Thyroid Disorder in Obese Children and Adolescents: A Cross-Sectional Study in a Tertiary Care Hospital in Bangladesh
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Procedures
2.3. Ethical Consideration
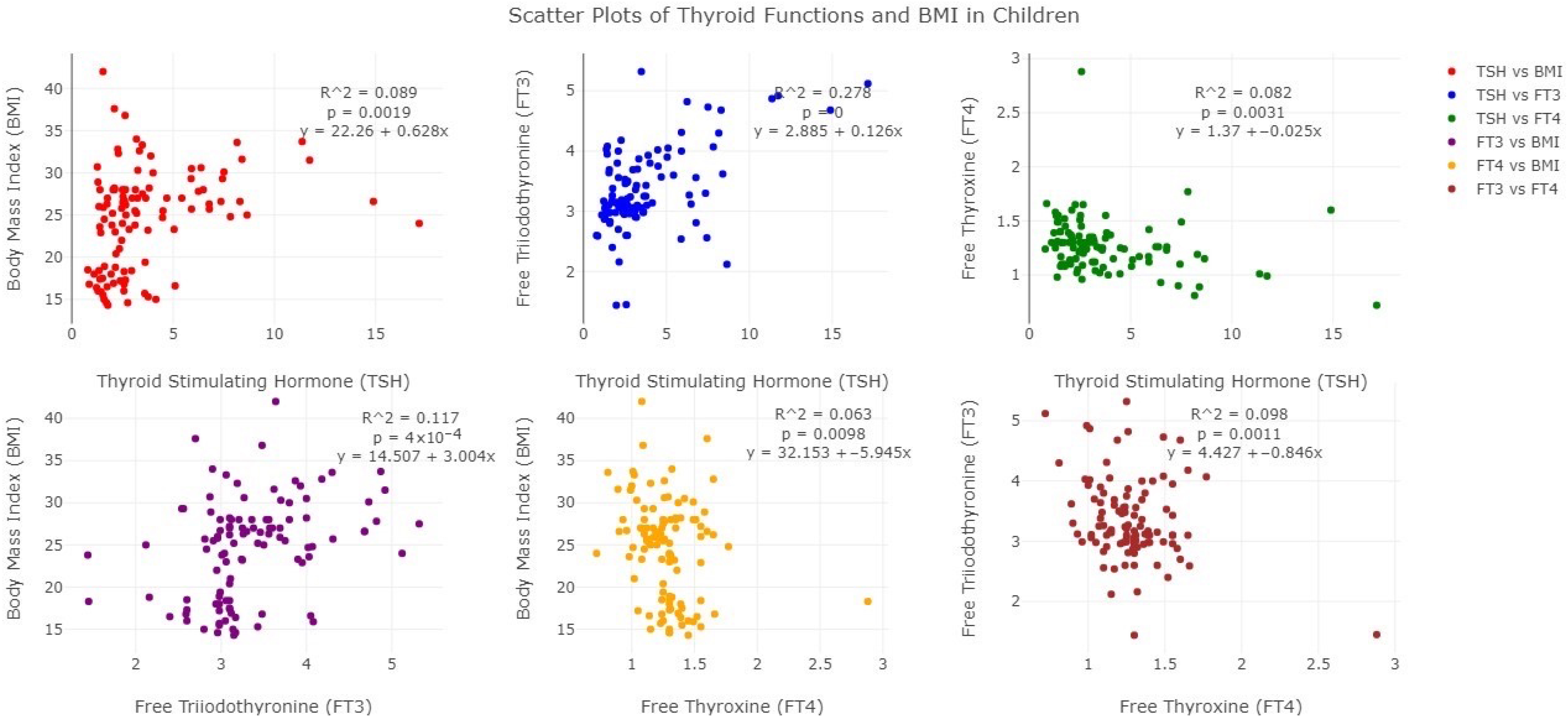
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stichel, H.; Allemand, D.; Grüters, A. Thyroid function and obesity in children and adolescents. Horm. Res. Paediatr. 2000, 54, 14–19. [Google Scholar] [CrossRef]
- The World Health Organization. Obesity and Overweight; The World Health Organization: Geneva, Switzerland, 2025.
- World Health Organization. The Double Burden of Malnutrition: Priority Actions on Ending Childhood Obesity; World Health Organization: Geneva, Switzerland; Regional Office for South-East Asia: New Delhi, India, 2020.
- World Health Organization. Prevalence of Overweight Among Children and Adolescents; World Health Organization: Geneva, Switzerland, 2016.
- Nannipieri, M.; Cecchetti, F.; Anselmino, M.; Camastra, S.; Niccolini, P.; Lamacchia, M.; Rossi, M.; Iervasi, G.; Ferrannini, E. Expression of thyrotropin and thyroid hormone receptors in adipose tissue of patients with morbid obesity and/or type 2 diabetes: Effects of weight loss. Int. J. Obes. 2009, 33, 1001–1006. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Platz, E.A.; Ladenson, P.W.; Mondul, A.M.; Menke, A.; de González, A.B. Body fatness and markers of thyroid function among US men and women. PLoS ONE 2012, 7, e34979. [Google Scholar] [CrossRef]
- Witkowska-Sędek, E.; Kucharska, A.; Rumińska, M.; Pyrżak, B. Thyroid dysfunction in obese and overweight children. Endokrynol. Pol. 2017, 68, 54–60. [Google Scholar] [CrossRef]
- Natah, T.M.; Wtwt, M.A.-A.; Hadi, M.A.; Farhood, H.F.; Al-Saadi, A.H. Thyroid metabolic hormones and its correlation with bmi and lipid profile in healthy people. Thyroid 2013, 18, 47–56. [Google Scholar]
- Muscogiuri, G.; Sorice, G.P.; Mezza, T.; Prioletta, A.; Lassandro, A.P.; Pirronti, T.; Della Casa, S.; Pontecorvi, A.; Giaccari, A. High-normal tsh values in obesity: Is it insulin resistance or adipose tissue’s guilt? Obesity 2013, 21, 101–106. [Google Scholar] [CrossRef]
- Pasquali, R.; Vicennati, V. Obesity and hormonal abnormalities. In International Textbook of Obesity; Björntorp, P., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2001; pp. 225–239. [Google Scholar]
- Björntorp, P. Endocrine abnormalities of obesity. Metabolism 1995, 44, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.S.; Pencina, M.J.; D’agostino, R.B.; Murabito, J.M.; Seely, E.W.; Pearce, E.N.; Vasan, R.S. Relations of thyroid function to body weight: Cross-sectional and longitudinal observations in a community-based sample. Arch. Intern. Med. 2008, 168, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Staníková, D.; Krajčovičová, L.; Lobotková, D.; Vitariušová, E.; Tichá, Ľ.; Pribilincová, Z.; Ukropcová, B.; Ukropec, J.; Staník, J. Thyroid hormone levels and BMI-SDS changes in adolescents with obesity. Front. Endocrinol. 2023, 14, 1304970. [Google Scholar] [CrossRef]
- Ghergherehchi, R.; Hazhir, N. Thyroid hormonal status among children with obesity. Ther. Adv. Endocrinol. Metab. 2015, 6, 51–55. [Google Scholar] [CrossRef]
- Grandone, A.; Santoro, N.; Coppola, F.; Calabrò, P.; Perrone, L.; del Giudice, E.M. Thyroid function derangement and childhood obesity: An Italian experience. BMC Endocr. Disord. 2010, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Walczak, K.; Sieminska, L. Obesity and thyroid axis. Int. J. Environ. Res. Public Health 2021, 18, 9434. [Google Scholar] [CrossRef]
- Laurberg, P.; Knudsen, N.; Andersen, S.; Carlé, A.; Pedersen, I.B.; Karmisholt, J. Thyroid function and obesity. Eur. Thyroid. J. 2012, 1, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Chikunguwo, S.; Brethauer, S.; Nirujogi, V.; Pitt, T.; Udomsawaengsup, S.; Chand, B.; Schauer, P. Influence of obesity and surgical weight loss on thyroid hormone levels. Surg. Obes. Relat. Dis. 2007, 3, 631–635. [Google Scholar] [CrossRef]
- Mahbuba, S.; Rahat, F.; Iman, K.; Sultana, A.; Parvin, R.; Afroze, S.; Ghosh, N.K. Disease Profile of Children with Endocrine Disorders: Experience in Dr. MR Khan Shishu Hospital, Dhaka, Bangladesh. J. Dr. MR Khan Shishu Child. Hosp. 2022, 3, 9–14. [Google Scholar]
- Åsvold, B.O.; Bjøro, T.; Vatten, L.J. Association of serum TSH with high body mass differs between smokers and never-smokers. J. Clin. Endocrinol. Metab. 2009, 94, 5023–5027. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. BMI; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2024.
- Özer, S.; Bütün, İ.; Sönmezgöz, E.; Yılmaz, R.; Demir, O. Relationships among thyroid hormones and obesity severity, metabolic syndrome and its components in Turkish children with obesity. Nutr. Hosp. 2015, 32, 645–651. [Google Scholar]
- Longhi, S.; Radetti, G. Thyroid function and obesity. J. Clin. Res. Pediatr. Endocrinol. 2013, 5, 40. [Google Scholar] [PubMed]
- Hari Kumar, K.; Verma, A.; Muthukrishnan, J.; Modi, K. Obesity and thyrotropinemia. Indian J. Pediatr. 2009, 76, 933–935. [Google Scholar] [CrossRef]
- Ali, B.A.-M.; Mahrous, D.M.; Ahmed, D.M. Thyroid function status in obese children. J. Diabetes Metab. 2016, 7, 665. [Google Scholar]
- An, Y.M.; Moon, S.J.; Kim, S.K.; Suh, Y.J.; Lee, J.E. Thyroid function in obese Korean children and adolescents: Korea National Health and Nutrition Examination Survey 2013–2015. Ann. Pediatr. Endocrinol. Metab. 2018, 23, 141. [Google Scholar] [CrossRef]
- Pacifico, L.; Anania, C.; Ferraro, F.; Andreoli, G.M.; Chiesa, C. Thyroid function in childhood obesity and metabolic comorbidity. Clin. Chim. Acta 2012, 413, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Marras, V.; Casini, M.R.; Pilia, S.; Carta, D.; Civolani, P.; Porcu, M.; Uccheddu, A.P.; Loche, S. Thyroid function in obese children and adolescents. Horm. Res. Paediatr. 2010, 73, 193–197. [Google Scholar] [CrossRef]
- Burman, K.D.; Latham, K.R.; Djuh, Y.-Y.; Smallridge, R.C.; Tseng, Y.-C.L.; Lukes, Y.G.; Maunder, R.; Wartofsky, L. Solubilized nuclear thyroid hormone receptors in circulating human mononuclear cells. J. Clin. Endocrinol. Metab. 1980, 51, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, F.; Uzuner, A.; Demet, M.C.; Turgut, B.; Tosun, N. Thyroid hormones and BMI in obese children: One year follow up results. Marmara Med. J. 2015, 28, 129–134. [Google Scholar] [CrossRef]
- Xu, R.; Huang, F.; Zhang, S.; Lv, Y.; Liu, Q. Thyroid function, body mass index, and metabolic risk markers in euthyroid adults: A cohort study. BMC Endocr. Disord. 2019, 19, 129. [Google Scholar] [CrossRef]
- Aeberli, I.; Jung, A.; Murer, S.B.; Wildhaber, J.; Wildhaber-Brooks, J.; Knöpfli, B.H.; Zimmermann, M.B. During rapid weight loss in obese children, reductions in TSH predict improvements in insulin sensitivity independent of changes in body weight or fat. J. Clin. Endocrinol. Metab. 2010, 95, 5412–5418. [Google Scholar] [CrossRef]
- Shimura, H.; Miyazaki, A.; Haraguchi, K.; Endo, T.; Onaya, T. Analysis of differentiation-induced expression mechanisms of thyrotropin receptor gene in adipocytes. Mol. Endocrinol. 1998, 12, 1473–1486. [Google Scholar] [CrossRef]
| Normal | Obese | p-Value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age (years) | |||||
| 10–13 | 27 | 34.18 | 52 | 65.82 | 0.967 |
| 14–18 | 9 | 34.62 | 17 | 65.38 | |
| Sex | |||||
| Male | 16 | 31.37 | 35 | 68.63 | 0.541 |
| Female | 20 | 37.04 | 34 | 62.96 | |
| Residence | |||||
| Rural | 20 | 43.48 | 26 | 56.52 | 0.059 |
| Urban | 15 | 25.86 | 43 | 74.14 | |
| Normal (Mean ± SD) | Obese (Mean ± SD) | p-Value | |
|---|---|---|---|
| Weight (kg) | 37.80 (±7.29) | 62.29 (±14.42) | <0.001 |
| Height (cm) | 147.68 (±9.30) | 148.80 (±10.26) | 0.5862 |
| BMI | 18.13 (±3.72) | 27.92 (±3.72) | <0.001 |
| Waist circumference (cm) | 71.21 (±7.05) | 91.71 (±9.41) | <0.001 |
| Father’s BMI | 24.59 (±2.64) | 25.71 (±2.94) | 0.0573 |
| Mother’s BMI | 23.90 (±3.44) | 26.65 (±4.04) | 0.0008 |
| FT3 (pg/mL) | 3.02 (±0.48) | 3.52 (±0.71) | 0.0003 |
| FT4 (ng/dL) | 1.38 (±0.30) | 1.23 (±0.21) | 0.0031 |
| TSH (µIU/mL) | 2.26 (±0.97) | 4.40 (±3.20) | 0.0002 |
| State of Thyroid Hormone Status | Number (n = 69) | Percentage (%) |
|---|---|---|
| Euthyroid | 49 | 71.01 |
| Subclinical hypothyroid | 19 | 27.54 |
| Overt hypothyroid | 01 | 1.45 |
| FT3 | FT4 | TSH | ||||
|---|---|---|---|---|---|---|
| Coefficient ± SE | p-Value | Coefficient ± SE | p-Value | Coefficient ± SE | p-Value | |
| BMI | 0.032 ± 0.011 | 0.004 | −0.010 ± 0.004 | 0.017 | 0.104 ± 0.044 | 0.020 |
| Age (ref. 10–13 years) | ||||||
| 14–18 years | −0.176 ± 0.142 | 0.218 | −0.066 ± 0.056 | 0.243 | −0.551 ± 0.578 | 0.342 |
| Sex (ref. Male) | ||||||
| Female | 0.032 ± 0.126 | 0.799 | −0.021 ± 0.050 | 0.672 | 0.842 ± 0.513 | 0.104 |
| Residence (ref. Rural) | ||||||
| Urban | −0.053 ± 0.126 | 0.675 | 0.047 ± 0.490 | 0.340 | 0.661 ± 0.511 | 0.199 |
| Mother’s BMI | −0.035 ± 0.024 | 0.154 | −0.003 ± 0.010 | 0.734 | 0.197 ± 0.099 | 0.048 |
| Father’s BMI | 0.027 ± 0.033 | 0.414 | −0.014 ± 0.013 | 0.286 | 0.076 ± 0.136 | 0.577 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharmin, F.; Chowdhury, A.T.; Hossian, M.; Rafiquzzaman, S.; Biswas, D.C.; Rupa, F.H.; Begum, S. Thyroid Disorder in Obese Children and Adolescents: A Cross-Sectional Study in a Tertiary Care Hospital in Bangladesh. Future 2025, 3, 18. https://doi.org/10.3390/future3040018
Sharmin F, Chowdhury AT, Hossian M, Rafiquzzaman S, Biswas DC, Rupa FH, Begum S. Thyroid Disorder in Obese Children and Adolescents: A Cross-Sectional Study in a Tertiary Care Hospital in Bangladesh. Future. 2025; 3(4):18. https://doi.org/10.3390/future3040018
Chicago/Turabian StyleSharmin, Farzana, Anika Tasneem Chowdhury, Mosharop Hossian, Shaima Rafiquzzaman, Dhiraj C. Biswas, Fatema Hashem Rupa, and Suraiya Begum. 2025. "Thyroid Disorder in Obese Children and Adolescents: A Cross-Sectional Study in a Tertiary Care Hospital in Bangladesh" Future 3, no. 4: 18. https://doi.org/10.3390/future3040018
APA StyleSharmin, F., Chowdhury, A. T., Hossian, M., Rafiquzzaman, S., Biswas, D. C., Rupa, F. H., & Begum, S. (2025). Thyroid Disorder in Obese Children and Adolescents: A Cross-Sectional Study in a Tertiary Care Hospital in Bangladesh. Future, 3(4), 18. https://doi.org/10.3390/future3040018





