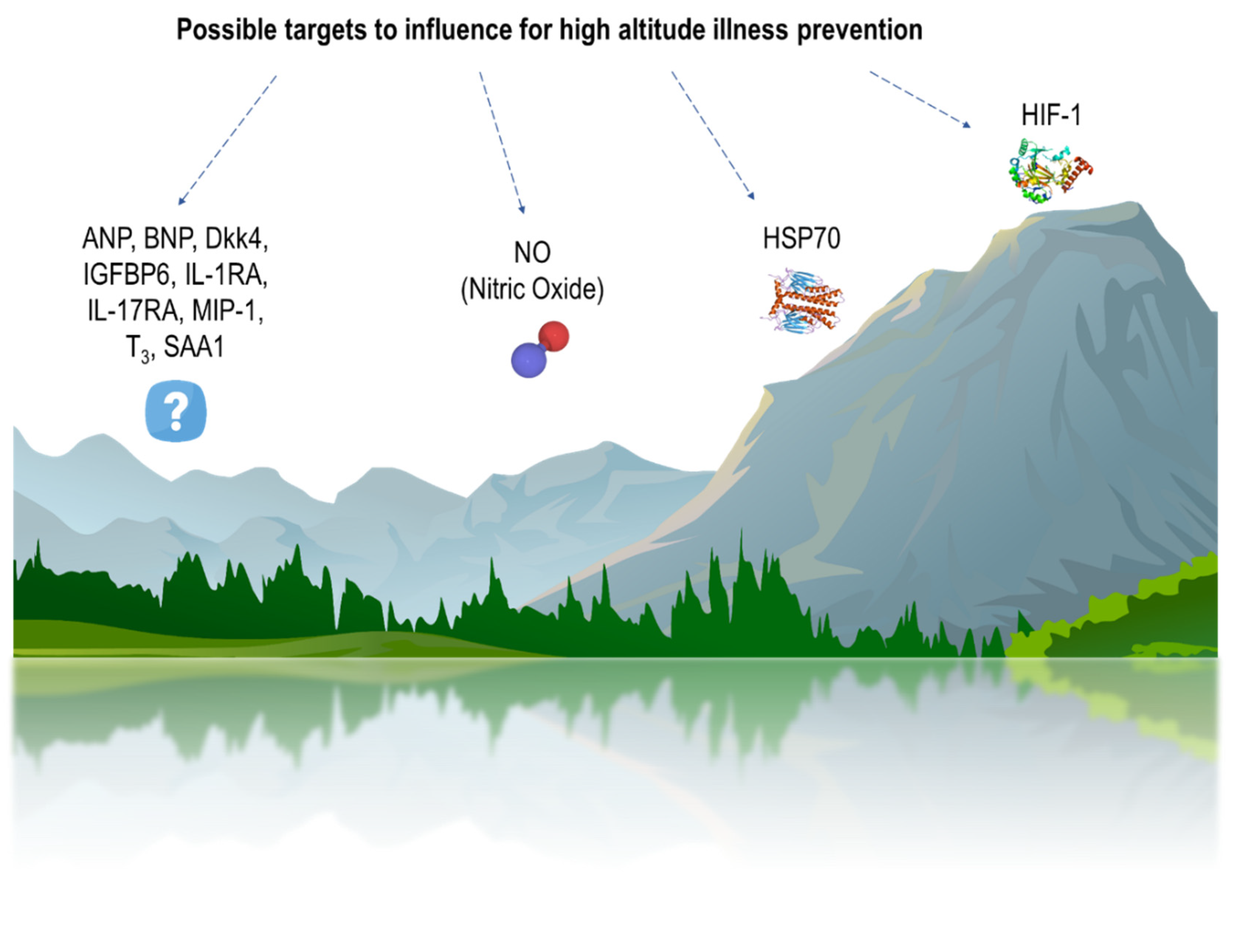
| Disorder | Exposure | Tolerant | Susceptible | Ref. |
|---|---|---|---|---|
| AMS | Low altitude | Higher blood plasma level of IGFBP6 | Higher blood plasma levels of Dkk4, IL-17RA, SAA1 | [99] |
| Low altitude and 3800 m for 3, 6, 9 and 12 h | Reduced level of proteins related to TCA, glycolysis, ribosome, and proteasome after 9 h | [101] | ||
| Low altitude and 4875 m for 10 h | Higher blood level of IL-1RA (after 4 and 9 h of exposure), HSP70 (before exposure to hypoxia) | Higher blood level of MIP-1 after 4 h of hypoxia | [123] | |
| Low altitude and 4559 m | Higher resting blood pressure at low altitude | [150] | ||
| Low altitude and over 9 consecutive altitudes during a progressive trek to 5140 m | Trait anxiety at low altitude was an independent predictor of future severe AMS development at high altitude | [152] | ||
| At rest in 1300 m, following exercise and at rest at 4270 and 5150 m | BNP is significantly greater at 5150 m | [153] | ||
| HAPE | Normoxia | Higher baseline serum HIF-1α level, the plasma concentration of T3 and ANP | [100] | |
| Low altitude and at 4559 m | Exaggerated sympathetic activation | [149] | ||
| Low altitude and 3100 m | Exaggerated pulmonary vascular response following ascent to high altitude | [163] | ||
| Low altitude and at 3810 m | The increase in pulmonary artery pressure during exercises | [165] | ||
| Low altitude and at 4500 m | Baseline higher levels of BNP, pulmonary artery pressure and reduced stroke volume | [167] | ||
| Exposure to 4500 m | Lower levels of exhaled NO | [170,171] |
| Targets | Methods | Tolerant | Susceptible | Ref. |
|---|---|---|---|---|
| Cerebral cortex cells | One month after a single measurement of tolerance to hypoxia | Higher content of mitochondria with more densely packed cristae and a dark matrix, number of small mitochondria, and concentration of SDHA, Cyt b, COX1, and succinate | Lower number of mitochondrial cristae | [17,21] |
| Liver cells | Higher rate of ATP-dependent K+ transport in the mitochondria Higher ability to hold Ca2+ in the mitochondria | [9,22] | ||
| Liver and heart cells | Higher rate of Ca2+ uptake by mitochondria | Higher amount of K+ in the mitochondria | [9,22] | |
| Blood plasma | Immediately after a single measurement of tolerance to hypoxia | Higher levels of norepinephrine, ACTH, and testosterone | Higher prolactin level | [126] |
| Immediately after three consecutive exposures at extreme altitude | Higher content of erythropoietin Higher level of NO | Higher levels of endothelin-1, corticosterone, ROS, and carbonylated proteins | [14,127] | |
| Myocardium | Immediately after three consecutive exposures at extreme altitude | Higher levels of superoxide dismutase and catalase Higher levels of NO, erythropoietin and activity of eNOS and iNOS Higher expression of HIF-1α, GLUT1, HSP27, HSP60, HSP70, and HSP90 | Higher activity of caspase-3, level of malondialdehyde, ROS, carbonylated proteins, expression of endothelin-1 and VEGF, the nuclear expression of NF-κB, and the expression of TNFα | [11,12,14] |
| Blood serum | 5 min after a single hypoxic exposure at extreme altitude | Higher content of VEGF, erythropoietin, and TGF-β | [194] | |
| 90 min after a single hypoxic exposure at extreme altitude | Higher content of TGF-β, level of the oxidative stress marker 8-isoprostane | [194] | ||
| After double determining the tolerance to hypoxia | Higher serum IL-10 level | [231] | ||
| Liver and neocortex | 5 min after a single hypoxic exposure at extreme altitude | Higher expression of HIF-1 and NF-kB | [194] | |
| One month after a single measurement of tolerance to hypoxia | Higher level of HIF-1 | [13,18] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzhalilova, D.; Makarova, O. Differences in Tolerance to Hypoxia: Physiological, Biochemical, and Molecular-Biological Characteristics. Biomedicines 2020, 8, 428. https://doi.org/10.3390/biomedicines8100428
Dzhalilova D, Makarova O. Differences in Tolerance to Hypoxia: Physiological, Biochemical, and Molecular-Biological Characteristics. Biomedicines. 2020; 8(10):428. https://doi.org/10.3390/biomedicines8100428
Chicago/Turabian StyleDzhalilova, Dzhuliia, and Olga Makarova. 2020. "Differences in Tolerance to Hypoxia: Physiological, Biochemical, and Molecular-Biological Characteristics" Biomedicines 8, no. 10: 428. https://doi.org/10.3390/biomedicines8100428





