Ileal Digestible and Metabolizable Energy of Corn, Wheat, and Barley in Growing Japanese Quail
Abstract
1. Introduction
2. Materials and Methods
2.1. Bird Management and Experimental Diets
2.2. Ileal Digestibility Measurements
2.3. Metabolizability Measurements
2.4. Chemical Analysis
2.5. Calculations
2.6. Statistical Analysis
3. Results
3.1. Ileal Digestibility and Total Tract Metabolizability
3.2. The IDE, AME, and AMEn of Feed Ingredients
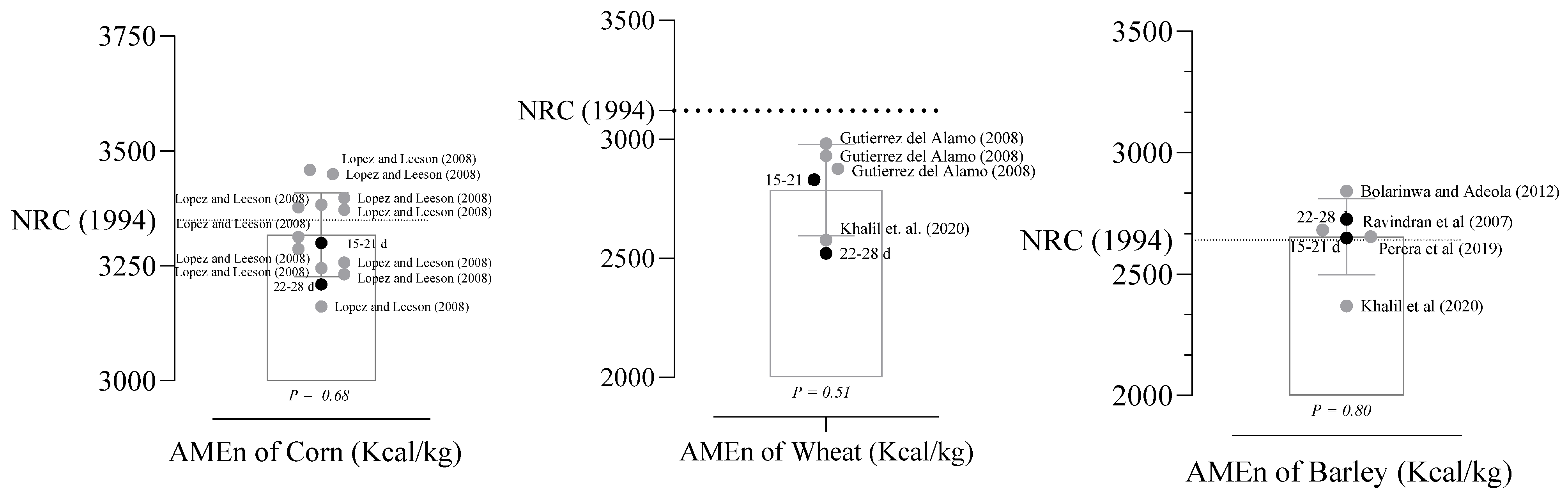
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du Sert, N.P.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar]
- NRC. Nutrient Requirements for Poultry, 9th ed.; National Academy Press: Washington, DC, USA, 1994. [Google Scholar]
- Hill, F.; Anderson, D. Comparison of metabolizable energy and productive energy determinations with growing chicks. J. Nutr. 1958, 64, 587–603. [Google Scholar] [CrossRef]
- Wu, S.-B.; Choct, M.; Pesti, G. Historical flaws in bioassays used to generate metabolizable energy values for poultry feed formulation: A critical review. Poult. Sci. 2020, 99, 385–406. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis; AOAC International: Arlington, VA, USA, 2006. [Google Scholar]
- Kong, C.; Adeola, O. Evaluation of amino acid and energy utilization in feedstuff for swine and poultry diets. Asian-Australas. J. Anim. Sci. 2014, 27, 917. [Google Scholar] [CrossRef]
- SAS. SAS/STAT 9.1 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2002. [Google Scholar]
- Anwar, U.; Chishti, F.; Bilal, M.; Farooq, U.; Mustafa, R.; Zamir, S.; Hussain, M.; Hussain, M.; Ashraf, M.; Qamar, S. Inclusion of Stored Wheat in the Feed of Broilers Influences Intake, Growth Performance, Nutrient Digestibility, and Digesta Viscosity from 1–21 Days of Age. Braz. J. Poult. Sci. 2023, 25, eRBCA-2022-1736. [Google Scholar] [CrossRef]
- Nóbrega, I.; Nogueira, H.; Lima, M.; Sakomura, N.; Peruzzi, N.; Artoni, S.; Suzuki, R.; Silva, E. Rate of feed passage in Japanese quail. Animal 2020, 14, s267–s274. [Google Scholar] [CrossRef]
- Alagawany, M.; Elnesr, S.S.; Farag, M. The role of exogenous enzymes in promoting growth and improving nutrient digestibility in poultry. Iran. J. Vet. Res. 2018, 19, 157. [Google Scholar]
- Tay-Zar, A.-C.; Wongphatcharachai, M.; Srichana, P.; Geraert, P.-A.; Noblet, J. Prediction of net energy of feeds for broiler chickens. Anim. Nutr. 2024, 16, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-B.; Swick, R.A.; Noblet, J.; Rodgers, N.; Cadogan, D.; Choct, M. Net energy prediction and energy efficiency of feed for broiler chickens. Poult. Sci. 2019, 98, 1222–1234. [Google Scholar] [CrossRef] [PubMed]
- Toghyani, M.; Rodgers, N.; Barekatain, M.R.; Iji, P.; Swick, R.A. Apparent metabolizable energy value of expeller-extracted canola meal subjected to different processing conditions for growing broiler chickens. Poult. Sci. 2014, 93, 2227–2236. [Google Scholar] [CrossRef]
- Khalil, M.; Abdollahi, M.; Zaefarian, F.; Chrystal, P.; Ravindran, V. Apparent metabolizable energy of cereal grains for broiler chickens is influenced by age. Poult. Sci. 2021, 100, 101288. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.R.; Wiltafsky-Martin, M.; Ravindran, V. Application of apparent metabolizable energy versus nitrogen-corrected apparent metabolizable energy in poultry feed formulations: A continuing conundrum. Animals 2021, 11, 2174. [Google Scholar] [CrossRef] [PubMed]
- Lopez, G.; Leeson, S. Relevance of nitrogen correction for assessment of metabolizable energy with broilers to forty-nine days of age. Poult. Sci. 2007, 86, 1696–1704. [Google Scholar] [CrossRef] [PubMed]
- Adeola, O.; Anwar, M.; Abdollahi, M.; Ravindran, V. Age-related energy values of meat and bone meal for broiler chickens. Poult. Sci. 2018, 97, 2516–2524. [Google Scholar] [CrossRef]
- Juanchich, A.; Urvoix, S.; Hennequet-Antier, C.; Narcy, A.; Mignon-Grasteau, S. Phenotypic timeline of gastrointestinal tract development in broilers divergently selected for digestive efficiency. Poult. Sci. 2021, 100, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Noy, Y.; Sklan, D. Nutrient use in chicks during the first week posthatch. Poult. Sci. 2002, 81, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Lopez, G.; Leeson, S. Assessment of the nitrogen correction factor in evaluating metabolizable energy of corn and soybean meal in diets for broilers. Poult. Sci. 2008, 87, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Del Alamo, A.G.; Verstegen, M.; Den Hartog, L.; De Ayala, P.P.; Villamide, M. Effect of wheat cultivar and enzyme addition to broiler chicken diets on nutrient digestibility, performance, and apparent metabolizable energy content. Poult. Sci. 2008, 87, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Abdollahi, M.; Zaefarian, F.; Ravindran, V. Measurement of ileal endogenous energy losses and true ileal digestible energy of cereal grains for broiler chickens. Poult. Sci. 2020, 99, 6809–6817. [Google Scholar] [CrossRef]
- Perera, W.; Abdollahi, M.; Ravindran, V.; Zaefarian, F.; Wester, T.; Ravindran, G. Nutritional evaluation of two barley cultivars, without and with carbohydrase supplementation, for broilers: Metabolisable energy and standardised amino acid digestibility. Br. Poult. Sci. 2019, 60, 404–413. [Google Scholar] [CrossRef]
- Ravindran, V.; Tilman, Z.; Morel, P.; Ravindran, G.; Coles, G. Influence of β-glucanase supplementation on the metabolisable energy and ileal nutrient digestibility of normal starch and waxy barleys for broiler chickens. Anim. Feed Sci. Technol. 2007, 134, 45–55. [Google Scholar] [CrossRef]
- Bolarinwa, O.; Adeola, O. Energy value of wheat, barley, and wheat dried distillers grains with solubles for broiler chickens determined using the regression method. Poult. Sci. 2012, 91, 1928–1935. [Google Scholar] [CrossRef] [PubMed]
- Sakomura, N.K. Modeling energy utilization in broiler breeders, laying hens and broilers. Braz. J. Poult. Sci. 2004, 6, 1–11. [Google Scholar] [CrossRef]
- Temim, S.; Chagneau, A.-M.; Guillaumin, S.; Michel, J.; Peresson, R.; Geraert, P.-A.; Tesseraud, S. Effects of chronic heat exposure and protein intake on growth performance, nitrogen retention and muscle development in broiler chickens. Reprod. Nutr. Dev. 1999, 39, 145–156. [Google Scholar] [CrossRef] [PubMed]

| Feed | Corn | Wheat | Barley |
|---|---|---|---|
| Dry matter | 905 | 922 | 902 |
| Crude protein (N × 6.25) | 79.6 | 101.0 | 110.0 |
| Ether extract | 37.0 | 22.0 | 26.0 |
| Ash | 12.0 | 18.0 | 22.0 |
| Crude fiber | 21.0 | 28.0 | 43.0 |
| Gross energy (kcal/kg) | 4025 | 4018 | 3952 |
| Ingredients | Amount (g/kg) |
|---|---|
| Corn | 340.0 |
| Wheat | 180.1 |
| Soybean meal | 386.6 |
| Corn gluten meal | 8.80 |
| Soybean oil | 24.4 |
| Cornstarch | 25.0 |
| Limestone | 14.2 |
| DCP | 6.20 |
| NaCl | 3.20 |
| NaHCO3 | 0.20 |
| DL-Methionine | 3.70 |
| L-Lysine.HCl | 1.40 |
| L-Threonine | 1.10 |
| Mineral premix 1 | 2.50 |
| Vitamin premix 2 | 2.50 |
| Nutrient composition | |
| AME (Kcal/kg) 3 | 2900 |
| CP (g/kg) 4 | 250 |
| Ca (g/kg) 3 | 8.00 |
| Pavailable (g/kg) 3 | 3.00 |
| Na (g/kg) 3 | 1.60 |
| Cl (g/kg) 3 | 2.60 |
| K (g/kg) 3 | 9.60 |
| DEB (mEq/kg) 5 | 270 |
| Ingredient | Age | iDM (%) | iCP (%) | IDE (kcal/kg) |
|---|---|---|---|---|
| Corn | I | 72.5 b | 56.1 c | 2954 |
| Wheat | I | 89.4 a | 65.3 b | 3441 |
| Barley | I | 85.1 a | 69.0 b | 3194 |
| Corn | II | 74.8 b | 64.0 b | 2894 |
| Wheat | II | 86.7 a | 63.7 b | 3439 |
| Barley | II | 84.0 a | 83.2 a | 3260 |
| SEM | 2.41 | 1.99 | 79.2 | |
| Ingredient | ||||
| Corn | 73.6 | 60.0 c | 2924 c | |
| Wheat | 88.1 | 64.5 b | 3440 a | |
| Barley | 84.6 | 76.1 a | 3227 b | |
| SEM | 1.70 | 1.41 | 56.0 | |
| Age | ||||
| I | 82.3 | 63.5 b | 3196 | |
| II | 81.8 | 70.3 a | 3198 | |
| SEM | 1.39 | 1.15 | 45.7 | |
| p-value | ||||
| Feed | <0.001 | <0.001 | <0.001 | |
| Age | 0.801 | <0.001 | 0.983 | |
| Feed × Age | 0.575 | 0.003 | 0.733 | |
| Ingredient | Age | mcDM (%) | mcCP (%) | NR (%) | AME (kcal/kg) | AMEn (kcal/kg) | ∆AME (kcal) |
|---|---|---|---|---|---|---|---|
| Corn | I | 83.7 | 82.5 | 86.2 a | 3340 | 3300 | 39.6 |
| Wheat | I | 78.5 | 83.9 | 74.9 b | 2899 | 2831 | 68.5 |
| Barley | I | 86.2 | 88.8 | 81.7 ab | 2663 | 2506 | 158 |
| Corn | II | 81.4 | 80.3 | 74.7 b | 3698 | 3665 | 33.3 |
| Wheat | II | 83.9 | 77.9 | 76.6 b | 3058 | 2976 | 82.6 |
| Barley | II | 87.0 | 80.8 | 87.8 a | 2757 | 2558 | 198 |
| SEM | 2.47 | 3.79 | 3.11 | 133 | 188 | 18.4 | |
| Ingredient | |||||||
| Corn | 82.6 | 81.4 | 80.5 ab | 3519 a | 3483 a | 36.3 c | |
| Wheat | 81.2 | 80.9 | 75.8 b | 2979 b | 2903 b | 75.5 b | |
| Barley | 86.6 | 84.8 | 84.7 a | 2710 b | 2532 c | 178 a | |
| SEM | 1.74 | 2.68 | 2.20 | 93.8 | 98.5 | 13.0 | |
| Age | |||||||
| I | 82.8 | 85.1 | 80.9 | 2968 | 2879 | 88.6 | |
| II | 84.1 | 79.7 | 79.7 | 3171 | 3066 | 105 | |
| SEM | 1.43 | 2.19 | 1.79 | 76.6 | 80.4 | 10.6 | |
| p-value | |||||||
| Feed | 0.101 | 0.549 | 0.033 | <0.001 | <0.001 | <0.001 | |
| Age | 0.529 | 0.098 | 0.631 | 0.077 | 0.117 | 0.297 | |
| Feed × Age | 0.313 | 0.737 | 0.029 | 0.592 | 0.530 | 0.465 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanipour, S.; Ghazaghi, M.; Abdollahi, M.R.; Mehri, M. Ileal Digestible and Metabolizable Energy of Corn, Wheat, and Barley in Growing Japanese Quail. Poultry 2024, 3, 190-199. https://doi.org/10.3390/poultry3030015
Khanipour S, Ghazaghi M, Abdollahi MR, Mehri M. Ileal Digestible and Metabolizable Energy of Corn, Wheat, and Barley in Growing Japanese Quail. Poultry. 2024; 3(3):190-199. https://doi.org/10.3390/poultry3030015
Chicago/Turabian StyleKhanipour, Sousan, Mahmoud Ghazaghi, Mohammad Reza Abdollahi, and Mehran Mehri. 2024. "Ileal Digestible and Metabolizable Energy of Corn, Wheat, and Barley in Growing Japanese Quail" Poultry 3, no. 3: 190-199. https://doi.org/10.3390/poultry3030015
APA StyleKhanipour, S., Ghazaghi, M., Abdollahi, M. R., & Mehri, M. (2024). Ileal Digestible and Metabolizable Energy of Corn, Wheat, and Barley in Growing Japanese Quail. Poultry, 3(3), 190-199. https://doi.org/10.3390/poultry3030015







