Managing Natural Extinctions
Simple Summary
Abstract
1. Introduction
2. What Defines a Species and Their Endpoint?
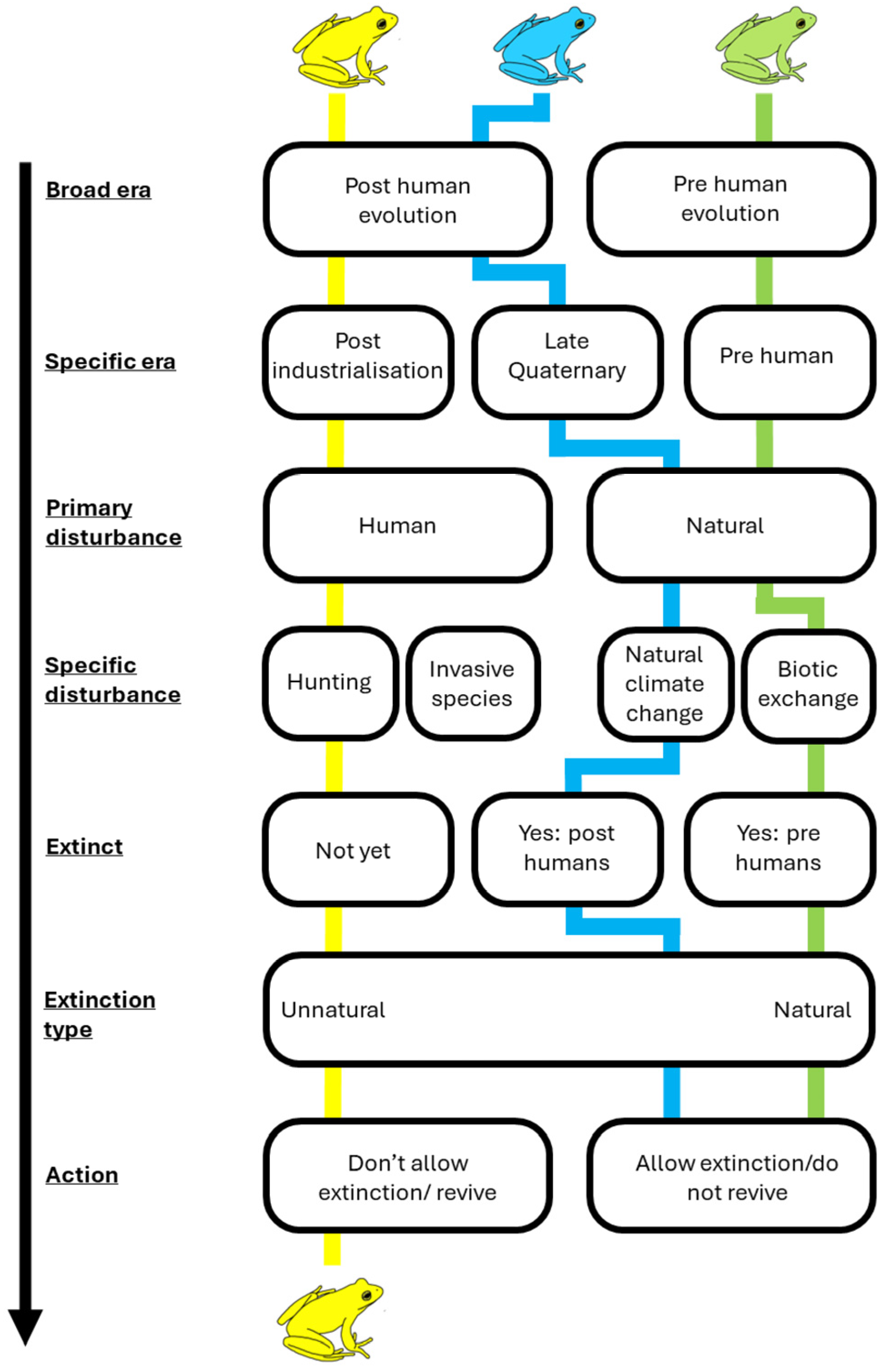
3. Are Human-Driven Extinctions Unnatural or Natural?
3.1. Our Interactions Within Ecosystems
3.2. Extinction Thresholds in Time
4. Preventing Extinctions in the Anthropocene
5. Extinctions Are a Part of Biotic Change
5.1. Evolutionary Transitions
5.2. Niche Availability
6. The Dilemma in Identifying Natural Extinctions Today
6.1. The Natural–Unnatural Continuum
6.2. When to Interfere
6.3. Case Study
7. Restoring Natural Extinction Cycles
7.1. Identifying Natural Extinctions and Implementation
7.2. Implementation
7.3. Dealing with Changes Already Felt in the Anthropocene
8. Future Extinctions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ceballos, G.; Ehrlich, P.R.; Barnosky, A.D.; García, A.; Pringle, R.M.; Palmer, T.M. Accelerated modern human–induced species losses: Entering the sixth mass extinction. Sci. Adv. 2015, 1, e1400253. [Google Scholar] [CrossRef]
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014, 344, 1246752. [Google Scholar] [CrossRef]
- Davis, M.A. Biotic globalization: Does competition from introduced species threaten biodiversity? Bioscience 2003, 53, 481–489. [Google Scholar] [CrossRef]
- Powers, R.P.; Jetz, W. Global habitat loss and extinction risk of terrestrial vertebrates under future land-use-change scenarios. Nat. Clim. Change 2019, 9, 323–329. [Google Scholar] [CrossRef]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.; De Siqueira, M.F.; Grainger, A.; Hannah, L. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Skerratt, L.F.; Berger, L.; Speare, R.; Cashins, S.; McDonald, K.R.; Phillott, A.D.; Hines, H.B.; Kenyon, N. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth 2007, 4, 125–134. [Google Scholar] [CrossRef]
- Crutzen, P.J. The “anthropocene”. In Earth System Science in the Anthropocene; Ehlers, E., Krafft, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 13–18. [Google Scholar]
- Flessa, K.W. The “facts” of mass extinctions. In Global Catastrophes in Earth History; An Interdisciplinary Conference on Impacts, Volcanism, and Mass Mortality; Geological Society of America Special Papers; Geological Society of America: Washington, DC, USA, 1990; Volume 247, pp. 1–7. [Google Scholar] [CrossRef]
- Jablonski, D. Background and mass extinctions: The alternation of macroevolutionary regimes. Science 1986, 231, 129–133. [Google Scholar] [CrossRef]
- Sepkoski, J.J., Jr. Mass extinctions in the Phanerozoic oceans: A review. In Geological Implications of Impacts of Large Asteroids and Comets on the Earth; Silver, L.T., Schulz, P.H., Eds.; Geological Society of America Special Paper; Geological Society of America: Washington, DC, USA, 1982; Volume 190, pp. 282–290. [Google Scholar]
- Louys, J.; Curnoe, D.; Tong, H. Characteristics of Pleistocene megafauna extinctions in Southeast Asia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 243, 152–173. [Google Scholar] [CrossRef]
- Ellwood, B.B.; Benoist, S.L.; El Hassani, A.; Wheeler, C.; Crick, R.E. Impact ejecta layer from the Mid-Devonian: Possible connection to global mass extinctions. Science 2003, 300, 1734–1737. [Google Scholar] [CrossRef]
- Scott, J.M.; Goble, D.D.; Haines, A.M.; Wiens, J.A.; Neel, M.C. Conservation-reliant species and the future of conservation. Conserv. Lett. 2010, 3, 91–97. [Google Scholar] [CrossRef]
- Poo, S.; Hinkson, K.M. Applying cryopreservation to anuran conservation biology. Conserv. Sci. Pract. 2019, 1, e91. [Google Scholar] [CrossRef]
- Hayward, M.W. Using the IUCN Red List to determine effective conservation strategies. Biodivs. Conserv. 2011, 20, 2563–2573. [Google Scholar] [CrossRef]
- De Meeûs, T.; Durand, P.; Renaud, F. Species concepts: What for? Trends Parasitol. 2003, 19, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, C. What is a species? Sci. Am. 2008, 298, 72–79. [Google Scholar] [CrossRef]
- Mayr, E. What is a species, and what is not? Philos. Sci. 1996, 63, 262–277. [Google Scholar] [CrossRef]
- Pimm, S.L.; Russell, G.J.; Gittleman, J.L.; Brooks, T.M. The future of biodiversity. Science 1995, 269, 347–350. [Google Scholar] [CrossRef]
- Crees, J.J.; Turvey, S.T. What constitutes a ‘native’ species? Insights from the Quaternary faunal record. Biol. Conserv. 2015, 186, 143–148. [Google Scholar] [CrossRef]
- Grayson, D.K. The archaeological record of human impacts on animal populations. J. World Prehist. 2001, 15, 1–68. [Google Scholar] [CrossRef]
- Faurby, S.; Silvestro, D.; Werdelin, L.; Antonelli, A. Brain expansion in early hominins predicts carnivore extinctions in East Africa. Ecol. Lett. 2020, 23, 537–544. [Google Scholar] [CrossRef]
- Cooper, A.; Turney, C.; Hughen, K.A.; Brook, B.W.; McDonald, H.G.; Bradshaw, C.J. Abrupt warming events drove Late Pleistocene Holarctic megafaunal turnover. Science 2015, 349, 602–606. [Google Scholar] [CrossRef]
- Sandom, C.; Faurby, S.; Sandel, B.; Svenning, J.-C. Global late Quaternary megafauna extinctions linked to humans, not climate change. Proc. R. Soc. B 2014, 281, 20133254. [Google Scholar] [CrossRef]
- Barnosky, A.D.; Koch, P.L.; Feranec, R.S.; Wing, S.L.; Shabel, A.B. Assessing the causes of late Pleistocene extinctions on the continents. Science 2004, 306, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Svenning, J.-C.; Lemoine, R.T.; Bergman, J.; Buitenwerf, R.; Le Roux, E.; Lundgren, E.; Mungi, N.; Pedersen, R.Ø. The late-Quaternary megafauna extinctions: Patterns, causes, ecological consequences and implications for ecosystem management in the Anthropocene. Camb. Prism. Extinct. 2024, 2, e5. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, R.T.; Buitenwerf, R.; Svenning, J.-C. Megafauna extinctions in the late-Quaternary are linked to human range expansion, not climate change. Anthropocene 2023, 44, 100403. [Google Scholar] [CrossRef]
- Svenning, J.-C.; Buitenwerf, R.; Le Roux, E. Trophic rewilding as a restoration approach under emerging novel biosphere conditions. Curr. Biol. 2024, 34, R435–R451. [Google Scholar] [CrossRef]
- Bibi, F.; Cantalapiedra, J.L. Plio-Pleistocene African megaherbivore losses associated with community biomass restructuring. Science 2023, 380, 1076–1080. [Google Scholar] [CrossRef]
- Prado, J.L.; Arroyo-Cabrales, J.; Johnson, E.; Alberdi, M.T.; Polaco, O.J. New World proboscidean extinctions: Comparisons between North and South America. Archaeol. Anthropol. Sci. 2015, 7, 277–288. [Google Scholar] [CrossRef]
- Jackson, S.T.; Weng, C. Late Quaternary extinction of a tree species in eastern North America. Proc. Natl. Acad. Sci. USA 1999, 96, 13847–13852. [Google Scholar] [CrossRef]
- Gill, J.L.; Williams, J.W.; Jackson, S.T.; Lininger, K.B.; Robinson, G.S. Pleistocene megafaunal collapse, novel plant communities, and enhanced fire regimes in North America. Science 2009, 326, 1100–1103. [Google Scholar] [CrossRef]
- Banks, P.B.; Hochuli, D.F. Extinction, de-extinction and conservation: A dangerous mix of ideas. Aust. Zool. 2017, 38, 390–394. [Google Scholar] [CrossRef]
- Austin, J.J.; Soubrier, J.; Prevosti, F.J.; Prates, L.; Trejo, V.; Mena, F.; Cooper, A. The origins of the enigmatic Falkland Islands wolf. Nat. Commun. 2013, 4, 1552. [Google Scholar] [CrossRef]
- Henderson, D.A. The eradication of smallpox–an overview of the past, present, and future. Vaccine 2011, 29, D7–D9. [Google Scholar] [CrossRef]
- Soulé, M.E. What is conservation biology? BioScience 1985, 35, 727–734. [Google Scholar] [CrossRef]
- Persson, E. What Is Wrong with Extinction? Ph.D. Thesis, Lund University, Lund, Sweden, 2008. [Google Scholar]
- Rea, A.W.; Munns, W.R., Jr. The value of nature: Economic, intrinsic, or both? Integr. Environ. Assess. Manag. 2017, 13, 953. [Google Scholar] [CrossRef] [PubMed]
- Valiente-Banuet, A.; Aizen, M.A.; Alcántara, J.M.; Arroyo, J.; Cocucci, A.; Galetti, M.; García, M.B.; García, D.; Gómez, J.M.; Jordano, P. Beyond species loss: The extinction of ecological interactions in a changing world. Funct. Ecol. 2015, 29, 299–307. [Google Scholar] [CrossRef]
- Jordán, F.; Takács-Sánta, A.; Molnar, I. A reliability theoretical quest for keystones. Oikos 1999, 86, 453–462. [Google Scholar] [CrossRef]
- Mills, L.S.; Soulé, M.E.; Doak, D.F. The keystone-species concept in ecology and conservation. BioScience 1993, 43, 219–224. [Google Scholar] [CrossRef]
- Ripple, W.J.; Beschta, R.L. Trophic cascades in Yellowstone: The first 15 years after wolf reintroduction. Biol. Conserv. 2012, 145, 205–213. [Google Scholar] [CrossRef]
- Huxtable, R.J. The pharmacology of extinction. J. Ethnopharmacol. 1992, 37, 1–11. [Google Scholar] [CrossRef]
- Marvier, M.; Kareiva, P. Extinction is a moral wrong but conservation is complicated. Biol. Conserv. 2014, 176, 281–282. [Google Scholar] [CrossRef]
- Walker, B.; Salt, D. Resilience Thinking: Sustaining Ecosystems and People in a Changing World; Island Press: Washington, DC, USA, 2012. [Google Scholar]
- Raup, D.M. The role of extinction in evolution. Proc. Natl. Acad. Sci. USA 1994, 91, 6758–6763. [Google Scholar] [CrossRef] [PubMed]
- Hull, P. Life in the aftermath of mass extinctions. Curr. Biol. 2015, 25, R941–R952. [Google Scholar] [CrossRef] [PubMed]
- Turvey, S.T.; Crees, J.J. Extinction in the Anthropocene. Curr. Biol. 2019, 29, R982–R986. [Google Scholar] [CrossRef] [PubMed]
- Pickett, S.T. The flux of nature: Changing worldviews and inclusive concepts. In Linking Ecology and Ethics for a Changing World. Ecology and Ethics; Rozzi, R., Pickett, S., Palmer, C., Armesto, J., Callicott, J., Eds.; Springer: Dordrecht, The Netherlands, 2013; Volume 1, pp. 265–279. [Google Scholar]
- Jablonski, D. Mass extinctions and macroevolution. Paleobiology 2005, 31, 192–210. [Google Scholar] [CrossRef]
- Jablonski, D. Evolutionary consequences of mass extinctions. In Patterns and Processes in the History of Life; Raup, D.M., Jablonski, D., Eds.; Springer: New York, NY, USA, 1986; pp. 313–329. [Google Scholar]
- Meredith, R.W.; Janečka, J.E.; Gatesy, J.; Ryder, O.A.; Fisher, C.A.; Teeling, E.C.; Goodbla, A.; Eizirik, E.; Simão, T.L.; Stadler, T. Impacts of the Cretaceous Terrestrial Revolution and KPg extinction on mammal diversification. Science 2011, 334, 521–524. [Google Scholar] [CrossRef]
- Maor, R.; Dayan, T.; Ferguson-Gow, H.; Jones, K.E. Temporal niche expansion in mammals from a nocturnal ancestor after dinosaur extinction. Nat. Ecol. Evol. 2017, 1, 1889–1895. [Google Scholar] [CrossRef]
- Lin, H.; Caley, M.J.; Sisson, S.A. Estimating global species richness using symbolic data meta-analysis. Ecography 2022, 2022, e05617. [Google Scholar] [CrossRef]
- Barnosky, A.D.; Matzke, N.; Tomiya, S.; Wogan, G.O.; Swartz, B.; Quental, T.B.; Marshall, C.; McGuire, J.L.; Lindsey, E.L.; Maguire, K.C. Has the Earth’s sixth mass extinction already arrived? Nature 2011, 471, 51–57. [Google Scholar] [CrossRef]
- May, R.M.; Lawton, J.H.; Stork, E. Assessing extinction rates. In Extinction Rates; Lawton, J.H., May, R.M., Eds.; Oxford University Press: Oxford, UK, 1995; pp. 1–24. [Google Scholar]
- Wolfe, J.D.; Luther, D.A.; Jirinec, V.; Collings, J.; Johnson, E.I.; Bierregaard, R.O., Jr.; Stouffer, P.C. Climate change aggravates bird mortality in pristine tropical forests. Sci. Adv. 2025, 11, eadq8086. [Google Scholar] [CrossRef]
- Venter, O.; Brodeur, N.N.; Nemiroff, L.; Belland, B.; Dolinsek, I.J.; Grant, J.W. Threats to endangered species in Canada. Bioscience 2006, 56, 903–910. [Google Scholar] [CrossRef]
- Menotti-Raymond, M.; O’Brien, S.J. Dating the genetic bottleneck of the African cheetah. Proc. Nati. Acad. Sci. USA 1993, 90, 3172–3176. [Google Scholar] [CrossRef]
- Woodford, J. The Wollemi Pine; Text Publishing Company: Melbourne, Australia, 2002. [Google Scholar]
- Offord, C.; Porter, C.; Meagher, P.; Errington, G. Sexual reproduction and early plant growth of the Wollemi pine (Wollemia nobilis), a rare and threatened Australian conifer. Ann. Bot. 1999, 84, 1–9. [Google Scholar] [CrossRef]
- Benson, J. Population ecology of the Wollemi pine. In Ecology 2002: Handbook of the Second Joint Meeting of the Ecological Society of Australia and the New Zealand Ecological Society; Landsberg, J., Ed.; Ecological Society of Australia: Canberra, Australia, 2002. [Google Scholar]
- Hogbin, P.M.; Peakall, R.; Sydes, M.A. Achieving practical outcomes from genetic studies of rare Australian plants. Aust. J. Bot. 2000, 48, 375–382. [Google Scholar] [CrossRef]
- Peakall, R.; Ebert, D.; Scott, L.J.; Meagher, P.F.; Offord, C.A. Comparative genetic study confirms exceptionally low genetic variation in the ancient and endangered relictual conifer, Wollemia nobilis (Araucariaceae). Mol. Ecol. 2003, 12, 2331–2343. [Google Scholar] [CrossRef] [PubMed]
- Fensom, G.; Offord, C. Propagation of the Wollemi pine. In Combined Proceedings; International Plant Propagators Society: Washington, DC, USA, 1997; Volume 47, pp. 66–67. [Google Scholar]
- Rigg, J.L.; Offord, C.A.; Zimmer, H.; Anderson, I.C.; Singh, B.K.; Powell, J.R. Conservation by translocation: Establishment of Wollemi pine and associated microbial communities in novel environments. Plant Soil 2017, 411, 209–225. [Google Scholar] [CrossRef]
- Trueman, S.J.; Pegg, G.S.; King, J. Domestication for conservation of an endangered species: The case of the Wollemi pine. Tree For. Sci. Biotechnol. 2007, 1, 1–10. [Google Scholar]
- Macphail, M.; Hill, K.; Partridge, A.; Truswell, E.; Foster, C. Wollemi Pine-Old pollen records for a newly discovered genus of gymnosperm. Geol. Today 1995, 11, 48–50. [Google Scholar]
- McLoughlin, S.; Vajda, V. Ancient Wollemi Pines resurgent: Ten years after its discovery, a vanishingly rare tree from the Cretaceous Period is a scientific darling and may soon become a commercial success too. Am. Sci. 2005, 93, 540–548. [Google Scholar] [CrossRef]
- Johnson, C.N. Fire, people and ecosystem change in Pleistocene Australia. Aust. J. Bot. 2016, 64, 643–651. [Google Scholar] [CrossRef]
- Karp, A.T.; Faith, J.T.; Marlon, J.R.; Staver, A.C. Global response of fire activity to late Quaternary grazer extinctions. Science 2021, 374, 1145–1148. [Google Scholar] [CrossRef]
- Miller, G.H.; Fogel, M.L.; Magee, J.W.; Gagan, M.K.; Clarke, S.J.; Johnson, B.J. Ecosystem collapse in Pleistocene Australia and a human role in megafaunal extinction. Science 2005, 309, 287–290. [Google Scholar] [CrossRef]
- Hannam, P. Incredible, Secret Firefighting Mission Saves Famous ‘Dinosaur Trees’. Sydney Morning Herald. 2020. Available online: https://fennerschool.anu.edu.au/news-events/news/fenner-news-incredible-secret-firefighting-mission-saves-famous-dinosaur-trees (accessed on 17 December 2024).
- McCauley, D.J.; Hardesty-Moore, M.; Halpern, B.S.; Young, H.S. A mammoth undertaking: Harnessing insight from functional ecology to shape de-extinction priority setting. Funct. Ecol. 2017, 31, 1003–1011. [Google Scholar] [CrossRef]
- Rohwer, Y.; Marris, E. An analysis of potential ethical justifications for mammoth de-extinction and a call for empirical research. Ethics Policy Environ. 2018, 21, 127–142. [Google Scholar] [CrossRef]
- Shapiro, B. How to Clone a Mammoth: The Science of De-Extinction; Princeton University Press: Princeton, NJ, USA, 2015. [Google Scholar]
- Genovesi, P.; Simberloff, D. “De-extinction” in conservation: Assessing risks of releasing “resurrected” species. J. Nat. Conserv. 2020, 56, 125838. [Google Scholar] [CrossRef]
- Scheele, B.C.; Heard, G.W.; Cardillo, M.; Duncan, R.P.; Gillespie, G.R.; Hoskin, C.J.; Mahony, M.; Newell, D.; Rowley, J.J.; Sopniewski, J. An invasive pathogen drives directional niche contractions in amphibians. Nat. Ecol. Evol. 2023, 7, 1682–1692. [Google Scholar] [CrossRef] [PubMed]
- Crawford, B.A.; Maerz, J.C.; Terrell, V.C.; Moore, C.T. Population viability analysis for a pond-breeding amphibian under future drought scenarios in the southeastern United States. Glob. Ecol. Conserv. 2022, 36, e02119. [Google Scholar] [CrossRef]
- Fantle-Lepczyk, J.; Taylor, A.; Duffy, D.C.; Crampton, L.H.; Conant, S. Using population viability analysis to evaluate management activities for an endangered Hawaiian endemic, the Puaiohi (Myadestes palmeri). PLoS ONE 2018, 13, e0198952. [Google Scholar] [CrossRef]
- Boyce, M.S. Population viability analysis. Ann. Rev. Ecol. Syst. 1992, 23, 481–506. [Google Scholar] [CrossRef]
- Galán-Acedo, C.; Verde Arregoitia, L.D.; Arasa-Gisbert, R.; Auliz-Ortiz, D.; Saldivar-Burrola, L.L.; Gouveia, S.F.; Correia, I.; Rosete-Vergés, F.A.; Dinnage, R.; Villalobos, F. Global primary predictors of extinction risk in primates. Proc. R. Soc. B Biol. Sci. 2024, 291, 20241905. [Google Scholar] [CrossRef]
- Howell, L.G.; Frankham, R.; Rodger, J.C.; Witt, R.R.; Clulow, S.; Upton, R.M.; Clulow, J. Integrating biobanking minimises inbreeding and produces significant cost benefits for a threatened frog captive breeding programme. Conserv. Lett. 2021, 14, e12776. [Google Scholar] [CrossRef]
- Byrne, K.; Nichols, R.A. Culex pipiens in London Underground tunnels: Differentiation between surface and subterranean populations. Heredity 1999, 82, 7–15. [Google Scholar] [CrossRef]
- Vellend, M.; Harmon, L.J.; Lockwood, J.L.; Mayfield, M.M.; Hughes, A.R.; Wares, J.P.; Sax, D.F. Effects of exotic species on evolutionary diversification. Trends Eco. Evol. 2007, 22, 481–488. [Google Scholar] [CrossRef]
- Dugatkin, L.A. Humans Are Driving a New Kind of Evolution in Animals; Scientific American: New York, NY, USA, 2024. [Google Scholar]
- Long, J. Australia’s Due to Collide with Asia… Whether We Like It or Not. Available online: https://www.curtin.edu.au/news/australias-due-to-collide-with-asia-whether-we-like-it-or-not/#:~:text=‘Australia%20is%20moving%20northwards%207cms,regularly%20and%20will%20happen%20again (accessed on 4 January 2024).
- Vermeij, G.J. When biotas meet: Understanding biotic interchange. Science 1991, 253, 1099–1104. [Google Scholar] [CrossRef]
- Webb, S.D. Ecogeography and the great American interchange. Paleobiology 1991, 17, 266–280. [Google Scholar] [CrossRef]
- Marshall, L.G.; Webb, S.D.; Sepkoski, J.J.; Raup, D.M. Mammalian evolution and the Great American Interchange. Science 1982, 215, 1351–1357. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gould, J.; Callen, A.; Beranek, C. Managing Natural Extinctions. Wild 2025, 2, 39. https://doi.org/10.3390/wild2040039
Gould J, Callen A, Beranek C. Managing Natural Extinctions. Wild. 2025; 2(4):39. https://doi.org/10.3390/wild2040039
Chicago/Turabian StyleGould, John, Alex Callen, and Chad Beranek. 2025. "Managing Natural Extinctions" Wild 2, no. 4: 39. https://doi.org/10.3390/wild2040039
APA StyleGould, J., Callen, A., & Beranek, C. (2025). Managing Natural Extinctions. Wild, 2(4), 39. https://doi.org/10.3390/wild2040039




