Abstract
Background: There is an urgent need for novel treatment options for Neisseria gonorrhoeae. Methenamine is an interesting urinary antiseptic with a very low propensity to induce antimicrobial resistance. Methods: We assessed the MICs of methenamine-hippurate for 18 N. gonorrhoeae isolates. We then assessed the in vivo efficacy of methenamine-hippurate against N. gonorrhoeae using the Galleria mellonella infection model. Results: We found that all the gonococcal isolates had a methenamine-hippurate MIC of 300 mg/L. This MIC was not higher in isolates with higher ceftriaxone MICs. No toxicity of methenamine at the doses tested was found, and doses as low as 200 mg/kg were effective in the G. mellonella model. Conclusions: Further studies in mice and humans are required to assess if methenamine-hippurate could be used to treat gonococcal urethritis alone or in combination with other agents such as ceftriaxone.
1. Introduction
Neisseria gonorrhoeae is a sexually transmitted infection whose main clinical manifestations are urethritis, cervicitis and proctitis [1]. Current therapy guidelines for gonorrhoea typically recommend ceftriaxone with or without azithromycin [2,3]. N. gonorrhoeae has developed antimicrobial resistance to all the classes of antimicrobials used to treat it [4]. This means that novel therapeutics are urgently required [5]. It would be particularly useful if these compounds would target novel targets that could be taken orally, are safe and cheap, and have a low probability of generating antimicrobial resistance [4,6]. One such compound is methenamine-hippurate which has been used as an oral urinary antiseptic to prevent urinary tract infections for over 100 years [7].
Methenamine is a urinary antiseptic first discovered in 1859 and first used as a urinary antiseptic in 1895 [7]. Methenamine is rapidly absorbed after oral administration [7]. More than 80% of methenamine is recovered in the urine of normal volunteers, which indicates excellent oral bioavailability [8]. Methenamine-hippurate undergoes hydrolysis in the acidic urine where it is converted into formaldehyde. Formaldehyde has a number of antimicrobial effects (Figure 1) [7,8]: (1) It reacts with the amino groups in proteins and thereby forms crosslinks that denature the proteins. (2) It forms covalent bonds between nucleotides or between nucleotides and proteins, thus inhibiting DNA replication, transcription, and repair. (3) It can compromise the integrity of cell membranes by crosslinking membrane proteins and lipids, increasing permeability and causing leakage of cellular contents. A dose of 1 g of methenamine-hippurate every 12 h results in a urine concentration of >500 μg/mL of methenamine, which exceeds the MICs of almost all urinary pathogens [8,9]. The elimination half-life is approximately 4.3 h, the volume of distribution is 0.56 L/kg, the renal clearance is about 1.46, and the total clearance is 1.58 mL/min/kg [9]. Between 70% and 90% of a 1 g dose of oral methenamine is excreted in the urine within 24 h [10]. The urinary antibacterial effects begin within 30 min of ingestion [10].
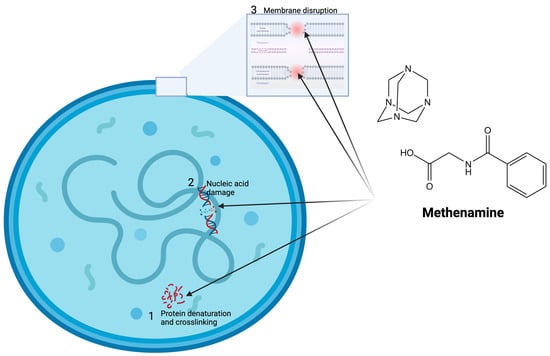
Figure 1.
Mechanism of action of methenamine-hippurate. Methenamine is broken down in the acid urine into formaldehyde, which has numerous antimicrobial actions including (1) protein denaturation and crosslinking, (2) nucleic acid damage, and (3) membrane disruption via crosslinking membrane proteins and lipids. Figure produced with BioRender.com (version 1.1).
Further advantages of methenamine-hippurate are its low cost and few contraindications or side effects. The most common side effects are nausea, vomiting, dyspepsia and diarrhea, which are generally mild [10,11]. Very rarely, hypersensitivity reactions have been reported [11]. We could not find any reports of treatment emergent resistance [7,8,9]. Two recent randomized controlled trials (RCTs) found methenamine to be as efficacious as long-term antibiotics at preventing recurrent urinary tract infections (rUTIs) but without the elevated risk of AMR found with antibiotic prophylaxis [12]. An obvious disadvantage of methenamine-hippurate is that because of poor penetration to other body sites, it could only be used to treat gonococcal infection of the urethra [4]. Adverse reactions occur in less than 4% of patients [11].
We have recently successfully introduced a methenamine-based regimen to treat recalcitrant, multidrug-resistant Mycoplasma genitalium urethritis [13]. A search in PubMed and Google Scholar could not find any evidence that methenamine has been evaluated for the treatment of N. gonorrhoeae. This provided the motivation for the current study, where we evaluated the in vitro and in vivo activity of methenamine against N. gonorrhoeae.
2. Materials and Methods
2.1. Neisseria gonorrhoeae Isolates
To assess the effects of methenamine-hippurate on Neisseria gonorrhoeae, a panel of 3 WHO reference strains (WHO X, Y, and Z), ATCC 49226, and 15 clinical isolates were chosen. All clinical isolates were from symptomatic clinical infections obtained from individuals attending the STI clinic at the Institute of Tropical Medicine, Antwerp, between 2023 and 2025. These isolates were chosen to incorporate both low and high ceftriaxone susceptible isolates. Eleven of these isolates had high ceftriaxone MICs and four isolates had low ceftriaxone MICs. Further information pertaining to the bacterial strains used in this study is provided in Table 1.

Table 1.
Methenamine-hippurate, ceftriaxone, ciprofloxacin and azithromycin Minimum Inhibitory Concentrations (MICs—µg/mL) of the Neisseria gonorrhoeae panel used in this study [10].
2.2. Antimicrobial Susceptibility Testing
A stock solution of 5 mg/mL methenamine hippurate (Mylan, NV, Canonsburg, PA, USA) was prepared by crushing two 1 g tablets and dissolving them in 5 mL distilled water (pH adjusted to 5.05), followed by gentle heating and preparations of the required dilutions.
The methenamine MICs were ascertained via agar dilution testing. This was performed using gonococcal (GC) agar base medium (BD Difco™, Becton Dickinson, Temse, Belgium) supplemented with 1% IsoVitaleX (BD, Temse, Belgium). This was performed according to the Clinical and Laboratory Standards Institute (CLSI) agar dilution method [14,15]. First, the N. gonorrhoeae strains were grown on Columbia blood agar with 5% sheep blood and incubated at 36 °C in 5% CO2 for 16 h. Second, a 0.5 McFarland suspension of each strain was prepared in a tube with 2 mL of Mueller Hinton Broth (BD Difco™, BD, Temse, Belgium). Third, approximately 104 CFU of a dilution of the suspension per spot was inoculated within 15 min of preparation onto the agar surface with a multipoint inoculator. Fourth, the plates were incubated at 36 °C in 5% CO2 for 20 to 24 h. The MICs were then interpreted by the standard method of reading growth inhibition.
2.3. Preparation of Live Microbial Inocula for G. mellonella Infection
N. gonorrhoeae 19.598 was used for these experiments. This isolate was chosen on the basis of it having the highest ceftriaxone MIC of all the clinical isolates in our study. It was cultured from frozen stocks onto blood agar (BA) plate for ≤16 h at 36 °C with 5% CO2. Single colonies were then taken from these cultures and plated onto fresh agar plates. These were incubated at 36 °C with 5% CO2 for 6 h. The N. gonorrhoeae were then injected into the larvae-hemocoel (30 μL of GC broth containing 108 CFU/larva). This dose of N. gonorrhoeae was similar to that used in previous analogous experiments [16,17].
2.4. Injection of G. mellonella Larvae
We used the last larval stage of Galleria mellonella (Terramania, Arnhem, The Netherlands) to assess the in vivo efficacy of methenamine. Healthy, non-discolored larvae weighing 250 to 450 mg were selected and placed into individual sterile Petri dishes in groups of 10 per Petri dish. The larvae were then kept in an incubator at 36 °C with a 5% CO2 atmosphere for the length of the experiments. Each experimental group consisted of at least 30 larvae.
The larvae were injected in the last right pro-leg with 30 μL of bacterial suspension, followed 5–20 min later by 10 μL of various doses of methenamine-hippurate (2 g/g and 4 g/g; Sanbio, Uden, The Netherlands), using 0.3 mL U-100 insulin syringes (BD Micro-Fine, Temse, Belgium). For each group of 10 larvae, one syringe and needle was used.
The larvae were then assessed at prespecified time points (16, 24, 32, 48, 64, 72 and 88 h) post inoculation for mortality (defined as a lack of movement in response to tactile stimulus [18]) and melanization [18].
2.5. Concentration of Methenamine Injected
We tested two doses of methenamine-hippurate: 2 g/g and 4 g/g. These doses were based on the MIC result for N. gonorrhoeae, 19.598, and a median larvae liquid volume of 192 µL (based on a median weight of 300 mg) [19]. Based on the formula,
Concin vivo = (Conccompound × 10 µL)/Vollarvae [20,21], this dose should translate into a hemolymph concentration of 104 µg/mL and 208 µg/mL for the 2 g/g and 4 g/g doses, respectively.
One positive control group was included that received the same protocol which consisted of bacterial inoculation (30 μL/larva) followed by 10 μL/larva of ceftriaxone (20 mg/kg; Sigma–Aldrich, St. Louis, MO, USA). One negative control group was included that received the same protocol: bacterial inoculation (30 μL/larva) followed by 10 μL/larva of phosphate-buffered saline (PBS). This dose of ceftriaxone was the same as that used in a previous study that found that this dose of ceftriaxone was effective against N. gonorrhoeae WHO P in G. mellonella [7]. One PBS control group was included. This control group received two injections of PBS: first 30 μL/larva followed by 10 μL/larva.
The surviving and dead G. mellonella, were kept at −80 °C overnight to sedate and kill them. They were then autoclaved at 121 °C for 15 min and disposed of according to local regulations.
2.6. Data Analysis
Statistical analyses and data visualization were conducted using GraphPad PrismV.10® with ANOVA or Mann–Whitney tests to compare groups. A Mantel–Cox test was performed to compare the degree of melanization and mortality rates between groups.
3. Results
3.1. Minimum Inhibitory Concentrations
3.1.1. In Vitro
The MIC of all the isolates was 300 µg/mL (Table 1).
3.1.2. Toxicity
At the doses tested (4, 8 and 12 g/g), methenamine-hippurate did not result in an increase in mortality or melanization of the larvae compared to PBS in the 4 days this was assessed (Figure 2).
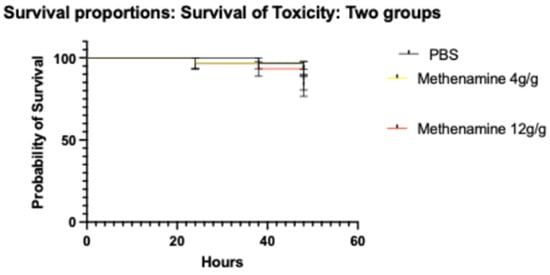
Figure 2.
Survival curves of Galleria mellonella treated with varying doses of methenamine-hippurate (4 to 12 g/g) or PBS (Phosphate Buffered Saline; symbols in each panel represent the mean survival from 3 independent experiments and the error bars represent the standard deviation of the mean).
3.1.3. Mortality
The ceftriaxone group as well as the 2 g/g and 4 g/g methenamine-hippurate groups had a statistically significant increase in survival compared to the no-treatment group (all p ≤ 0.01; Figure 3).
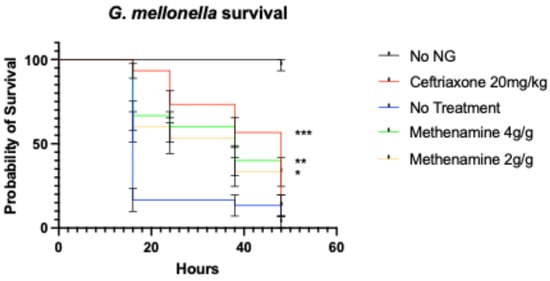
Figure 3.
Survival curves of Galleria mellonella infected with N. gonorrhoeae (NG) and treated with varying doses of methenamine-hippurate, ceftriaxone (20 mg/kg) or PBS (*** p < 0.0001, ** p < 0.001, * p < 0.01; symbols in each panel represent the mean survival from 3 independent experiments and the error bars represent the standard deviation of the mean).
4. Discussion
We found that all the gonococcal isolates had a methenamine-hippurate MIC of 300 mg/L. This MIC was not higher in isolates with higher ceftriaxone MICs. No toxicity of methenamine at the doses tested was found, and doses as low as 2 g/g were effective in the G. mellonella model. While these findings are promising, the efficacy observed in an invertebrate model should be interpreted cautiously. Further validation in mammalian infection models is necessary to assess its clinical relevance.
A single oral dose of 1 g methenamine-hippurate results in a mean urinary concentration of methenamine-hippurate of 792 mg/L within 2 h, increases to 1000 mg/L at 4 h, and then steadily declines to 230 mg/L. Dosing 1 g every 12 h results in a steady state above 500 mg/L [9]. These concentrations are higher than the methenamine MIC and the doses of methenamine assessed in our in vivo model.
These findings provide preliminary evidence that methenamine-hippurate could be used for the treatment of N. gonorrhoeae urethritis. We also found that methenamine is safe. More importantly, the safety of methenamine in humans has been well established in humans in other studies [11]. There are however a number of limitations to our study and this conclusion. As noted above, methenamine could only be used to treat urethritis, which is only one of multiple presentations of N. gonorrhoeae. We only evaluated the effect of methenamine on a relatively limited panel of N. gonorrhoeae isolates and in a single strain in the in vivo model. We did not assess the probability of resistance emerging to methenamine. Interactions with other antimicrobials were also not assessed [22]. We only investigated the in vivo efficacy of methenamine in a G. mellonella model of infection. There are a number of differences between gonococcal infections in this model and humans. These include the fact that the larvae do not have urethras, they have different immune systems, and the pharmacokinetics and pharmacodynamics likely vary [19,20]. The pH of urine varies according to a number of factors such as diet and co-medications [7,11]. Since methenamine requires an acidic pH for conversion to formaldehyde, this can affect its efficacy. Studies of methenamine for other conditions have not found that this has made much of an impact on its clinical efficacy but this would need to be investigated in future studies using methenamine for gonococcal urethritis [8,10].
There is an important need to search for companion antimicrobials to prevent the emergence of gonococcal resistance to ceftriaxone [5]. Methenamine may be useful in this regard. However, in vitro and in vivo interaction assessments should first be performed [23].
5. Conclusions
Methenamine is an interesting urinary antiseptic with a very low propensity to induce AMR. As such, it could be useful to treat gonococcal urethritis alone or in combination with other agents such as ceftriaxone [5]. It is crucial to acknowledge that our results represent early preclinical results. As such, further assessments in mammalian models including humans would be required before methenamine could be considered for clinical use.
Author Contributions
C.K., S.S.M.-B., I.K. and S.A. conceptualized the study. C.K. and S.A. conducted the experiments, and C.K. was responsible for the statistical analyses. C.K. wrote the first draft. Visualization: C.K., S.S.M.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable because the work was limited to G. mellonella.
Data Availability Statement
The data reported in this article is available from the authors on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript
| MIC | Minimum Inhibitory Concentration |
| RCT | Randomized Controlled Trial |
References
- Unemo, M.; Bradshaw, C.S.; Hocking, J.S.; de Vries, H.J.C.; Francis, S.C.; Mabey, D.; Marrazzo, J.M.; Sonder, G.J.B.; Schwebke, J.R.; Hoornenborg, E.; et al. Sexually transmitted infections: Challenges ahead. Lancet Infect. Dis. 2017, 17, 235–279. [Google Scholar] [CrossRef] [PubMed]
- Cyr, S.S.; Barbee, L.; Workowski, K.A.; Bachmann, L.H.; Pham, C.; Schlanger, K.; Torrone, E.; Weinstock, H.; Kersh, E.N.; Thorpe, P. Update to CDC’s treatment guidelines for gonococcal infection. Morb. Mortal. Wkly. Rep. 2020, 69, 1911–1916. [Google Scholar] [CrossRef]
- Unemo, M.; Ross, J.; Serwin, A.B.; Gomberg, M.; Cusini, M.; Jensen, J.S. 2020 European guideline for the diagnosis and treatment of gonorrhoea in adults. Int. J. STD AIDS 2020, 69, 345–351. Available online: https://www.ncbi.nlm.nih.gov/pubmed/33121366 (accessed on 12 March 2025). [CrossRef]
- Alirol, E.; Wi, T.E.; Bala, M.; Bazzo, M.L.; Chen, X.-S.; Deal, C.; Dillon, J.-A.R.; Kularatne, R.; Heim, J.; Huijsduijnen, R.H.v.; et al. Multidrug-resistant gonorrhea: A research and development roadmap to discover new medicines. PLoS Med. 2017, 14, 1002366. Available online: https://www.ncbi.nlm.nih.gov/pubmed/28746372 (accessed on 13 March 2025). [CrossRef] [PubMed]
- Kanesaka, I.; Kong, F.Y.S.; Vanbaelen, T.; Santhini Manoharan-Basil, S.; Kenyon, C. An overview of potential combination therapies with ceftriaxone as a treatment for gonorrhoea. Expert Rev. Anti Infect. Ther. 2025, 23, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Unemo, M. Current and future antimicrobial treatment of gonorrhoea—The rapidly evolving Neisseria gonorrhoeae continues to challenge. BMC Infect. Dis. 2015, 15, 364. Available online: https://www.ncbi.nlm.nih.gov/pubmed/26293005 (accessed on 16 March 2025). [CrossRef]
- Altinoz, M.A.; Ozpinar, A.; Ozpinar, A.; Perez, J.L.; Elmaci, İ. Methenamine’s journey of 160 years: Repurposal of an old urinary antiseptic for treatment and hypoxic radiosensitization of cancers and glioblastoma. Clin. Exp. Pharmacol. Physiol. 2019, 46, 407–412. [Google Scholar] [CrossRef]
- Lo, T.S.; Hammer, K.D.; Zegarra, M.; Cho, W.C. Methenamine: A forgotten drug for preventing recurrent urinary tract infection in a multidrug resistance era. Expert Rev. Anti Infect. Ther. 2014, 12, 549–554. [Google Scholar] [CrossRef]
- Klinge, E.; Männistö, P.; Mäntylä, R.; Lamminsivu, U.; Ottoila, P. Pharmacokinetics of methenamine in healthy volunteers. J. Antimicrob. Chemother. 1982, 9, 209–216. [Google Scholar] [CrossRef]
- Li, J.M.; Cosler, L.E.; Harausz, E.P.; Myers, C.E.; Kufel, W.D. Methenamine for urinary tract infection prophylaxis: A systematic review. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2024, 44, 197–206. [Google Scholar] [CrossRef]
- Mary Ann Liebert, Inc. Final Report on the Safety Assessment of Methenamine. J. Am. Coll. Toxicol. 1992, 11, 531–558. [Google Scholar]
- Gu, C.; Ackerman, A.L. An oldie but a goodie: Methenamine as a nonantibiotic solution to the prevention of recurrent urinary tract infections. PLoS Pathog. 2023, 19, 1011405. [Google Scholar] [CrossRef]
- Vanbaelen, T.; Huis in ‘t Veld, D.; Visser, B.J.; De Baetselier, I.; Van Praet, J.T.; Santhini Manoharan-Basil, S.; Van den Bossche, D.; Kenyon, C. Combination therapy for multidrug-resistant Mycoplasma genitalium infections: A case series. Sex. Transm. Infect. 2025. [Google Scholar] [CrossRef]
- Liu, H.; Taylor, T.H.; Pettus, K.; Trees, D. Assessment of Etest as an alternative to agar dilution for antimicrobial susceptibility testing of Neisseria gonorrhoeae. J. Clin. Microbiol. 2014, 52, 1435–1440. [Google Scholar] [CrossRef]
- Lewis II, J.S.; Weinstein, M.P.; Bobenchik, A.M.; Campeau, S.; Cullen, S.K.; Galas, M.F.; Gold, H.; Humphries, R.M.; Kirn, T.J.; Limbago, B. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Third Informational Supplement, 2022. Clinical and Laboratory Standards Institute. Available online: https://clsi.org/shop/standards/m100/ (accessed on 18 May 2025).
- Hofkens, N.; Gestels, Z.; Abdellati, S.; De Baetselier, I.; Gabant, P.; Martin, A.; Kenyon, C.; Santhini Manoharan-Basil, S. Microbisporicin (NAI-107) protects Galleria mellonella from infection with Neisseria gonorrhoeae. Microbiol. Spectr. 2023, 11, 0282523. [Google Scholar] [CrossRef] [PubMed]
- Gestels, Z.; De Baetselier, I.; Abdellati, S.; Santhini Manoharan-Basil, S.; Kenyon, C. Ramoplanin as a novel therapy for Neisseria gonorrhoeae infection: An in vitro and in vivo study in Galleria mellonella. J. Med. Microbiol. 2024, 73, 001785. [Google Scholar] [CrossRef] [PubMed]
- Dijokaite, A.; Humbert, M.V.; Borkowski, E.; La Ragione, R.M.; Christodoulides, M. Establishing an invertebrate Galleria mellonella greater wax moth larval model of Neisseria gonorrhoeae infection. Virulence 2021, 12, 1900–1920. Available online: https://www.ncbi.nlm.nih.gov/pubmed/34304706 (accessed on 11 February 2025). [CrossRef]
- Andrea, A.; Krogfelt, K.A.; Jenssen, H. Methods and challenges of using the greater wax moth (Galleria mellonella) as a model organism in antimicrobial compound discovery. Microorganisms 2019, 7, 85. [Google Scholar] [CrossRef]
- Serrano, I.; Verdial, C.; Tavares, L.; Oliveira, M. The virtuous Galleria mellonella model for scientific experimentation. Antibiotics 2023, 12, 505. [Google Scholar] [CrossRef] [PubMed]
- Ménard, G.; Rouillon, A.; Cattoir, V.; Donnio, P.-Y. Galleria mellonella as a suitable model of bacterial infection: Past, present and future. Front. Cell Infect. Microbiol. 2021, 11, 782733. [Google Scholar] [CrossRef]
- Wind, C.M.; De Vries, H.J.C.; Van Dam, A.P. Determination of in vitro synergy for dual antimicrobial therapy against resistant Neisseria gonorrhoeae using Etest and agar dilution. Int. J. Antimicrob. Agents 2015, 45, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Pascual, F.; Au, C.; Chikwari, C.D.; Daram, P.; Deal, C.; Espinosa Miranda, A.; Grad, Y.H.; Hook, E.W.; Kittiyaowamarn, R.; Luckey, A.; et al. Recommendations for the optimal introduction of novel antibiotics to treat uncomplicated gonorrhoea in the face of increasing antimicrobial resistance: A case study with zoliflodacin. BMC Glob. Public Health 2024, 2, 58. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).