Assessment of a Natural Phytobiotic Mixture as Feed Additive for Broiler Chicken: Studies on Animal Performance, Gut Health, and Antioxidant Status After Experimental Infection with Eimeria spp.
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics and Procedures
2.2. Experimental Design
2.3. Dietary Treatments
2.4. Characterization of the Basal Diet and the Phytobiotic Mixture
2.5. Eimeria Challenge
2.6. Performance Parameters
2.7. Anticoccidial Efficacy Evaluation
2.8. Histomorphometrical and Immunohistochemical Analysis of the Intestine
2.9. Determination of Oxidative Status of the Intestine, Thigh, and Breast Meat
2.10. Statistical Analysis
3. Results
3.1. Performance
3.2. Anticoccidial Index
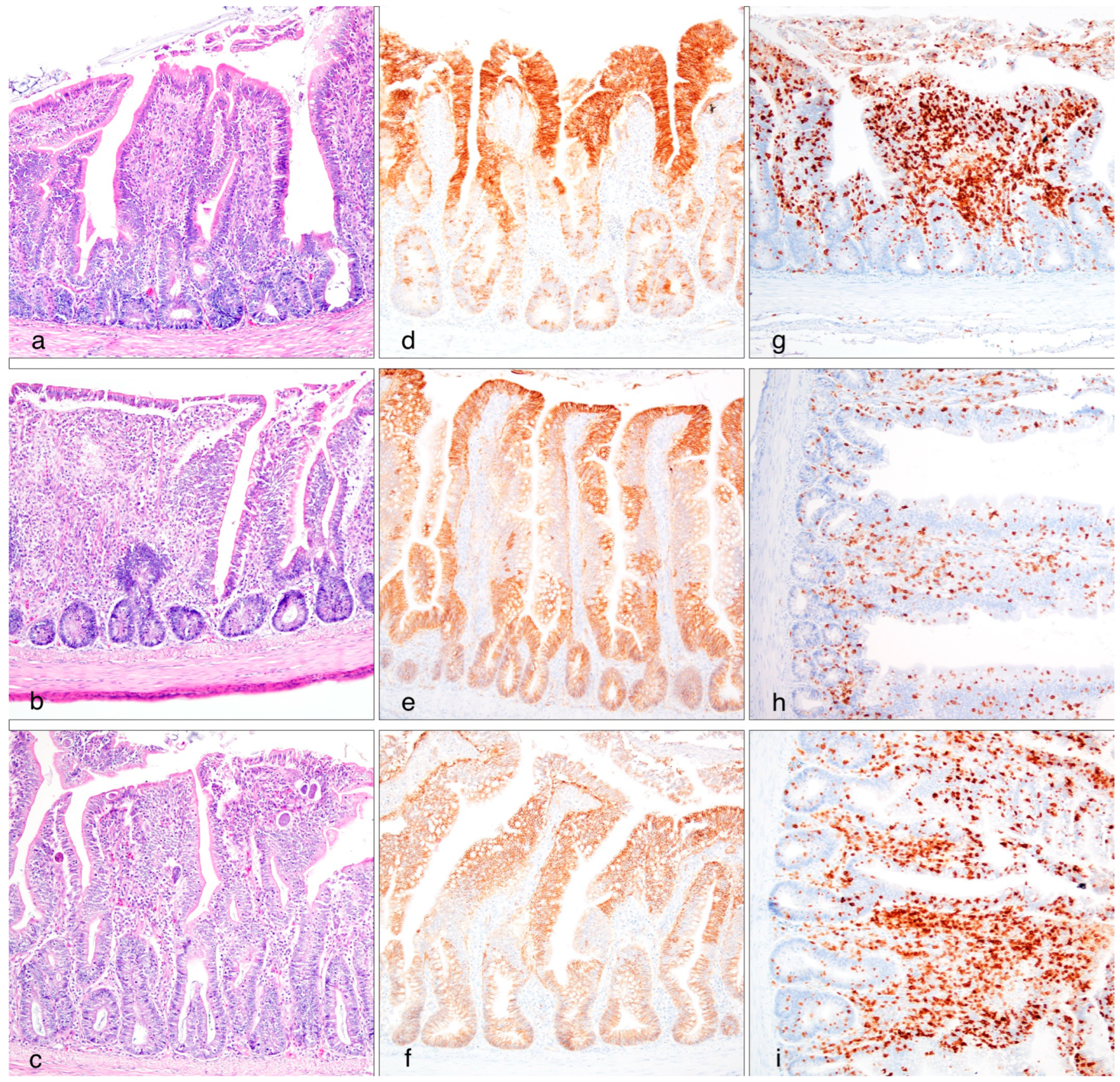
3.3. Histomorphometrical and Immunohistochemical Analysis
3.4. Antioxidant Status Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blake, D.P.; Knox, J.; Dehaeck, B.; Huntington, B.; Rathinam, T.; Ravipati, V.; Ayoade, S.; Gilbert, W.; Adebambo, A.O.; Jatau, I.D.; et al. Re-Calculating the Cost of Coccidiosis in Chickens. Vet. Res. 2020, 51, 115. [Google Scholar] [CrossRef] [PubMed]
- Aarthi, S.; Dhinakar Raj, G.; Raman, M.; Gomathinayagam, S.; Kumanan, K. Molecular Prevalence and Preponderance of Eimeria Spp. among Chickens in Tamil Nadu, India. Parasitol. Res. 2010, 107, 1013–1017. [Google Scholar] [CrossRef]
- Taylor, M.A.; Coop, R.L.; Wall, R.L. Veterinary Parasitology, 4th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2015; pp. 1–1006. [Google Scholar] [CrossRef]
- Snyder, R.P.; Guerin, M.T.; Hargis, B.M.; Kruth, P.S.; Page, G.; Rejman, E.; Rotolo, J.L.; Sears, W.; Zeldenrust, E.G.; Whale, J.; et al. Restoration of Anticoccidial Sensitivity to a Commercial Broiler Chicken Facility in Canada. Poult. Sci. 2021, 100, 663–674. [Google Scholar] [CrossRef]
- Mesa-Pineda, C.; Navarro-Ruíz, J.L.; López-Osorio, S.; Chaparro-Gutiérrez, J.J.; Gómez-Osorio, L.M. Chicken Coccidiosis: From the Parasite Lifecycle to Control of the Disease. Front. Vet. Sci. 2021, 8, 787653. [Google Scholar] [CrossRef]
- Jamil, M.; Aleem, M.T.; Shaukat, A.; Khan, A.; Mohsin, M.; Rehman, T.U.; Abbas, R.Z.; Saleemi, M.K.; Khatoon, A.; Babar, W.; et al. Medicinal Plants as an Alternative to Control Poultry Parasitic Diseases. Life 2022, 12, 449. [Google Scholar] [CrossRef]
- Abd El-Ghany, W.A. Phytobiotics in Poultry Industry as Growth Promoters, Antimicrobials and Immunomodulators-A Review. Sci. Publ. J. World Poult. Res. 2020, 10, 571–579. [Google Scholar] [CrossRef]
- Abdelli, N.; Solà-Oriol, D.; Pérez, J.F. Phytogenic Feed Additives in Poultry: Achievements, Prospective and Challenges. Animals 2021, 11, 3471. [Google Scholar] [CrossRef]
- Tsiouris, V.; Giannenas, I.; Bonos, E.; Papadopoulos, E.; Stylianaki, I.; Sidiropoulou, E.; Lazari, D.; Tzora, A.; Ganguly, B.; Georgopoulou, I. Efficacy of a Dietary Polyherbal Formula on the Performance and Gut Health in Broiler Chicks after Experimental Infection with Eimeria spp. Pathogens 2021, 10, 524. [Google Scholar] [CrossRef]
- Bozkurt, M.; Ege, G.; Aysul, N.; Akşit, H.; Tüzün, A.E.; Küçükyllmaz, K.; Borum, A.E.; Uygun, M.; Akşit, D.; Aypak, S.; et al. Effect of Anticoccidial Monensin with Oregano Essential Oil on Broilers Experimentally Challenged with Mixed Eimeria spp. Poult. Sci. 2016, 95, 1858–1868. [Google Scholar] [CrossRef]
- Mączka, W.; Twardawska, M.; Grabarczyk, M.; Wińska, K. Carvacrol—A Natural Phenolic Compound with Antimicrobial Properties. Antibiotics 2023, 12, 824. [Google Scholar] [CrossRef]
- Felici, M.; Tugnoli, B.; Ghiselli, F.; Massi, P.; Tosi, G.; Fiorentini, L.; Piva, A.; Grilli, E. In Vitro Anticoccidial Activity of Thymol, Carvacrol, and Saponins. Poult. Sci. 2020, 99, 5350–5355. [Google Scholar] [CrossRef]
- Sidiropoulou, E.; Marugán-Hernández, V.; Skoufos, I.; Giannenas, I.; Bonos, E.; Aguiar-Martins, K.; Lazari, D.; Papagrigoriou, T.; Fotou, K.; Grigoriadou, K.; et al. In Vitro Antioxidant, Antimicrobial, Anticoccidial, and Anti-Inflammatory Study of Essential Oils of Oregano, Thyme, and Sage from Epirus, Greece. Life 2022, 12, 1783. [Google Scholar] [CrossRef] [PubMed]
- Hikal, W.M.; Tkachenko, K.G.; Ahl, H.A.H.S.-A.; Sany, H.; Sabra, A.S.; Baeshen, R.S.; Bratovcic, A.; Hikal, W.M.; Tkachenko, K.G.; Ahl, H.A.H.S.-A.; et al. Chemical Composition and Biological Significance of Thymol as Antiparasitic. Open J. Ecol. 2021, 11, 240–266. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, D.H.; Kim, Y.B.; Jeong, S.B.; Oh, S.T.; Cho, S.Y.; Lee, K.W. Dietary Encapsulated Essential Oils Improve Production Performance of Coccidiosis-Vaccine-Challenged Broiler Chickens. Animals 2020, 10, 481. [Google Scholar] [CrossRef]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and Terpenoids as Main Bioactive Compounds of Essential Oils, Their Roles in Human Health and Potential Application as Natural Food Preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Sahak, M.K.A.; Kabir, N.; Abbas, G.; Draman, S.; Hashim, N.H.; Hasan Adli, D.S. The Role of Nigella sativa and Its Active Constituents in Learning and Memory. Evid.-Based Complement. Altern. Med. 2016, 2016, 6075679. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, D.M.; Silva, A.V.F.; Borges, S.A.; Maiorka, F.A.; Vargas, S.; Santin, E. Use of Yucca Schidigera Extract in Broiler Diets and Its Effects on Performance Results Obtained with Different Coccidiosis Control Methods. J. Appl. Poult. Res. 2007, 16, 248–254. [Google Scholar] [CrossRef]
- Tonda, R.M.; Rubach, J.K.; Lumpkins, B.S.; Mathis, G.F.; Poss, M.J. Effects of Tannic Acid Extract on Performance and Intestinal Health of Broiler Chickens Following Coccidiosis Vaccination and/or a Mixed-Species Eimeria Challenge. Poult. Sci. 2018, 97, 3031–3042. [Google Scholar] [CrossRef] [PubMed]
- Marzo, F.; Urdaneta, E.; Santidrián, S. Liver Proteolytic Activity in Tannic Acid-Fed Birds. Poult. Sci. 2002, 81, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Pyrka, I.; Mantzouridou, F.T.; Nenadis, N. Optimization of Olive Leaves’ Thin Layer, Intermittent near-Infrared-Drying. Innov. Food Sci. Emerg. Technol. 2023, 84, 103264. [Google Scholar] [CrossRef]
- Velkers, F.C.; Blake, D.P.; Graat, E.A.M.; Vernooij, J.C.M.; Bouma, A.; de Jong, M.C.M.; Stegeman, J.A. Quantification of Eimeria Acervulina in Faeces of Broilers: Comparison of McMaster Oocyst Counts from 24 h Faecal Collections and Single Droppings to Real-Time PCR from Cloacal Swabs. Vet. Parasitol. 2010, 169, 1–7. [Google Scholar] [CrossRef] [PubMed]
- De Pablos, L.M.; Dos Santos, M.F.B.; Montero, E.; Garcia-Granados, A.; Parra, A.; Osuna, A. Anticoccidial Activity of Maslinic Acid against Infection with Eimeria Tenella in Chickens. Parasitol. Res. 2010, 107, 601–604. [Google Scholar] [CrossRef]
- Johnson, J.; Reid, W.M. Anticoccidial Drugs: Lesion Scoring Techniques in Battery and Floor-Pen Experiments with Chickens. Exp. Parasitol. 1970, 28, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Gava, M.S.; de Moraes, L.B.; Carvalho, D.; Chitolina, G.Z.; Fallavena, L.C.B.; Moraes, H.L.S.; Herpich, J.; Salle, C.T.P. Determining the Best Sectioning Method and Intestinal Segment for Morphometric Analysis in Broilers. Rev. Bras. Cienc. Avic./Braz. J. Poult. Sci. 2015, 17, 145–150. [Google Scholar] [CrossRef]
- Chieh Sung, W.; Haryono, M. Quality Changes after Oven-Drying and Frozen Storage of Bluestripe Herring (Herklotsichthys quadrimaculatus). J. Food Nutr. Res. 2017, 5, 935–940. [Google Scholar] [CrossRef]
- Vyncke, W. Evaluation of the Direct Thiobarbituric Acid Extraction Method for Determining Oxidative Rancidity in Mackerel (Scomber scombrus L.). Fette Seifen Anstrichm. 1975, 77, 239–240. [Google Scholar] [CrossRef]
- Charan, J.; Kantharia, N. How to Calculate Sample Size in Animal Studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef]
- Grover, J.K.; Yadav, S.; Vats, V. Medicinal Plants of India with Anti-Diabetic Potential. J. Ethnopharmacol. 2002, 81, 81–100. [Google Scholar] [CrossRef]
- Tadjbakhsh, H. Traditional Methods Used for Controlling Animal Diseases in Iran. Rev. Sci. Tech. 1994, 13, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Kikusato, M. Phytobiotics to Improve Health and Production of Broiler Chickens: Functions beyond the Antioxidant Activity. Anim. Biosci. 2021, 34, 345–353. [Google Scholar] [CrossRef]
- Pandey, S.; Kim, E.S.; Cho, J.H.; Song, M.; Doo, H.; Kim, S.; Keum, G.B.; Kwak, J.; Ryu, S.; Choi, Y.; et al. Cutting-Edge Knowledge on the Roles of Phytobiotics and Their Proposed Modes of Action in Swine. Front. Vet. Sci. 2023, 10, 1265689. [Google Scholar] [CrossRef] [PubMed]
- Pandian, S.C.; Krishna Murthy, G.T.; Rao, A.V. Antimicrobial Activity of Selected Phytobiotics Individually and in Combination against Gram Positive and Gram Negative Bacteria. J. Entomol. Zool. Stud. 2021, 9, 2255–2260. [Google Scholar]
- Skoufos, I.; Bonos, E.; Anastasiou, I.; Tsinas, A.; Tzora, A. Effects of Phytobiotics in Healthy or Disease Challenged Animals. Feed. Addit. Aromat. Plants Herbs Anim. Nutr. Health 2020, 311–337. [Google Scholar] [CrossRef]
- Mohiti-Asli, M.; Ghanaatparast-Rashti, M. Dietary Oregano Essential Oil Alleviates Experimentally Induced Coccidiosis in Broilers. Prev. Vet. Med. 2015, 120, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Bortoluzzi, C.; Lumpkins, B.; Mathis, G.F.; França, M.; King, W.D.; Graugnard, D.E.; Dawson, K.A.; Applegate, T.J. Zinc Source Modulates Intestinal Inflammation and Intestinal Integrity of Broiler Chickens Challenged with Coccidia and Clostridium Perfringens. Poult. Sci. 2019, 98, 2211–2219. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lu, M.; Lillehoj, H.S. Coccidiosis: Recent Progress in Host Immunity and Alternatives to Antibiotic Strategies. Vaccines 2022, 10, 215. [Google Scholar] [CrossRef]
- Pop, L.M.; Varga, E.; Coroian, M.; Nedisan, M.E.; Mircean, V.; Dumitrache, M.O.; Farczádi, L.; Fülöp, I.; Croitoru, M.D.; Fazakas, M.; et al. Efficacy of a Commercial Herbal Formula in Chicken Experimental Coccidiosis. Parasit. Vectors 2019, 12, 343. [Google Scholar] [CrossRef]
- Giannenas, I.A.; Florou-Paneri, P.; Botsoglou, N.A.; Christaki, E.; Spais, A.B. Effect of Supplementing Feed with Oregano and/or α-Tocopheryl Acetate on Growth of Broiler Chickens and Oxidative Stability of Meat. J. Anim. Feed Sci. 2005, 14, 521–535. [Google Scholar] [CrossRef]
- Aljedaie, M.M.; Al-Malki, E.S. Anticoccidial Activities of Salvadora Persica(Arak), Zingiber Officinale (Ginger) and Curcuma Longa (Turmeric) Extracts on the Control of Chicken Coccidiosis. J. King Saud Univ. Sci. 2020, 32, 2810–2817. [Google Scholar] [CrossRef]
- Oikeh, I.; Sakkas, P.; Taylor, J.; Giannenas, I.; Blake, D.P.; Kyriazakis, I. Effects of Reducing Growth Rate via Diet Dilution on Bone Mineralization, Performance and Carcass Yield of Coccidia-Infected Broilers. Poult. Sci. 2019, 98, 5477–5487. [Google Scholar] [CrossRef]
- Villar-Patiño, G.; del C. Camacho-Rea, M.; Olvera-García, M.E.; Baltazar-Vázquez, J.C.; Gómez-Verduzco, G.; Téllez, G.; Labastida, A.; Ramírez-Pérez, A.H. Effect of an Alliaceae Encapsulated Extract on Growth Performance, Gut Health, and Intestinal Microbiota in Broiler Chickens Challenged with Eimeria spp. Animals 2023, 13, 3884. [Google Scholar] [CrossRef]
- Han, M.; Hu, W.; Chen, T.; Guo, H.; Zhu, J.; Chen, F. Anticoccidial Activity of Natural Plants Extracts Mixture against Eimeria Tenella: An in Vitro and in Vivo Study. Front. Vet. Sci. 2022, 9, 1066543. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.-Y.; Di, K.-Q.; Xu, J.; Chen, Y.-F.; Xi, J.-Z.; Wang, D.-H.; Hao, E.-Y.; Xu, L.-J.; Chen, H.; Zhou, R.-Y. Effect of Natural Garlic Essential Oil on Chickens with Artificially Infected Eimeria Tenella. Vet. Parasitol. 2021, 300, 109614. [Google Scholar] [CrossRef]
- Nabian, S.; Arabkhazaeli, F.; Seifouri, P.; Farahani, A. Morphometric Analysis of the Intestine in Experimental Coccidiosis in Broilers Treated with Anticoccidial Drugs. Iran J. Parasitol. 2018, 13, 493–499. [Google Scholar]
- Kiela, P.R.; Ghishan, F.K. Physiology of Intestinal Absorption and Secretion. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 145. [Google Scholar] [CrossRef] [PubMed]
- Prakash, B.; Kumar, A.; Singh, P.P.; Songachan, L.S. Antimicrobial and Antioxidant Properties of Phytochemicals: Current Status and Future Perspective. Funct. Preserv. Prop. Phytochem. 2020, 1–45. [Google Scholar] [CrossRef]
- Chodkowska, K.A.; Abramowicz-Pindor, P.A.; Tuśnio, A.; Gawin, K.; Taciak, M.; Barszcz, M. Effect of Phytobiotic Composition on Production Parameters, Oxidative Stress Markers and Myokine Levels in Blood and Pectoral Muscle of Broiler Chickens. Animals 2022, 12, 2625. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.; Kareem, K.Y.; Matuszewski, A.; Bień, D.; Ciborowska, P.; Lutostański, K.; Michalczuk, M. Enhancing Broiler Chicken Health and Performance: The Impact of Phytobiotics on Growth, Gut Microbiota, Antioxidants, and Immunity. Phytochem. Rev. 2024, 1–15. [Google Scholar] [CrossRef]
| Samples | ||
|---|---|---|
| Phytobiotic Mixture | Basal Diet | |
| TPC | 359.13 | 13.18 |
| Age of Chickens | Parameters | Groups | SEM | p | ||||
|---|---|---|---|---|---|---|---|---|
| CN | PN | CI | PI | SI | ||||
| Day 1 | BW | 44.3 | 42.5 | 44.1 | 43.6 | 44.7 | 0.4 | 0.322 |
| Day 7 | BW | 120.1 | 126.1 | 121.4 | 121.9 | 123.0 | 2.0 | 0.909 |
| BWG | 75.8 | 83.6 | 77.3 | 78.3 | 78.3 | 2.0 | 0.799 | |
| FI | 867.7 | 857.7 | 825.3 | 836.7 | 796.8 | 15.8 | 0.675 | |
| FCR | 1.17 | 1.04 | 1.11 | 1.07 | 1.03 | 0.03 | 0.636 | |
| Day 14 | BW | 320.8 | 327.2 | 326.3 | 309.1 | 336.0 | 5.6 | 0.651 |
| BWG | 200.8 | 201.1 | 204.8 | 187.2 | 213.0 | 5.6 | 0.706 | |
| FI | 2714 | 2668.8 | 2770.8 | 2600.5 | 2892.2 | 56.5 | 0.577 | |
| FCR | 1.36 | 1.33 | 1.37 | 1.40 | 1.36 | 0.03 | 0.958 | |
| Day 21 | BW | 719.7 ab | 744.9 a | 637.7 bc | 624.8 c | 731.0 a | 11.9 | <0.001 |
| BWG | 398.8 a | 417.8 a | 311.4 b | 315.7 b | 395.0 a | 8.1 | <0.001 | |
| FI | 5750.3 ab | 5926.2 a | 5356.8 bc | 5008.7 c | 5786.8 ab | 70.0 | <0.001 | |
| FCR | 1.45 b | 1.43 b | 1.73 a | 1.59 ab | 1.47 b | 0.03 | 0.006 | |
| Day 28 | BW | 1326.5 a | 1332.8 a | 1093.8 c | 1140.17 bc | 1263.8 ab | 21.0 | <0.001 |
| BWG | 606.8 a | 587.9 a | 456.1 b | 515.4 ab | 532.8 ab | 13.0 | <0.001 | |
| Day 35 | BW | 2019.0 ab | 2066.9 a | 1721.4 c | 1843.8 bc | 1919.4 abc | 27.1 | <0.001 |
| BWG | 692.5 ab | 734.08 a | 627.67 b | 703.58 ab | 655.67 ab | 12.0 | 0.041 | |
| FI | 21,038 a | 20,151.5 bc | 20,558.3 ab | 20,272.2 bc | 19,776.7 c | 107.9 | <0.001 | |
| FCR | 1.62 b | 1.53 b | 1.91 a | 1.66 b | 1.66 b | 0.03 | <0.001 | |
| Days 1–14 | BWG | 276.5 | 284.7 | 282.2 | 265.5 | 291.3 | 5.5 | 0.661 |
| FI | 3581.7 | 3526.5 | 3596.2 | 3437.2 | 3689.0 | 52.6 | 0.747 | |
| FCR | 1.30 | 1.25 | 1.28 | 1.30 | 1.27 | 0.02 | 0.858 | |
| Days 14–35 | BWG | 1698.2 ab | 1739.8 a | 1395.2 c | 1534.7 b | 1583.4 ab | 24.6 | <0.001 |
| FI | 26,788.3 a | 26,077.6 ab | 25,915.2 bc | 25,280.8 c | 25,563.5 bc | 120.8 | <0.001 | |
| FCR | 1.58 b | 1.50 b | 1.87 a | 1.65 b | 1.62 b | 0.03 | <0.001 | |
| Days 1–35 | BWG | 1974.7 ab | 2024.4 a | 1677.3 c | 1800.2 bc | 1874.8 abc | 27.0 | <0.001 |
| FI | 30,370.0 a | 29,604.2 ab | 29,511.3 abc | 28,718.0 c | 29,252.5 bc | 133.2 | <0.001 | |
| FCR | 1.54 b | 1.47 b | 1.77 a | 1.60 b | 1.56 b | 0.02 | <0.001 | |
| Days (Post Infection) | CN | PN | CI | PI | SI | SEM | p-Value | |
|---|---|---|---|---|---|---|---|---|
| OPG | 4 | 0 b | 0 b | 1900 a | 300 b | 200 b | 152.3 | <0.001 |
| 5 | 0 b | 0 b | 32000 a | 1850 b | 2050 b | 2343.4 | <0.001 | |
| 6 | 0 b | 0 b | 150,500 a | 11,500 b | 14,500 b | 10,884.1 | <0.001 | |
| 7 | 0 c | 0 c | 304,000 a | 21,000 bc | 27,500 b | 21,905.6 | <0.001 | |
| CLS | 0.0 a | 0.0 a | 3.46 b | 3.13 b | 5.17 c | <0.001 | ||
| ACI | 21 | 200 a | 200 a | 93.36 c | 188.45 ab | 136.91 b | 10.1 | <0.001 |
| Gut Segment | Groups | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CN | PN | CI | PI | SI | ||||
| Duodenum | Villus Height | 695.6 | 742.2 | 611.1 | 648.0 | 690.6 | 21.8 | 0.404 |
| Crypt Depth | 88.2 c | 150.2 ab | 189.0 a | 148.2 ab | 126.2 ab | 8.0 | <0.001 | |
| VH/CD | 7.89 | 4.94 | 3.23 | 4.37 | 5.47 | |||
| Jejunum | Villus Height | 558.2 a | 571.4 a | 436.1 b | 524.1 ab | 531.9 ab | 14.5 | 0.015 |
| Crypt Depth | 97.4 c | 107.9 c | 134.4 b | 167.3 a | 152.7 ab | 5.9 | <0.001 | |
| VH/CD | 5.73 | 5.3 | 3.24 | 3.13 | 3.48 | |||
| Ileum | Villus Height | 222.7 a | 232.2 a | 163.3 b | 232.7 a | 178.1 ab | 17.4 | 0.003 |
| Crypt Depth | 81.9 c | 91.3 c | 100.0 bc | 106.3 b | 141.4 a | 4.6 | <0.001 | |
| VH/CD | 2.72 | 2.54 | 1.63 | 2.19 | 1.26 | |||
| Antioxidant Analysis | Body Part | Day (Post-Sampling) | Groups | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| CN | PN | CI | PI | SI | |||||
| MDA | breast | 1 | 7.155 | 10.059 | 7.387 | 6.098 | 7.038 | 0.747 | 0.527 |
| 5 | 17.782 | 21.512 | 17.95 | 16.165 | 17.535 | 1 | 0.529 | ||
| thigh | 1 | 13.459 | 14.449 | 12.075 | 11.282 | 13.473 | 0.772 | 0.722 | |
| 5 | 26.132 | 27.321 | 25.538 | 23.953 | 26.84 | 1.355 | 0.952 | ||
| intestine | 1 | 57.287 | 81.181 | 59.863 | 52.898 | 71.448 | 5.14 | 0.399 | |
| Protein Carbonyls | breast | 1 | 4.5 | 4.4 | 4.5 | 4.55 | 3.97 | 0.57 | 0.897 |
| thigh | 1 | 3.99 | 3.94 | 3.89 | 3.84 | 3.91 | 0.158 | 0.782 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galamatis, D.; Panitsidis, I.; Mantzios, T.; Sioutas, G.; Stylianaki, I.; Papadopoulos, E.; Raj, J.; Vasiljević, M.; Bošnjak-Neumüller, J.; Blake, D.; et al. Assessment of a Natural Phytobiotic Mixture as Feed Additive for Broiler Chicken: Studies on Animal Performance, Gut Health, and Antioxidant Status After Experimental Infection with Eimeria spp. Poultry 2025, 4, 4. https://doi.org/10.3390/poultry4010004
Galamatis D, Panitsidis I, Mantzios T, Sioutas G, Stylianaki I, Papadopoulos E, Raj J, Vasiljević M, Bošnjak-Neumüller J, Blake D, et al. Assessment of a Natural Phytobiotic Mixture as Feed Additive for Broiler Chicken: Studies on Animal Performance, Gut Health, and Antioxidant Status After Experimental Infection with Eimeria spp. Poultry. 2025; 4(1):4. https://doi.org/10.3390/poultry4010004
Chicago/Turabian StyleGalamatis, Dimitrios, Ioannis Panitsidis, Tilemachos Mantzios, Georgios Sioutas, Ioanna Stylianaki, Elias Papadopoulos, Jog Raj, Marko Vasiljević, Jasna Bošnjak-Neumüller, Damer Blake, and et al. 2025. "Assessment of a Natural Phytobiotic Mixture as Feed Additive for Broiler Chicken: Studies on Animal Performance, Gut Health, and Antioxidant Status After Experimental Infection with Eimeria spp." Poultry 4, no. 1: 4. https://doi.org/10.3390/poultry4010004
APA StyleGalamatis, D., Panitsidis, I., Mantzios, T., Sioutas, G., Stylianaki, I., Papadopoulos, E., Raj, J., Vasiljević, M., Bošnjak-Neumüller, J., Blake, D., Tsiouris, V., & Giannenas, I. (2025). Assessment of a Natural Phytobiotic Mixture as Feed Additive for Broiler Chicken: Studies on Animal Performance, Gut Health, and Antioxidant Status After Experimental Infection with Eimeria spp. Poultry, 4(1), 4. https://doi.org/10.3390/poultry4010004











