Abstract
Background: Puberty is a life milestone that marks the transition from childhood to adulthood. An ambispective Chongqing Pubertal Timing (CQPT) cohort was started in 2014 to understand pubertal timing and identify environmental risk factors. Methods: A total of 1429 children and adolescents were recruited and have been followed up once every 6 months for 8 years in a district of Chongqing, China. Data were collected via questionnaires for social and family environment, health conditions, gestational and maternal information, and in-person physical examinations by trained medical school graduate students in follow-ups. Environmental exposures of polycyclic aromatic hydrocarbons (PAHs), neonicotinoids, and heavy metals in urine samples were measured at different time points. Results: The mean ages at pubertal onset were 10.20 for thelarche, 11.62 for pubic hair development, and 11.84 for menarche in girls, and 11.16 for genital development, 11.66 for testicular enlargement, and 12.71 for first spermatorrhea in boys. Four OH-PAHs were associated with delayed timing of menarche, thelarche, pubic hair, and axillary hair development in girls, and thiacloprid was found to potentially impact genital stages in boys and axillary hair development in girls. Conclusions: We built a cohort to provide evidence of regional pubertal timing of boys and girls and the significant environmental factors. Further health outcomes, especially mental health and women’s health and its long-term health implications, will be followed.
1. Introduction
Puberty is a life milestone that marks the transition from childhood to adulthood with a series of events of physical and psychological changes [1]. Puberty starting before 8 years old in girls and 9 years old in boys is commonly defined as precocious puberty [2]. Pubertal timing indicates the age at which children entering these pubertal events, which is relative according to sex and same-age peers [3]. Tanner staging evaluates breast development, genital ratings, and pubic hair development to estimate pubertal timing and is often used in pediatric care and epidemiology studies [4]. Moreover, peak height velocity, voice break, first spermatorrhea, beard development, menarche, axillary hair development, and acne are also used as characteristics of pubertal timing [5]. As these pubertal events may develop swiftly in a relative sequence and some events are mostly recorded using self-reporting, which may introduce bias, studies with short follow-up intervals covering comprehensive pubertal characteristics could better represent regional pubertal timing.
In recent years, it is widely discussed that there might be a secular trend of altered pubertal timing around the world [6,7,8]. With a trend for earlier pubertal timing, more evidence is needed for medical community to define “precocious puberty”, which might be outdated. Moreover, compared to girls, there are fewer studies of boys, and the results from limited studies on the pubertal timing trend in boys are not consistent [9]. Genetics are regarded as the main factor of pubertal timing. Studies also suggested that life behaviors and health problems in pregnancy, endocrine-disrupting chemicals (EDCs) [10], metals [11], family environment [12], and social environment [13] might influence pubertal timing. However, the weight of these factors in influencing pubertal timing and how they have been associated with each other are still unclear.
Until now, most studies have reported pubertal timing from cross-sectional studies or retrieved data from longitudinal database not designed for pubertal timing covering only partial pubertal events; have used self-reporting pubertal events data; have focused on limited environmental exposures at a time; and have followed up for a relatively short period. Little is known about interactions between environmental factors and other factors such as gestational status, BMI, exercise time, screen time, and mental health. Hence, long-term follow-up throughout puberty covering a wide range of pubertal events with various risk factors is needed.
The Chongqing Pubertal Timing (CQPT) cohort was designed to overcome the limitations of existing studies, observe pubertal timing, and identify key risk factors and their interactions in a district of Chongqing, southwest China, one of China’s four direct-controlled municipalities. As high level of heavy metals [14], different neonicotinoids [15], and PAHs [16] were detected in Yangtze River Area where Chongqing is located, we sought to address the hypothesis that polycyclic aromatic hydrocarbons (PAHs) acting as EDCs, neonicotinoids, and heavy metals combined with the family environment and social environment may affect pubertal timing. On one hand, EDCs such as PAHs [17,18] and neonicotinoids [19,20] usually have estrogenic and anti-androgenic effects, which could lead to hormonal imbalance on the hypothalamic–pituitary–gonadal (HPG) axis or hypothalamic–pituitary-adrenal (HPA) axis, which play crucial roles in the regulation of puberty [21]. On the other hand, children are more susceptible to the harmful health effects of air pollution than adults [22].
The CQPT is a school-based cohort study aimed at defining the relationship between factors including environment, exercise time, screen time, sleep, mental health, BMI, gestational status, and a wide range of pubertal events. More specifically, the goals of the CQPT study are as follows: (1) Monitor pubertal timing with a series of in-person measured pubertal events to evaluate adolescents’ pubertal timing in a city in China. (2) Evaluate each environmental exposure (PAHs, neonicotinoids, heavy metals, family environment, and social environment) in relation to pubertal timing. (3) Identify the association of environmental exposures, lifestyle (exercise time, screen time, and sleep), other health factors (mental health, BMI, etc.), gestational status, and pubertal timing. (4) Understand the relationship between pubertal timing and health implications through subsequent follow-ups and provide a potential disease etiology research approach.
2. Materials and Methods
2.1. Study Participants
The CQPT study is an ongoing ambispective cohort in the JLP district of Chongqing since December 2014. Chongqing is a city with over 40 districts and a population of 30 million with typical urban–rural dual economic structure. To better represent the city, the JLP district was picked in this study as it was a demonstration zone for integrating urban and rural areas per the government. In the district, we selected four schools, two located near the site of a former thermal power plant, one located right by the main roads with much traffic, and one located in a residential area, to reflect different levels of environmental pollution. The schools near a former thermal power plant and main roads were regarded as highly polluted areas, and the one in the residential area, similar to a community school, was considered to be in a less polluted area.
As an observational study, the eligibility criteria were students who did not have medical conditions with no reported endocrine disease, genetic disorders, or chromosome disorders that might interfere with normal growth processes with returned consent forms in the four targeted primary schools. Before recruitment, we estimated the sample size based on our preliminary research, which showed approximately 26% earlier puberty onset in the exposure group and 14% incidence in the control group. This calculation used a two-sided α of 0.05 and a β of 0.1 in the formula for cohort studies. After considering 50% loss to follow-up, the theoretical total sample size was approximately 348 participants per group, resulting in a total of 696 participants. In the recruitment phase, 2953 consent forms were sent out in printed paper and brought home by children. In April 2014 and May 2014, 695 boys and 542 girls were recruited to collect basic information and to conduct the physical examination for baseline assessment. To balance the number of boys and girls, we added another round of recruitment, and 192 girls consented to the cohort in December 2014. When we first started the recruitment in April and May 2014 (Spring Semester), participants were from grades 1–4. By the time we officially finished recruitment in December 2014, they entered into grades 2–5 (Fall Semester). Hence, 1429 participants with 695 boys (ages 8.7 ± 1.2) and 734 girls (ages 9.2 ± 1.2) were officially included into the cohort.
From 2015 to 2022, we conducted 15 in-person follow-ups every 6 months on a voluntary basis with the participants. Every visit was conducted at the schools during the school day during breaks for in-person examination and questionnaires. Due to the pubertal timing of different events developing at distinct time points, some participants might have reached pubertal onset on some outcomes at baseline, and we excluded them in the analysis, causing different baseline sample sizes. For those lost to follow-up, we might have followed their pubertal onset already, which is the main outcome of the study before we lost them, and they were included in our pubertal timing analysis. Hence, Figure 1 presents the sub-sample sizes for different pubertal outcomes at baseline, drop-out number without the onset event observed, and the total number we observed in terms of pubertal onset for each outcome.
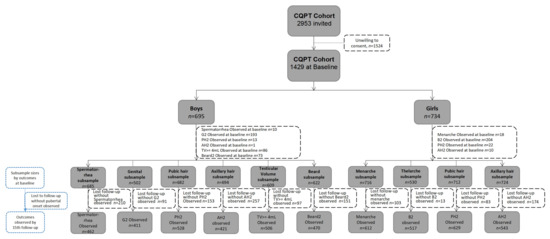
Figure 1.
Presentation of sample size of the CQPT cohort according to pubertal development characteristics.
As reported in Table 1, at the fourth follow-up, participants dropped out dramatically, resulting in a response from 98.0% to 83.1% when students started to graduate from primary school to middle school as expected. To control the drop-out rate, three measures were taken immediately as follows: (1) investigating students’ middle school list with teachers and parents and finalizing the eight middle school campuses for further in-person follow-ups; (2) re-establishing connections with students and their families through alternative contact information; (3) showing full respect to the participants during each follow-up visit. Response rates dropped out greatly again in the 10th follow-up as students graduated from eight targeted middle schools to high schools, and students were scattered around the city, making the in-person follow-ups difficult. We started to send out electronic questionnaires to students who were not conveniently able to participate in the on-site follow-ups from then. Although the response rates were rebounded, the rates dropped dramatically soon after, which might have been affected by the COVID-19 pandemic. As this cohort is still in progress, we will be continuing follow-up for adolescents’ pubertal development, physical growth, and health outcomes, especially in terms of mental health and women’s health once a year.

Table 1.
Summary of data collected from the CQPT cohort in each follow-up.
2.2. Measurements
To monitor changes of pubertal stages, pubertal events, anthropometric measurement variables, and various environmental exposures, data were obtained from physical examinations, questionnaires, and urine samples through follow-ups. We monitored pubertal and physical development as well as environmental factors in every follow-up. Stressful life events were collected in the first seven follow-ups as a potential influence factor. With the participants growing up and the increasing awareness of pubertal timing and tempo influences on health, we started to monitor participants’ health conditions including mental health and other health outcomes (see Figure 2).
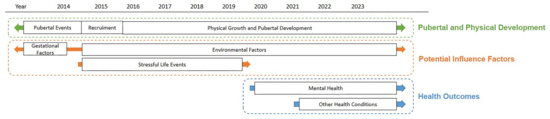
Figure 2.
Overview and data collection process of the CQPT cohort.
All the investigators were medical school graduate students trained with standard protocol before each follow-up. Data were double-entered using Epidata 3.0 (Jens M. Lauritsen, Michael Bruus, and Mark Myatt, Odense, Denmark). Table 1 specifies the information collected at the baseline and at each follow-up.
2.2.1. Physical Examinations
Anthropometric measurement and pubertal development stages were measured by investigators utilizing standardized protocols at baseline and all follow-ups. Children and adolescents were asked to remove their jackets and shoes before the examination. The height and weight were measured using the calibrated mechanical height stadiometer and electronic weight scale. BMI was calculated using the formula [weight (kg)/height2 (m2)], and the threshold of overweight and obesity was based on the Chinese Standard of screening for overweight and obesity among school-age children and adolescents (WS/T 586-2018) [23]. Before the waist and hip circumference examination, children and adolescents were required to stand up straight and relax their abdomen and arms. The waist circumference and hip circumference were measured using a soft tape. All results were accurate to one decimal place.
Pubertal development data were inspected and recorded by an investigator in the same gender in every follow-up. Puberty outcomes including breast palpation and pubic hair in girls and genital development and pubic hair in boys were assessed through Tanner stage classification (1, prepuberty; 2, onset; 3, on-going; 4, nearly complete; 5, adult-like and complete) [24]. The axillary hair and beard development was divided into three stages (1, no hair; 2, onset/small amount of hair; 3, adult-like hair is approximately adult). Testicular volume (TV) was measured by Prader orchidometer, and TV ≥ 4 mL indicates the onset [4]. Other pubertal event (first spermatorrhea, menarche) timing was asked by the inspector during physical examination (the first spermatorrhea question can be found in Appendix A). Table 2 shows the physical examination characteristics at baseline.

Table 2.
Physical examination characteristics at baseline.
2.2.2. Urine Sample Measurements
Urine samples were collected every year during fall/winter follow-ups. Internal exposure of PAHs, heavy metals, and neonicotinoid insecticides were measured at various time points (see Table 1). Urine samples were collected in the morning at schools during the follow-up under instructions. Midstream urine samples were collected in 10 mL glass tubes and stored at −20 °C in Research Center for Environment and Human Health, Chongqing Medical University.
We modified the high-performance liquid chromatography mass spectrometry (HPLS-MS) method to detect four PAH metabolites [25] and the solid-phase extraction-ultrahigh performance liquid chromatography-tandem mass spectrometry (SPE-UPLC-MS/MS) method for measuring neonicotinoid insecticides to robustly and simultaneously quantify these compounds in urine samples [26]. Four PAH metabolites (1-hydroxypyrene [1-OHPyr], 2-hydroxynaphthalene [2-OHNap], 2-hydroxyfluorine [2-OHFlu], and 9-hydroxyphenanthrene [9-OHPhe]) were measured at baseline (girls) and at the second follow-up (boys). Boys’ internal heavy metal levels of arsenic (As), zinc (Zn), lead (Pb), iron (Fe), manganese (Mn), aluminum (Al), copper (Cu), cobalt (Co), rubidium (Rb), cadmium (Cd), and magnesium (Mg) were measured at the fourth follow-up using inductively-coupled plasma–mass spectrometry (ICP-MS). Neonicotinoid analytes including imidacloprid (IMI), nitenpyram (NIT), acetamiprid (ACE), thiacloprid (THIA), imidaclothiz (IMID), thiamethoxam (TMX), clothianidin (CLO), dinotefuran (DIN), flonicamid (FLO), sulfoxaflor (SUL), and metabolite N-desmethyl-acetamiprid (NACE) were detected at the 8th and 10th follow-ups.
2.2.3. Questionnaires
Questionnaires and scales were completed by participants and their parents for basic information, potential environmental and lifestyle risk factors, and diverse health-related questions. The characteristics of participants of baseline are listed in Table 3. A student survey was administered to collect family factors. A parent survey was administered to collect the mother’s menarche age and gestational information at baseline and students’ environmental exposures (e.g., cigarettes exposure, smoked and fried food exposure) and screen time in each follow-up until the 10th follow-up. Since we stopped collecting parent surveys after December 2019, the environmental exposures and screen time were moved to the student survey, and exercise time, sleep time, and self-reported myopia were added later.

Table 3.
Description of baseline characteristics.
The pubertal development scale (Chinese version) [27] was used to learn the consistency between trained medical students and self-reported Tanner stages. Scale of Stressful Life Events for Primary School Students (SSLEPSS) was developed by our team based on household lifestyle, peer relationships, teacher–student relationship, life adaptation, and study adaptation dimensions and was used from the first to the seventh follow-ups to assess primary school students’ psychological stress level [28]. Since students entered middle schools after the seventh follow-up, SSLEPSS was not applicable for participants who remained in the cohort. We started to send out various suitable questionnaires and scales to monitor adolescents’ psychological and physical health from the eighth follow-up.
Since puberty is closely related to sexual development, we first used the Adolescent Psychosexual Health questionnaire [29] and Chinese Youth Risk Behavior Survey (CYRBS) [30] to monitor adolescents’ psychosexual health and potential risk behaviors. Youth Self-Report (YSR) and Child Behavior Checklist (CBCL) [31] were the similar scales with a child version and a parent version conducted to understand adolescents internalizing and externalizing problems, including the socializing problems we were monitoring in previous follow-ups. Due to the limited space of the survey, we monitored adolescents’ depression, anxiety, and social anxiety using the Children’s Depression Inventory (CDI) [32], Screen for Child Anxiety Related Emotional Disorders (SCARED) [33], and Social Anxiety Subscale of the Self-Consciousness Scale [34] every year.
Other than these main psychological health scales, the Inventory of Parent and Peer Attachment (IPPA) [35], Chinese version of the Internet Addiction Scale [36], Index of Well-Being [37], Adolescent Students’ Life Satisfaction Scale [38], Chinese version of the Children’s Loneliness Scale (CLS) [39], Chinese version of the Self-esteem Scale (SES) [40], Chinese version of the Buss–Warren Aggression Questionnaire (AQ) [41], Social Support Scale [42], Family Assessment Device (FAD) [43], Revised Prosocial Tendencies Measure (PTM) [44], Multidimensional Sub-health Questionnaire of Adolescents (MSQA) [45], Adolescents Self-Harm Scale [46], and Mental Health Inventory of Middle-School Students [47] were sent out to understand adolescents’ psychological status and potential co-factors. Since some of the tools were developed in Chinese, we provided a brief introduction, validity, and reliability of these tools in this study in Table A2 in Appendix B. Time points of all tools can be found in Table 1.
3. Results
Out of the 1429 participants, 827 (390 boys) participants were retained in the cohort after the 5 year follow-up and 439 (211 boys) participants remained after the 8 year follow-up, with boys’ mean age being 15.9 ± 1.2 and girls’ mean age 16.3 ± 1.1. Among the baseline characteristics collected (age, gender, parents passed away, parental marital status, father’s/mother’s education, household monthly income per capita, moved to this area from another district, father/mother working out of town), we only found significance for participants who moved to this area from another district between those who remained in the cohort and those who were lost to follow-up. In general, we observed 67.4% first spermatorrhea, 81.9% genital onset (G2), 77.4% pubic hair onset (PH2), 60.7% axillary hair onset (AH2), 83.1% testicular enlargement (TV ≥ 4 mL), and 75.6% beard onset (Beard2) in boys, and 85.5% menarche, 97.5% thelarche (B2), 88.3% pubic hair onset (PH2), and 75.0% axillary hair onset (AH2) in girls by the 15th follow-up until June 2022 (See Figure 1). Until now, our analysis focused on pubertal timing and its risk factors. More than 10 manuscripts (see Table A1 in Appendix B) are online or in submission, and the main findings are briefly described below.
3.1. Pubertal Development
P25 and P75 of each pubertal timing were used to decide early and delayed pubertal onset by age. The mean ages at pubertal onset were 10.20 for thelarche, 11.62 for pubic hair development, and 11.84 for menarche in girls and 11.16 for genital development, 11.66 for testicular enlargement, and 12.71 for first spermatorrhea in boys. The consistency between self-reported pubertal development by the Pubertal Development Scale and Tanner staging was low, and the Cronbach’s alpha was less than 0.7 for both sexes. The concordance of puberty self-assessment using Realistic Color Images and physical examination was almost perfect in girls (wk > 0.800). But the concordance was not constant in boys.
3.2. Risk Factors
In the risk factor research, we studied stressful life events, family factors, and gestational factors. The mean SSLEPSS score of the first follow-up (first time) and the seventh follow-up (last time) were 25.47 ± 18.57 and 19.89 ± 18.96, respectively, which decreased gradually through follow-ups.
In the environmental factors research, OH-PAH levels during early puberty were higher than those reported in other countries. Participates who were female, older, attended school near an industrial site, frequently consumed smoked food, and had lower family income were found to have a higher OH-PAH concentration. Further analysis showed that these four OH-PAHs were associated with delayed timing of menarche, thelarche, pubic hair, and axillary hair development in girls. In later research, we detected high neonicotinoids exposure with a detection rate of 72–100% and found negative association between neonicotinoids and genitalia development. Thiacloprid thus might impact genital stages in boys and axillary hair development in girls.
3.3. Health Outcomes
Based on our interest in health outcomes, we analyzed mental health. We detected depressive symptoms with a rate of 15.63%, anxiety symptoms with a rate of 14.03% ,and average score of emotional and behavioral problems of 37.9 ± 24.7 in early- to mid-puberty. Through further association analysis, boys that had first spermatorrhea and girls that had menarche or advanced pubic hair development were more likely to have depressive symptoms.
4. Discussion
China started the pubertal development study relatively late compared to Europe and North America. Studies such as the German Dortmund Nutritional and Anthropometric Longitudinally Designed Study (DONALD) [48], Avon Longitudinal Study of Parents and Children (ALSPAC) [49], and Health Assessment of Mothers and Children of Salinas (CHAMACOS) [50] were birth cohorts that started in the 1990s that followed participants through puberty and reported findings on pubertal timing. A Hong Kong Chinese birth cohort “Children of 1997” was the first Chinese birth cohort that reported pubertal timing and its early life impact factors [51]. However, these birth cohorts were not designed to study puberty, which might introduce bias, and the results can be very different depending on race. As far as we know, there is one growth and development cohort study starting in March 2016 in Anhui, China, studying early life adversity, psychological factors, and puberty and its potential polygenic risk [52,53]. The Xiamen Pubertal Growth Cohort was another Chinese puberty cohort set up in 2017 to analyze pubertal onset and cardiovascular disease risk in later life. They measured phthalates and sex hormone through follow-ups [54]. Along with these cohorts, we could provide more evidence of Chinese pubertal timing and study various influence factors for future genes and environment interactions and phenotype attribution.
In terms of Chinese cohort studies, our boys’ pubertal onset age was similar to that in Hong Kong [55], Xiamen [56], and Sichuan [57]. Girls’ thelarche age ranges from 9 to 10, and Sichuan [57] reported the earliest age at 9.1, while we had the latest age at 10.2. Compared with a Chongqing cross-sectional study, children in our cohort had earlier pubertal timing [58]. Studies have shown that both early and delayed puberty can have adverse physiological and psychological effects on children [59]. Early puberty leads to accelerated physical growth, which, if untreated, initially results in tall stature during childhood but may ultimately cause short stature in adulthood due to premature closure of the growth plates, impairing final adult height [2]. The significant differences in pubertal development appearance may also impact psychological aspects, leading to long-term psychological issues and social adaptation difficulties. This includes negative emotions such as anxiety, depression, and low self-esteem, and may result in problematic behaviors such as smoking, alcohol abuse, and violence [60]. Furthermore, the hormonal changes associated with early or delayed puberty can affect health in adulthood, increasing the risk of metabolic syndrome and malignancies such as breast and prostate cancer [61].
Like studies in Denmark [62]; the USA [63]; and Hebei, China [64], we found that early maternal menarchal age and prepubertal BMI may induce early pubertal timing in boys and girls [1,65]. Interestingly, thiacloprid concentration was associated with delayed genitalia development in boys and early axillary hair development in girls [66]. As genitalia development is regulated by the hypothalamic–pituitary–gonadal (HPG) axis and axillary hair is regulated by the hypothalamic–pituitary-adrenal (HPA) axis, the different axes might be one reason for these distinct impacts [67,68]. Moreover, sex differences of neonicotinoids on pubertal timing may be linked to the different impacts of endocrine disruptors on the hypothalamic regions responsible for controlling puberty in males and females [69,70]. Interestingly, we found similar sex-specific inverse phenomenon on PAHs and pubertal timing [71,72]. As we detected high PAHS and neonicotinoids, there might be potential mixed effects between compounds, and the mixed effects will be our next main research target.
A leading strength of the CQPT cohort is that it is one of the few studies specifically designed for exploring pubertal timing by accurately recording a wide range of pubertal events through in-person follow-ups, and it assesses the impact of internal and external factors on puberty. We tried to include comprehensive pubertal events such as first spermatorrhea, which is not commonly used to reflect overall pubertal timing. The pubertal timing from in-person examinations and environmental factors from various sources were continuously monitored every 6 months, even during the COVID-19 pandemic, as well as gestational factors back to pregnancy. With detailed data collected over a span of 8 years under intense follow-ups, we were able to describe pubertal timing more comprehensively than previous ventures as pubertal events occur at several time points. The longitudinal data have the advantages of monitoring not only pubertal timing but also pubertal tempo and relating the different environmental exposures to particular pubertal development stages. With multiple environmental factors included in this single study, data supported us to study factor interactions. Additionally, to ensure data quality, pubertal development examinations and anthropometric measurement were all completed in person by trained same-gender medical graduates in all follow-ups.
Nonetheless, a major limitation of our cohort is that it was conducted in only four schools in a region of Chongqing, China, and did not include a large sample size. Also, some participants from grade 2 to grade 5 might have experienced menarche or first spermatorrhea for a while, which might have introduced recalling bias at baseline. Moreover, a few children felt uncomfortable during in-person examinations at follow-ups. If participants refused the physical examination, we respected their request, which caused additional drop-outs. As in-person examination might reduce the self-report bias, we traced the consistency between self-ratings and trained medical students in rating pubertal development stages longitudinally to provide more methodology evidence. Self-rating results indicated that our PDS had low Cronbach’s alpha. Self-rating by RCI was more reliable in girls, while the result was not constant in boys. Therefore, self-rated pubertal development will be further investigated.
During follow-ups, the drop-out rate increased dramatically after graduation from primary schools due to only a few schools having both primary and secondary education. We have proposed effective actions to lower the drop-out rate in this manuscript. However, this might still be a popular issue in any cohort with similar situations. Further studies are needed to investigate methodology to reduce drop-out rate. Moreover, each environmental factor from urine samples was only measured a few times, even though we collected urine samples every year. With the current findings from the CQPT cohort, monitoring repetitive internal exposures and understand its mechanism would be an important goal in our following work. Moreover, nutrition and diet play a crucial role in determining the timing and progression of puberty [73]. We only collected dietary information related to compounds rather than a nutrition perspective, which is another limitation. Another key factor potentially influencing pubertal onset is vitamin D, which is regarded as a regulator of neuroendocrine and ovarian physiology, and we did not collect its measurements [74].
Similar to overall pubertal timing research, we had more findings among girls up to now. Our cohort experiences suggested that boys’ pubertal stage classification is vaguer than girls, and hence this is a limitation of this study. As Tanner described in the pubertal changes in boys, the figures for PH2 should be the first appearance of pubic hair, which might not be seen on Tanner stages photographs. Therefore, estimated age at PH2 might be later than the true PH2 age [75], which could introduce ambiguity. Furthermore, the first spermatorrhea reported by boys might introduce recalling bias. Moreover, we observed earlier Tanner stage G2 development than testicular volume ≥ 4 mL, contrary to the majority of studies [21,75]. The reason might be that Tanner stage G2 is relatively subjective in terms of observing scrotum and testes enlargement and scrotal skin, rather than being an objective measurement. Even though we tried to control the quality to have one trained same-gender medical school graduate student to complete the ratings at each follow-up, there were still discrepancies between different inspectors throughout multiple years. Since both G2 and testicular enlargement mark gonadarche [76], our research will be using testicular volume ≥ 4 mL as the main outcome of gonadarche.
5. Conclusions
This study is one of few pubertal timing studies in China providing evidence of environmental factors’ impact on pubertal timing. We will continue to follow pubertal development; physical growth; and health outcomes, especially mental health and women’s health, to reveal the pubertal development trajectory and its long-term health implications.
Author Contributions
Conceptualization, Q.L.; methodology, Q.L., H.W. and D.W.; formal analysis, D.W., S.L., X.X. and J.Z.; investigation, Q.L., J.L., Q.Z., D.W., S.L., X.X., W.W., Y.Z., Y.T., Y.W. and Z.H.; writing—original draft preparation, D.W.; writing—review and editing, Q.L., D.W., J.L. and Q.Z; project administration, Q.L.; funding acquisition, Q.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [grant number: 81973067]; National Natural Science Foundation of China Youth Project [grant number:81502825]; Venture & Innovation Support Program for Chongqing Overseas Returnees, China [grant number cx2018105]; Basic and Frontier Research Project of Chongqing Science and Technology Commission [grant number: cstc2013jcyjA10001]; CQMU Program for Youth Innovation in Future Medicine [grant number: W0054].
Institutional Review Board Statement
This study involves human participants and was performed in line with the principles of the Declaration of Helsinki. Approval (protocol code: 2023003) was granted by the Ethics Committee of Chongqing Medical University in March 2014, and we made amendments in March 2019 and February 2023 according to the latest progress of the cohort.
Informed Consent Statement
Written consent forms were obtained from participants and their guardians for their participation at baseline and the year when they graduated from primary schools. The follow-up process of the study is entirely based on voluntary participation. Oral consent from participants was received under two investigators’ witness at each follow-up, and participants were documented as drop-outs if they did not consent at the follow-up.
Data Availability Statement
Currently, our data are not open access. The data are stored and used among Chongqing Medical University research teams, and we are working on data sharing policies and a website for collaborators’ data access. We look forward to future collaborations in exploring adolescents’ pubertal development. Any data access request or questions should be sent to the principal investigator Professor Qin Liu (liuqin@cqmu.edu.cn).
Acknowledgments
We thank four primary schools, eight middle schools, and their teachers for their support in our recruitment and follow-ups. We also thank all the participants and their guardians in the CQPT study since 2014.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
Spermatorrhea question asked during examination:
“Have you had spermatorrhea so far? Do you remember the first time you experienced it?” We would do further explanation if participants were unable to understand the answer as “spermatorrhea refers to a wet dream, where sticky fluid leaked out from your body, and you probably found it on your underwear after waking up”.
Appendix B

Table A1.
The list of cohort publications.
Table A1.
The list of cohort publications.
| Publication Title | DOI | Key Findings | Language |
|---|---|---|---|
| Risk Factors of Pubertal Development | |||
| Levels and risk factors for urinary metabolites of polycyclic aromatic hydrocarbons in children living in Chongqing, China [25] | 10.1016/j.scitotenv.2017.04.103 | (1) Urinary concentrations of OH-PAHs were elevated in Chongqing compared to children in other countries. (2) Being female, older age, having a school location near an industrial site, consuming smoked foods, and low family income were associated with higher OH-PAH concentrations. | English |
| Association of prepubertal obesity with pubertal development in Chinese girls and boys: A longitudinal study [65] | 10.1002/ajhb.23195 | Higher prepubertal BMI was associated with earlier puberty in both Chinese boys and girls. | English |
| Maternal Age at Menarche and Pubertal Timing in Boys and Girls: A Cohort Study From Chongqing, China [1] | 10.1016/j.jadohealth.2020.06.036 | Earlier maternal age at menarche was related to earlier pubertal timing in Chongqing. | English |
| Measuring urinary concentrations of neonicotinoid insecticides by modified solid-phase extraction-ultrahigh performance liquid chromatography-tandem mass spectrometry: Application to human exposure and risk assessment [26] | 10.1016/j.chemosphere.2021.129714 | (1) A robust method for quantification of urinary neonicotinoids was developed. (2) Girls had significantly higher urinary concentrations of clothianidin than boys. | English |
| Polycyclic aromatic hydrocarbons are associated with later puberty in girls: A longitudinal study [72] | 10.1016/j.scitotenv.2022.157497 | (1) Girls with higher concentrations of 1-OHPyr and 2-OHFlu were at risk of delayed pubic hair. (2) Girls with higher concentrations of 2-OHNap were at risk of delayed breast and pubic hair. (3) Girls with higher concentrations of 9-OHPhe were at risk of delayed breast, pubic hair and axillary hair development. | English |
| Urinary neonicotinoid concentrations and pubertal development in Chinese adolescents: A cross-sectional study [66] | 10.1016/j.envint.2022.107186 | (1) Higher thiacloprid concentration was associated with delayed genitalia development in boys and early axillary hair development in girls. (2) Neonicotinoid mixture was negatively associated with genitalia stage in the joint effect. | English |
| The influence of the trajectory of obesity indicators on the age of pubertal onset and pubertal tempo in girls: A longitudinal study in Chongqing, China [77] | 10.3389/fpubh.2023.1025778 | (1) Overweight and obesity (BMI scale) before pubertal onset can influence pubertal onset age and B2-B5 pubertal tempo. (2) Overweight (BMI scale) and high WC before menarche have an impact on the age of menarche. (3) Overweight (WHtR scale) before menarche is associated with B2-B5 pubertal tempo. | English |
| Relevant factors of early puberty timing in urban primary schools in Chongqing [58] | 10.19813/j.cnki.weishengyanjiu.2016.03.013. | Gender, parents’ relationship, and hair product use have an essential impact on early puberty timing. | Chinese |
| Determination of polycyclic aromatic hydrocarbons in girls and association between polycyclic aromatic hydrocarbons exposure and puberty timing [78] | 10.19813/j.cnki.weishengyanjiu.2017.05.011 | (1) We developed a method for simultaneous determination of four metabolites of polycyclic of aromatic hydrocarbons. (2) Early exposure to PAHs might be one of the factors causing early puberty timing in girls. | Chinese |
| Relationship between puberty growth and sexual development of boys [79] | 10.16835/j.cnki.1000-9817.2020.06.006 | (1) The height growth of boys reached its peak one year before the first ejaculation and began to decrease after first ejaculation. (2) The first ejaculation age of boys was negatively correlated with the increment of height in the following year. (3) The testicular development of boys was positively correlated with height, weight, and BMI. | Chinese |
| Prospective cohort study on the association between family factors and the puberty timing in children [80] | 10.16835/j.cnki.1000-9817.2020.06.004. | Left behind children, self-perceived parental relationship, and family economic conditions were the influencing factors of pubic hair of girls. | Chinese |
| Health Outcomes | |||
| Co-Development and Bidirectional Associations Between Psychological Stress and Obesity in School-Aged Children: A Longitudinal Study in China [81] | 10.1097/PSY.0000000000001212 | Different aspects of psychological stress were differentially associated with obesity. There may be a clear reciprocal relationship between peer interaction psychological stress and obesity. | English |
| Urinary neonicotinoid concentrations and obesity: A cross-sectional study among Chinese adolescents [82] | 10.1016/j.envpol.2024.123516 | (1) Nitenpyram, neonicotinoid mixtures, and clothianidin were positively associated with obesity. (2) Imidacloprid and thiacloprid were inversely associated with general obesity. (3) Acetamiprid and its metabolite were differently associated with obesity measures. (4) Sex differences were seen with nitenpyram and clothianidin and obesity. | English |
| Predictive effect of psychological stress in early puberty on subsequent anxiety and depression [83] | 10.16835/j.cnki.1000-9817.2020.06.008 | The psychological stress level and age during early puberty had a positive predictive effect on anxiety and depression after 4 years. | Chinese |
| Associations between psychosocial stress in early and middle adolescence with emotional and behavioral problems one year later [84] | 10.16835/j.cnki.1000-9817.2022.05.002 | Psychological stress levels in early and middle puberty have a positive predictive effect on emotional and behavioral problems in the following year. | Chinese |
| Depressive symptoms and associated factors among adolescents in different pubertal stages in a district of Chongqing [85] | 10.16835/j.cnki.1000-9817.2022.05.018 | (1) The detection rate of depressive symptoms among adolescents is relatively low. (2) Boys who have had the first spermatorrhea and girls with advanced pubic hair development or who have had menarche are more likely to suffer from depressive symptoms. | Chinese |
| Predictive effects of psychological stress in early and middle puberty on adolescent health-risk behaviors [86] | 10.16835/j.cnki.1000-9817.2022.05.004 | (1) Unreasonable physical activity and food preferences are the most common health-risk behaviors among adolescents. (2) Psychological stress during early to middle puberty is predictive of adolescent health-risk behaviors. | Chinese |
| Effects of screen time and internet addiction on social anxiety among adolescents in a district of Chongqing [87] | 10.12173/j.issn.1004-5511.202304013 | Daily screen time might increase adolescents’ social anxiety, and more attention should be paid to teenage girls. | Chinese |
| Effects of passive smoking exposure on adolescent emotional behaviors in household environment [88] | 10.16168/j.cnki.issn.1002-9982.2023.07.008 | (1) About 50% of adolescents had been exposed to second-hand smoke in their family environment. (2) Second-hand smoke exposure in the family environment is an important influencing factor of internalizing behaviors and overall emotional behavior. | Chinese |
| Tool Development | |||
| Development of the Stressful Life Events for Primary School Students [28] | NA | The Scale of Stressful Life Events for Primary School Students has good validity and reliability, and it could be used as an assessment tool for psychological stress level in primary school students. | Chinese |

Table A2.
The list of survey tools developed in China.
Table A2.
The list of survey tools developed in China.
| Tool Name | Description | KMO in This Study | Cronbach α in This Study | Reference No. |
|---|---|---|---|---|
| Scale of Stressful Life Events for Primary School Students | The scale includes 45 items, with the score of stressful life events ranging from 1 to 5 points. | 0.85 | 0.88 | [17] |
| Adolescent Psychosexual Health Questionnaire | The questionnaire includes 46 items with three sub-scales of sexual knowledge, sexual values, and sexual adaptation. | 0.92 | 0.89 | [18] |
| Adolescent Students’ Life Satisfaction Scale | This scale includes 6 dimensions of friendship, family, study, freedom, school, and environment, with a total of 36 items. | 0.95 | 0.95 | [27] |
| Social Support Scale | The scale includes 17 items based on a social support theory (subjective support, objective support, and support utilization). | 0.94 | 0.96 | [31] |
| Multidimensional Sub-health Questionnaire of Adolescents | The questionnaire includes 71 items with physical sub-health with a total of 32 items and psychological sub-health areas with a total of 39 items. | 0.78–0.97 * | 0.93–0.98 * | [34] |
| Adolescents Self-Harm Scale | The scale includes 18 items and 1 open-ended question with each item divided into 4 self-harm grades and 5 physical injury grades. | 0.93 | 0.99 | [35] |
| Mental Health Inventory of Middle-School Students | The scale has a total of 60 items, composed of 10 sub-scales, evaluating 10 common psychological problems of middle school students. | 0.97 | 0.98 | [36] |
* The value ranges of the questionnaire and its various dimensions.
References
- Yang, B.; Ostbye, T.; Huang, X.; Li, Y.; Fang, B.; Wang, H.; Liu, Q. Maternal Age at Menarche and Pubertal Timing in Boys and Girls: A Cohort Study from Chongqing, China. J. Adolesc. Health 2021, 68, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Bradley, S.H.; Lawrence, N.; Steele, C.; Mohamed, Z. Precocious puberty. BMJ 2020, 368, l6597. [Google Scholar] [CrossRef] [PubMed]
- German, A.; Shmoish, M.; Belsky, J.; Hochberg, Z. Outcomes of pubertal development in girls as a function of pubertal onset age. Eur. J. Endocrinol. 2018, 179, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, M.; Bokor, B.R. Tanner Stages. In StatPearls; StatPearls Publishing, StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Chen, L.; Su, B.; Zhang, Y.; Ma, T.; Liu, J.; Yang, Z.; Li, Y.; Gao, D.; Chen, M.; Ma, Y.; et al. Association between height growth patterns in puberty and stature in late adolescence: A longitudinal analysis in chinese children and adolescents from 2006 to 2016. Front. Endocrinol. 2022, 13, 882840. [Google Scholar] [CrossRef] [PubMed]
- Eckert-Lind, C.; Busch, A.S.; Petersen, J.H.; Biro, F.M.; Butler, G.; Bräuner, E.V.; Juul, A. Worldwide Secular Trends in Age at Pubertal Onset Assessed by Breast Development Among Girls: A Systematic Review and Meta-analysis. JAMA Pediatr. 2020, 174, e195881. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Shi, D.; Dang, J.J.; Zhong, P.L.; Liu, Y.F.; Cai, S.; Dong, Y.H.; Hu, P.J.; Ma, J.; Song, Y. Secular trends and urban-rural disparities in the median age at menarche among Chinese han girls from 1985 to 2019. World J. Pediatr. 2023, 19, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, C.; Bygdell, M.; Celind, J.; Sondén, A.; Tidblad, A.; Sävendahl, L.; Kindblom, J.M. Secular Trends in Pubertal Growth Acceleration in Swedish Boys Born From 1947 to 1996. JAMA Pediatr. 2019, 173, 860–865. [Google Scholar] [CrossRef] [PubMed]
- de Muinich Keizer, S.M.; Mul, D. Trends in pubertal development in Europe. Hum. Reprod. Update 2001, 7, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Predieri, B.; Iughetti, L.; Bernasconi, S.; Street, M.E. Endocrine Disrupting Chemicals’ Effects in Children: What We Know and What We Need to Learn? Int. J. Mol. Sci. 2022, 23, 11899. [Google Scholar] [CrossRef]
- De Craemer, S.; Croes, K.; van Larebeke, N.; De Henauw, S.; Schoeters, G.; Govarts, E.; Loots, I.; Nawrot, T.; Nelen, V.; Den Hond, E.; et al. Metals, hormones and sexual maturation in Flemish adolescents in three cross-sectional studies (2002–2015). Environ. Int. 2017, 102, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lam, S.P.; Kong, A.P.; Ma, R.C.; Li, S.X.; Chan, J.W.; Yu, M.W.; Zhou, J.; Chan, M.H.; Ho, C.S.; et al. Family conflict and lower morning cortisol in adolescents and adults: Modulation of puberty. Sci. Rep. 2016, 6, 22531. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.T.; DiLalla, L.F.; Corley, R.P.; Dorn, L.D.; Berenbaum, S.A. Family environmental antecedents of pubertal timing in girls and boys: A review and open questions. Horm. Behav. 2022, 138, 105101. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.Q.; Wang, D.Y.; He, M.; Zhang, C. Monitoring of atmospheric heavy metal deposition in Chongqing, China—Based on moss bag technique. Environ. Monit. Assess. 2009, 148, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Song, S.; Bai, X.; He, Y.; Zhang, B.; Gui, M.; Kannan, K.; Lu, S.; Huang, Y.; Sun, H. A nationwide survey of urinary concentrations of neonicotinoid insecticides in China. Environ. Int. 2019, 132, 105114. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.-Y.; Luo, Y.-M.; Zhang, G.-Y.; Teng, Y.; Li, Z.-G.; Wu, L.-H. Contamination of PAHs in Sludge Samples from the Yangtze River Delta Area1 1Project supported by the National Basic Research Program (973 Program) of China (No. 2002CB410810). Pedosphere 2007, 17, 373–382. [Google Scholar] [CrossRef]
- Sievers, C.K.; Shanle, E.K.; Bradfield, C.A.; Xu, W. Differential action of monohydroxylated polycyclic aromatic hydrocarbons with estrogen receptors α and β. Toxicol. Sci. 2013, 132, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Wenger, D.; Gerecke, A.C.; Heeb, N.V.; Schmid, P.; Hueglin, C.; Naegeli, H.; Zenobi, R. In vitro estrogenicity of ambient particulate matter: Contribution of hydroxylated polycyclic aromatic hydrocarbons. J. Appl. Toxicol. 2009, 29, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Mikolić, A.; Karačonji, I.B. Imidacloprid as reproductive toxicant and endocrine disruptor: Investigations in laboratory animals. Arch. Ind. Hyg. Toxicol. 2018, 69, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Caron-Beaudoin, E.; Viau, R.; Hudon-Thibeault, A.A.; Vaillancourt, C.; Sanderson, J.T. The use of a unique co-culture model of fetoplacental steroidogenesis as a screening tool for endocrine disruptors: The effects of neonicotinoids on aromatase activity and hormone production. Toxicol. Appl. Pharmacol. 2017, 332, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.P.; Kaiser, U.B. Pubertal development and regulation. Lancet Diabetes Endocrinol. 2016, 4, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Millman, A.; Tang, D.; Perera, F.P. Air pollution threatens the health of children in China. Pediatrics 2008, 122, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Commission, N.H. Screening for Overweight and Obesity among School-Age Children and Adolescents; National Health Commission: Beijing, China, 2018. [Google Scholar]
- Tanner, J.M. Normal growth and techniques of growth assessment. Clin. Endocrinol. Metab. 1986, 15, 411–451. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, Q.; Ostbye, T.; Story, M.; Deng, X.; Chen, Y.; Li, W.; Wang, H.; Qiu, J.; Zhang, J. Levels and risk factors for urinary metabolites of polycyclic aromatic hydrocarbons in children living in Chongqing, China. Sci. Total Environ. 2017, 598, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Yue, M.; Liu, Q.; Wang, F.; Liu, L.; Wang, L.; Liu, X.; Zheng, M.; Xiao, H.; Bai, Q.; et al. Measuring urinary concentrations of neonicotinoid insecticides by modified solid-phase extraction-ultrahigh performance liquid chromatography-tandem mass spectrometry: Application to human exposure and risk assessment. Chemosphere 2021, 273, 129714. [Google Scholar] [CrossRef] [PubMed]
- Chan, N.P.; Sung, R.Y.; Nelson, E.A.; So, H.K.; Tse, Y.K.; Kong, A.P. Measurement of pubertal status with a Chinese self-report Pubertal Development Scale. Matern. Child Health J. 2010, 14, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Shu-Dan, L.; Qin, L.; Yan, L.; Yi, W. Development of the Stressful Life Events for Primary School Students. Chin. Ment. Health J. 2016, 30, 745–751. [Google Scholar]
- Yi, L. A Preliminary Design of the Adolescent Psychosexual Health Questionnaire. Master’s Thesis, Southwest China Normal University, Chongqing, China, 2005. [Google Scholar]
- Ji, C.; Xing, Y.; Zhang, L.; Chen, T.; Song, Y.; Hu, P. Chinese Youth Risk Behavior Survey in 2005, 1st ed.; Ji, C., Ed.; Peking University Medical Press: Beijing, China, 2007. [Google Scholar]
- Achenbach, T.M.; McConaughy, S.H.; Ivanova, M.Y.; Rescorla, L.A. Manual for the ASEBA School-Age Forms and Profiles; University of Vermont, Research Center for Children, Youth, and Families: Burlington, VT, USA, 2001. [Google Scholar]
- Fristad, M.A.; Emery, B.L.; Beck, S.J. Use and abuse of the Children’s Depression Inventory. J. Consult. Clin. Psychol. 1997, 65, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Birmaher, B.; Khetarpal, S.; Brent, D.; Cully, M.; Balach, L.; Kaufman, J.; Neer, S.M. The Screen for Child Anxiety Related Emotional Disorders (SCARED): Scale construction and psychometric characteristics. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Burnkrant, R.E.; Page, T.J., Jr. A modification of the Fenigstein, Scheier, and Buss Self-Consciousness Scales. J. Pers. Assess. 1984, 48, 629–637. [Google Scholar] [CrossRef]
- Armsden, G.C.; Greenberg, M.T. The inventory of parent and peer attachment: Individual differences and their relationship to psychological well-being in adolescence. J. Youth Adolesc. 1987, 16, 427–454. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.; Ou, W.; Zhen-qing, Z.; Fang, G. Validation Study of Young’s Chinese Version of Internet Addiction Scale. Inj. Med. Electron. Ed. 2019, 8, 17–23. [Google Scholar]
- Campbell, A. Subjective measures of well-being. Am. Psychol. 1976, 31, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Xinggui, Z.; Liguo, H.; Xue, Z. Adolescent Students’ Life Satisfaction: Its Construct and Scale Development. Psychol. Sci. 2004, 27, 1257–1260. [Google Scholar] [CrossRef]
- Asher, S.R.; Wheeler, V.A. Children’s loneliness: A comparison of rejected and neglected peer status. J. Consult. Clin. Psychol. 1985, 53, 500. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Yu, X. The Self-Esteem Scale. Chin. Ment. Health J. 1993, 318–320. [Google Scholar]
- Maxwell, J.P. Psychometric properties of a Chinese version of the Buss–Warren Aggression Questionnaire. Personal. Individ. Differ. 2008, 44, 943–953. [Google Scholar] [CrossRef]
- Ye, Y.; Dai, X. Development of Social Support Scale for University Students. Chin. J. Clin. Psychol. 2008, 05, 456–458. [Google Scholar]
- Mansfield, A.K.; Keitner, G.I.; Dealy, J. The family assessment device: An update. Fam. Process 2015, 54, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Hui-fang, H.; Chen, T.; Lei, L. Revisioning Prosocial Tendencies Measure for Adolescent. Psychol. Dev. Educ. 2007, 23, 112–117. [Google Scholar]
- Xing, C. Development and Psychometric Analysis of the Multidimensional Sub-Health Questionnaire of Adolescents. Master’s Thesis, Anhui Medical University, Anhui, China, 2009. [Google Scholar]
- Yu, F. The Relation of Adolescents’ Self-Harm Behaviors, Individual Emotion Characteristics and Family Environmental Factors. Master’s Thesis, Central China Norm University, Wuhan, China, 2008. [Google Scholar]
- Wang, J.; Li, Y.; He, E. Development and standardization of Mental Health Inventory of Middle-school students in China. Sci. Soc. Psychol. 1997, 4, 15–20. [Google Scholar]
- Kroke, A.; Manz, F.; Kersting, M.; Remer, T.; Sichert-Hellert, W.; Alexy, U.; Lentze, M.J. The DONALD Study. History, current status and future perspectives. Eur. J. Nutr. 2004, 43, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.; Golding, J.; Macleod, J.; Lawlor, D.A.; Fraser, A.; Henderson, J.; Molloy, L.; Ness, A.; Ring, S.; Davey Smith, G. Cohort Profile: The ‘children of the 90s’--the index offspring of the Avon Longitudinal Study of Parents and Children. Int. J. Epidemiol. 2013, 42, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Berger, K.; Eskenazi, B.; Balmes, J.; Holland, N.; Calafat, A.M.; Harley, K.G. Associations between prenatal maternal urinary concentrations of personal care product chemical biomarkers and childhood respiratory and allergic outcomes in the CHAMACOS study. Environ. Int. 2018, 121, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Schooling, C.M.; Hui, L.L.; Ho, L.M.; Lam, T.H.; Leung, G.M. Cohort profile: ‘children of 1997’: A Hong Kong Chinese birth cohort. Int. J. Epidemiol. 2012, 41, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Fang, J.; Wan, Y.; Su, P.; Tao, F. Role of polygenic risk in susceptibility to accelerated pubertal onset following chronic stress exposure. Eur. J. Endocrinol. 2019, 181, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Fang, J.; Wan, Y.; Su, P.; Tao, F. Association of Early-Life Adversity with Measures of Accelerated Biological Aging Among Children in China. JAMA Netw. Open 2020, 3, e2013588. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Gao, D.; Yang, Z.; Dong, B.; Dong, Y.; Zou, Z.; Ma, J. Cohort Profile: The Xiamen Pubertal Growth Cohort Longitudinal Study. Future 2023, 1, 3. [Google Scholar] [CrossRef]
- Kwok, M.K.; Leung, G.M.; Schooling, C.M. Pubertal testis volume, age at pubertal onset, and adolescent blood pressure: Evidence from Hong Kong’s “Children of 1997” birth cohort. Am. J. Hum. Biol. 2017, 29, e22993. [Google Scholar] [CrossRef]
- Li, Y.; Ma, T.; Ma, Y.; Gao, D.; Chen, L.; Chen, M.; Liu, J.; Dong, B.; Dong, Y.; Ma, J. Adiposity Status, Trajectories, and Earlier Puberty Onset: Results from a Longitudinal Cohort Study. J. Clin. Endocrinol. Metab. 2022, 107, 2462–2472. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Xu, Y.; Liu, X.; Wang, X.; Shan, S.; Crabbe, M.J.C.; Zhao, L.; Fang, H.; Cheng, G. Prospective association of dietary soy and fibre intake with puberty timing: A cohort study among Chinese children. BMC Med. 2022, 20, 145. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Qin, L.; Yi, W.; Shudan, L.; Xun, L.; Hong, W. Relevant factors of early puberty timing in urban primary schools in Chongqing. J. Hyg. Res. 2016, 45, 430–435. [Google Scholar] [CrossRef]
- Gohil, A.; Eugster, E.A. Delayed and Precocious Puberty: Genetic Underpinnings and Treatments. Endocrinol. Metab. Clin. N. Am. 2020, 49, 741–757. [Google Scholar] [CrossRef] [PubMed]
- Mendle, J.; Beam, C.R.; McKone, K.M.P.; Koch, M.K. Puberty and Transdiagnostic Risks for Mental Health. J. Res. Adolesc. 2020, 30, 687–705. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Crowne, E. The impact of childhood cancer and its treatment on puberty and subsequent hypothalamic pituitary and gonadal function, in both boys and girls. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101291. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, S.; Brix, N.; Ernst, A.; Lauridsen, L.L.B.; Ramlau-Hansen, C.H. Maternal age at menarche and pubertal development in sons and daughters: A Nationwide Cohort Study. Hum. Reprod. 2018, 33, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Aris, I.M.; Perng, W.; Dabelea, D.; Ganiban, J.M.; Liu, C.; Marceau, K.; Robertson, O.C.; Hockett, C.W.; Mihalopoulos, N.L.; Kong, X.; et al. Analysis of Early-Life Growth and Age at Pubertal Onset in US Children. JAMA Netw. Open 2022, 5, e2146873. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Guo, J.; Zhang, X.; Lu, Y.; Miao, J.; Xue, H. Obesity is a risk factor for central precocious puberty: A case-control study. BMC Pediatr. 2021, 21, 509. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Q.; Deng, X.; Chen, Y.; Yang, B.; Huang, X.; Østbye, T. Association of prepubertal obesity with pubertal development in Chinese girls and boys: A longitudinal study. Am. J. Hum. Biol. 2018, 30, e23195. [Google Scholar] [CrossRef] [PubMed]
- Yue, M.; Liu, Q.; Wang, F.; Zhou, W.; Liu, L.; Wang, L.; Zou, Y.; Zhang, L.; Zheng, M.; Zeng, S.; et al. Urinary neonicotinoid concentrations and pubertal development in Chinese adolescents: A cross-sectional study. Environ. Int. 2022, 163, 107186. [Google Scholar] [CrossRef] [PubMed]
- Biro, F.M.; Huang, B.; Daniels, S.R.; Lucky, A.W. Pubarche as well as thelarche may be a marker for the onset of puberty. J. Pediatr. Adolesc. Gynecol. 2008, 21, 323–328. [Google Scholar] [CrossRef]
- Schubert, C.M.; Chumlea, W.C.; Kulin, H.E.; Lee, P.A.; Himes, J.H.; Sun, S.S. Concordant and discordant sexual maturation among U.S. children in relation to body weight and BMI. J. Adolesc. Health 2005, 37, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.C.; Shryne, J.E.; Gorski, R.A. Structural sexual dimorphisms in the anteroventral periventricular nucleus of the rat hypothalamus are sensitive to gonadal steroids perinatally, but develop peripubertally. Neuroendocrinology 1996, 63, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Orikasa, C.; Kondo, Y.; Hayashi, S.; McEwen, B.S.; Sakuma, Y. Sexually dimorphic expression of estrogen receptor beta in the anteroventral periventricular nucleus of the rat preoptic area: Implication in luteinizing hormone surge. Proc. Natl. Acad. Sci. USA 2002, 99, 3306–3311. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, W.; Bravo, M.A.; Liu, S.; Xi, X.; Zhou, Y.; Zhang, Q.; Liu, Q. Prepubertal exposure to polycyclic aromatic hydrocarbons are associated with early pubertal development onset in boys: A longitudinal study. J. Hazard Mater. 2024, 470, 134160. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Bravo, M.A.; Wang, H.; Sheng, L.; Wu, W.; Zhou, Y.; Xi, X.; Østbye, T.; Liu, Q. Polycyclic aromatic hydrocarbons are associated with later puberty in girls: A longitudinal study. Sci. Total Environ. 2022, 846, 157497. [Google Scholar] [CrossRef] [PubMed]
- Norris, S.A.; Frongillo, E.A.; Black, M.M.; Dong, Y.; Fall, C.; Lampl, M.; Liese, A.D.; Naguib, M.; Prentice, A.; Rochat, T.; et al. Nutrition in adolescent growth and development. Lancet 2022, 399, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Magenes, V.C.; Tagi, V.M.; Grazi, R.; Bianchi, A.; Cena, H.; Zuccotti, G.; Fabiano, V. Association between Vitamin D Levels, Puberty Timing, and Age at Menarche. Children 2023, 10, 1243. [Google Scholar] [CrossRef] [PubMed]
- Marshall, W.A.; Tanner, J.M. Variations in the pattern of pubertal changes in boys. Arch. Dis. Child. 1970, 45, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Wohlfahrt-Veje, C.; Tinggaard, J.; Juul, A.; Toppari, J.; Skakkebæk, N.E.; Main, K.M. Pubarche and Gonadarche Onset and Progression Are Differently Associated with Birth Weight and Infancy Growth Patterns. J. Endocr. Soc. 2021, 5, bvab108. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.; Wu, D.; Wu, W.; Zhou, Y.; Zhang, Q.; Wang, Y.; Wang, H.; Liu, Q. The influence of the trajectory of obesity indicators on the age of pubertal onset and pubertal tempo in girls: A longitudinal study in Chongqing, China. Front. Public Health 2023, 11, 1025778. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Liu, Q.; Liu, S.; Li, W.; Chen, Y.; Wen, Y.; Luo, Y. Determination of polycyclic aromatic hydrocarbons in girls and association between polycyclic aromatic hydrocarbon exposure and puberty timing. J. Hyg. Res. 2017, 46, 743–748. [Google Scholar] [CrossRef]
- Fang, B.; Liu, Q.; Yang, B.; Huang, X.; Li, Y.; Sheng, L. Relationship between puberty growth and sexual development of boys. Chin. J. Sch. Health 2020, 41, 821–823+829. [Google Scholar] [CrossRef]
- Sheng, L.; Liu, Q.; Huang, X.; Yang, B.; Li, Y.; Fang, B. Prospective cohort study on the association between family factors and the puberty timing in children. Chin. J. Sch. Health 2020, 41, 811–814+820. [Google Scholar] [CrossRef]
- Tang, L.; Yin, R.; Xi, X.; Hu, Q.; Zhang, F.; Liu, Q. Co-Development and Bidirectional Associations Between Psychological Stress and Obesity in School-Aged Children: A Longitudinal Study in China. Psychosom. Med. 2023, 85, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Qin, L.; Yin, L.; Min, Y.; Qian, S.; Jinzhu, L.; Yang, L.; Shaohua, Z.; Jieying, G. Urinary neonicotinoid concentrations and obesity: A cross-sectional study among Chinese adolescents. Environ. Pollut. 2024, 345, 123516. [Google Scholar] [CrossRef]
- Yueyue, L.; Qin, L.; Xin, H.; Bo, Y.; Bo, F.; Lulu, S. Predictive effect of psychological stress in early puberty on subsequent anxiety and depression. J. Sch. Health 2020, 41, 830–832+836. [Google Scholar] [CrossRef]
- Wenyi, W.; Qin, L.; Yueyue, L.; Bo, F.; Lulu, S. Associations between psychosocial stress in early and middle adolescence with emotional and behavioral problems one year later. J. Sch. Health 2022, 43, 644–647. [Google Scholar] [CrossRef]
- Xuan, X.; Qin, L.; Yueyue, L.; Bo, F.; Lulu, S.; Wenyi, W.; Yuanke, Z.; Qin, Z. Depressive symptoms and associated factors among adolescents in different pubertal stages in a district of Chongqing. J. Sch. Health 2022, 43, 718–721+726. [Google Scholar] [CrossRef]
- Qin, Z.; Qin, L.; Yueyue, L.; Bo, F.; Lulu, S.; Wenyi, W.; Yuuanke, Z.; Xuan, X. Predictive effects of psychological stress in early and middle puberty on adolescent health-risk behaviors. J. Sch. Health 2022, 43, 653–657+662. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Q.; Zhou, Y.K.; Wu, W.Y.; Xi, X.; Zhang, Q.; He, Z.W.; Luo, J.; Tian, Y. Effects of screen time and internet addiction on social anxiety among adolescents in a district of Chongqing. Yixue Xinzhi Zazhi. 2023, 33, 409–416. [Google Scholar] [CrossRef]
- Wenyi, W.; Yixin, C.; Yueyue, L.; Bo, F.; Lulu, S.; Yuanke, Z.; Xuan, X.; Qin, L. Effects of passive smoking exposure on adolescent emotional behaviors in household environment. Chin. J. Health Educ. 2023, 39, 618–622. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).