Secondary Syphilis: Pathophysiology, Clinical Manifestations, and Diagnostic Testing
Abstract
:1. Syphilis: More Than just an STI
2. Pathogenesis of Secondary Syphilis
3. Clinical Features of Secondary Syphilis
4. Testing for Syphilis
4.1. Serological Assays
4.2. Morphological Testing
4.3. Nucleic Acid Amplification Testing
5. Conclusions
6. Methodology
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsuboi, M.; Evans, J.; Davies, E.P.; Rowley, J.; Korenromp, E.L.; Clayton, T.; Chico, R.M. Prevalence of syphilis among men who have sex with men: A global systematic review and meta-analysis from 2000–20. Lancet Glob. Health 2021, 9, e1110–e1118. [Google Scholar] [CrossRef] [PubMed]
- Office of Disease Prevention and Health Promotion. Reduce the Syphilis Rate in Females—STI 03. Healthy People 2030. U.S. Department of Health and Human Services. 2023. Available online: https://health.gov/healthypeople/objectives-and-data/browse-objectives/sexually-transmitted-infections/reduce-syphilis-rate-females-sti-03 (accessed on 3 January 2023).
- Office of Disease Prevention and Health Promotion. Reduce the Syphilis Rate in Men Who Have Sex with Men—STI 05. Healthy People 2030. U.S. Department of Health and Human Services. 2023. Available online: https://health.gov/healthypeople/objectives-and-data/browse-objectives/sexually-transmitted-infections/reduce-syphilis-rate-men-who-have-sex-men-sti-05 (accessed on 3 January 2023).
- CDC. Preliminary 2021 STD Surveillance Data. 2022. Available online: https://www.cdc.gov/std/statistics/2021/default.htm (accessed on 3 January 2023).
- Wright, S.S.M.; Kreisel, K.M.; Hitt, J.C.M.; Pagaoa, M.A.M.; Weinstock, H.S.M.; Thorpe, P.G.M. Impact of the COVID-19 Pandemic on Centers for Disease Control and Prevention–Funded Sexually Transmitted Disease Programs. Sex. Transm. Dis. 2022, 49, e61–e63. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on Prevention and Control of Sexually Transmitted Infections in the United States. Sexually Transmitted Infections: Adopting a Sexual Health Paradigm; Crowley, J.S., Geller, A.B., Vermund, S.H., Eds.; National Academies Press: Washington, DC, USA, 2021. [CrossRef]
- Beale, M.A.; Marks, M.; Cole, M.J.; Lee, M.K.; Pitt, R.; Ruis, C.; Thomson, N.R. Global phylogeny of Treponema pallidum lineages reveals recent expansion and spread of contemporary syphilis. Nat. Microbiol. 2021, 6, 1549–1560. [Google Scholar] [CrossRef]
- Mao, C.; Wang, L.; Li, L.M. Historical perspective of progress and achievement on epidemiology in the past 70 years in China. Chin. J. Endem. 2019, 40, 1173–1179. [Google Scholar] [CrossRef]
- Gong, X.D.; Yue, X.L.; Teng, F.; Jiang, N.; Men, P.X. Syphilis in China from 2000 to 2013: Epidemiological trends and characteristics. Chin. J. Dermatol. 2014, 47, 310–315. Available online: https://pesquisa.bvsalud.org/portal/resource/pt/wpr-447025 (accessed on 3 January 2023).
- Kidd, S.; Torrone, E.; Su, J.; Weinstock, H. Reported Primary and Secondary Syphilis Cases in the United States: Implications for HIV Infection. Sex. Transm. Dis. 2018, 45, S42–S47. [Google Scholar] [CrossRef]
- Adawiyah, R.; Saweri, O.P.M.; Boettiger, D.C.; Applegate, T.L.; Probandari, A.; Guy, R.; Guinness, L.; Wiseman, V. The costs of scaling up HIV and syphilis testing in low- and middle-income countries: A systematic review. Heal. Policy Plan. 2021, 36, 939–954. [Google Scholar] [CrossRef] [PubMed]
- Remington, J.S. Infectious Diseases of the Fetus and Newborn Infant, 7th ed; Saunders/Elsevier: Philadelphia, PA, USA, 2011. [Google Scholar]
- WHO. Data on Syphilis. World Health Organization. 2020. Available online: https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/data-on-syphilis (accessed on 3 January 2023).
- Tiecco, G.; Degli Antoni, M.; Storti, S.; Marchese, V.; Focà, E.; Torti, C.; Castelli, F.; Quiros-Roldan, E. A 2021 Update on Syphilis: Taking Stock from Pathogenesis to Vaccines. Pathogens 2021, 10, 1364. [Google Scholar] [CrossRef]
- Mahmud, S.; Mohsin, M.; Muyeed, A.; Hossain, S.; Islam, M.M.; Islam, A. Prevalence of HIV and Syphilis and Their Co-Infection among Men Having Sex with Men in Asia: A Systematic Review and Meta-Analysis. medRxiv 2021, 12, 21.21268191. Available online: https://www.medrxiv.org/content/10.1101/2021.12.21.21268191v1 (accessed on 3 January 2023).
- Sarigül, F.; Sayan, M.; Inan, D.; Deveci, A.; Ceran, N.; Çelen, M.K.; Çağatay, A.; Özdemir, H.; Kuşcu, F.; Karagöz, G.; et al. Current status of HIV/AIDS-syphilis co-infections: A retrospective multicentre study. Central Eur. J. Public Heal. 2019, 27, 223–228. [Google Scholar] [CrossRef] [Green Version]
- Klausner, J.D. The great imitator revealed: Syphilis. Top. Antivir. Med. 2019, 27, 71–74. [Google Scholar]
- Salazar, J.C.; Cruz, A.R.; Pope, C.D.; Valderrama, L.; Trujillo, R.; Saravia, N.G.; Radolf, J.D. Treponema pallidumElicits Innate and Adaptive Cellular Immune Responses in Skin and Blood during Secondary Syphilis: A Flow-Cytometric Analysis. J. Infect. Dis. 2007, 195, 879–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LaFond, R.E.; Lukehart, S.A. Biological Basis for Syphilis. Clin. Microbiol. Rev. 2006, 19, 29–49. [Google Scholar] [CrossRef] [Green Version]
- Peeling, R.W.; Mabey, D.; Kamb, M.L.; Chen, X.-S.; Radolf, J.D.; Benzaken, A.S. Primer: Syphilis. Nat. Rev. Dis. Prim. 2017, 3, 17073. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, R.; Cervantes, J.; Hawley, K.L.; Cruz, A.R.; Babapoor, S.; Murphy, M.; Dadras, S.S.; Salazar, J.C. Inflammation and immune evasion coexist in Treponema pallidum–infected skin. JAAD Case Rep. 2018, 4, 462–464. [Google Scholar] [CrossRef]
- Cruz, A.R.; Ramirez, L.G.; Zuluaga, A.V.; Pillay, A.; Abreu, C.; Valencia, C.A.; Vake, C.L.; Cervantes, J.L.; Dunham-Ems, S.; Cartun, R.; et al. Immune Evasion and Recognition of the Syphilis Spirochete in Blood and Skin of Secondary Syphilis Patients: Two Immunologically Distinct Compartments. PLoS Negl. Trop. Dis. 2012, 6, e1717. Available online: https://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0001717 (accessed on 3 January 2023). [CrossRef] [PubMed]
- Gisondi, P.; Bellinato, F.; Girolomoni, G.; Albanesi, C. Pathogenesis of Chronic Plaque Psoriasis and Its Intersection with Cardio-Metabolic Comorbidities. Front. Pharmacol. 2020, 11, 117. [Google Scholar] [CrossRef]
- Rendon, A.; Schäkel, K. Psoriasis pathogenesis and treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef] [Green Version]
- Baughn, R.E.; Musher, D.M. Secondary Syphilitic Lesions. Clin. Microbiol. Rev. 2005, 18, 205–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wormser, G.P. Hematogenous dissemination in early Lyme disease. Wien. Klin. Wochenschr. 2006, 118, 634–637. [Google Scholar] [CrossRef]
- Yang, W.-J.; Hu, H.-H.; Yang, Y.; Li, J.-H.; Guo, H. Unusual erythematous plaque with white scales, a case of acquired syphilis in a child and literature review. BMC Infect. Dis. 2021, 21, 1–6. [Google Scholar] [CrossRef]
- Emma, C.; Andre, F.; Andrei, B. Secondary syphilis. Clevel. Clin. J. Med. 2017, 84, 510–511. [Google Scholar] [CrossRef]
- Krishnaswamy, M.; Arunprasath, P.; Rai, R. Synchronous primary and secondary syphilis—An uncommon presentation. Indian J. Sex. Transm. Dis. AIDS 2021, 42, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Hira, S.K.; Patel, J.S.; Bhat, S.G.; Chilikima, K.; Mooney, N. Clinical Manifestations of Secondary Syphilis. Int. J. Dermatol. 1987, 26, 103–107. [Google Scholar] [CrossRef]
- Chung, H.J.; Marley-Kemp, D.; Keller, M. Rupioid psoriasis and other skin diseases with rupioid manifestations. Cutis 2014, 94, 119–121. Available online: https://www.ncbi.nlm.nih.gov/pubmed/25279472 (accessed on 3 January 2023).
- Liu, J.-W.; Ma, D.-L. Oyster Shell-Like Skin Lesions in a Young Man. Mayo Clin. Proc. 2021, 96, 1120–1121. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Che, J.; Song, J.; Duan, X.; Yang, J. Annular rupioid secondary syphilis confined to the face. Int. J. Infect. Dis. 2022, 122, 644–646. [Google Scholar] [CrossRef]
- Ehlers, S.; Sergent, S.; Ashurst, J. Secondary Syphilis. Clin. Pract. Cases Emerg. Med. 2020, 4, 675–676. [Google Scholar] [CrossRef]
- Pourang, A.; Fung, M.A.; Tartar, D.; Brassard, A. Condyloma lata in secondary syphilis. JAAD Case Rep. 2021, 10, 18–21. [Google Scholar] [CrossRef]
- Tayal, S.; Shaban, F.; Dasgupta, K.; Tabaqchali, M.A. A case of syphilitic anal condylomata lata mimicking malignancy. Int. J. Surg. Case Rep. 2015, 17, 69–71. [Google Scholar] [CrossRef] [Green Version]
- Zawar, V.; Chuh, A. Mucous patches and arthralgia. J. R. Soc. Med. 2004, 97, 79–80. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, P.L.F.; Silva, S.J.P.; Fonseca, O.M.T.; Francisco, D.A.; Darceny, Z.-B. Oral Manifestations of Secondary Syphilis. Int. J. Infect. Dis. 2015, 35, 40–42. [Google Scholar] [CrossRef] [Green Version]
- Mindel, A.; Tovey, S.J.; Timmins, D.J.; Williams, P. Primary and secondary syphilis, 20 years’ experience. 2. Clinical features. Sex. Transm. Infect. 1989, 65, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Bi, M.Y.; Cohen, P.R.; Robinson, F.W.; Gray, J.M. Alopecia syphi- litica-report of a patient with secondary syphilis presenting as moth-eaten alopecia and a review of its common mimickers. Dermatol. Online J. 2009, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Cuozzo Daniel, W.; Benson Paul, M.; Sperling Leonard, C.; Skelton Henry, G. Essential syphilitic alopecia revisited. J. Am. Acad. Dermatol. 1995, 32 (5 PART 2), 840–843. [Google Scholar] [CrossRef]
- Paulraj, S.; Kumar, P.A.; Gambhir, H.S. Eyes As the Window to Syphilis: A Rare Case of Ocular Syphilis As the Initial Presentation of Syphilis. Cureus 2020, 12, e6998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, X.; Gao, Y.; Yan, Y.; Marks, M.; Zhu, L.; Lu, H.; Guan, Z.; Shi, M.; Ni, L.; Peng, R.; et al. The importance of proper and prompt treatment of ocular syphilis: A lesson from permanent vision loss in 52 eyes. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1569–1578. [Google Scholar] [CrossRef]
- Zhang, X.; Du, Q.; Ma, F.; Lu, Y.; Wang, M.; Li, X. Characteristics of syphilitic uveitis in northern China. BMC Ophthalmol. 2017, 17, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaza, H.; Tyagi, M.; Pathengay, A.; Agrawal, H.; Behera, S.; Lodha, D.; Pappuru, R.R.; Basu, S.; Murthy, S. Clinical manifestations and outcomes of ocular syphilis in Asian Indian population: Analysis of cases presenting to a tertiary referral center. Indian J. Ophthalmol. 2020, 68, 1881–1886. [Google Scholar] [CrossRef]
- Shah, J.; Lingiah, V.; Niazi, M.; Olivo-Salcedo, R.; Pyrsopoulos, N.; Galan, M.; Shahidullah, A. Acute Liver Injury as a Manifestation of Secondary Syphilis. ACG Case Rep. J. 2021, 8, e00668. [Google Scholar] [CrossRef]
- Al Dallal, H.A.; Narayanan, S.; Alley, H.F.; Eiswerth, M.J.; Arnold, F.W.; Martin, B.A.; Shandiz, A.E. Case Report: Syphilitic Hepatitis–A Rare and Underrecognized Etiology of Liver Disease with Potential for Misdiagnosis. Front. Med. 2021, 8, 789250. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.V.; Thornton, G.F.; Conn, H.O. Liver Disease Associated with Secondary Syphilis. New Engl. J. Med. 1971, 284, 1423–1425. [Google Scholar] [CrossRef]
- Alemam, A.; Ata, S.; Shaikh, D.; Leuzzi, B.; Makker, J. Syphilitic Hepatitis: A Rare Cause of Acute Liver Injury. Cureus 2021, 13, e14800. [Google Scholar] [CrossRef]
- Ao, X.; Chen, J.H.; Kata, P.; Kanukuntla, A.; Bommu, V.; Rothberg, M.; Cheriyath, P. The Great Impostor Did It Again: Syphilitic Arthritis. Cureus 2021, 13, e17344. [Google Scholar] [CrossRef]
- Williams, W.C.; Marion, G.S. Secondary syphilis presenting with arthritis, hepatitis, and glucose intolerance. J. Fam. Pr. 1987, 25, 509–511. [Google Scholar]
- Seña, A.C.; Zhang, X.-H.; Li, T.; Zheng, H.-P.; Yang, B.; Yang, L.-G.; Salazar, J.C.; Cohen, M.S.; Moody, M.A.; Radolf, J.D.; et al. A systematic review of syphilis serological treatment outcomes in HIV-infected and HIV-uninfected persons: Rethinking the significance of serological non-responsiveness and the serofast state after therapy. BMC Infect. Dis. 2015, 15, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, K.; Shen, X.; Lin, Y.; Zhu, X.-Z.; Lin, L.-R.; Tong, M.-L.; Xiao, Y.; Zhang, H.-L.; Liang, X.-M.; Niu, J.-J.; et al. Origin of Nontreponemal Antibodies During Treponema pallidum Infection: Evidence From a Rabbit Model. J. Infect. Dis. 2018, 218, 835–843. [Google Scholar] [CrossRef]
- Morshed, M.G.; Singh, A.E. Recent Trends in the Serologic Diagnosis of Syphilis. Clin. Vaccine Immunol. 2014, 22, 137–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuddenham, S.; Katz, S.S.; Ghanem, K.G. Syphilis Laboratory Guidelines: Performance Characteristics of Nontreponemal Antibody Tests. Clin. Infect. Dis. 2020, 71, S21–S42. [Google Scholar] [CrossRef]
- Lin, L.-R.; Zhu, X.-Z.; Liu, D.; Liu, L.-L.; Tong, M.-L.; Yang, T.-C. Are nontreponemal tests suitable for monitoring syphilis treatment efficacy? Evidence from rabbit infection models. Clin. Microbiol. Infect. 2020, 26, 240–246. [Google Scholar] [CrossRef]
- Marra, C.M.; Tantalo, L.C.; Maxwell, C.L.; Ho, E.L.; Sahi, S.K.; Jones, T. The Rapid Plasma Reagin Test Cannot Replace the Venereal Disease Research Laboratory Test for Neurosyphilis Diagnosis. Sex. Transm. Dis. 2012, 39, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Park, I.U.; Tran, A.; Pereira, L.; Fakile, Y. Sensitivity and Specificity of Treponemal-specific Tests for the Diagnosis of Syphilis. Clin. Infect. Dis. 2020, 71, S13–S20. [Google Scholar] [CrossRef]
- Binnicker, M.J.; Jespersen, D.J.; Rollins, L.O. Treponema-Specific Tests for Serodiagnosis of Syphilis: Comparative Evaluation of Seven Assays. J. Clin. Microbiol. 2011, 49, 1313–1317. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Xie, Y.; Xiao, Y. Laboratory Diagnostic Tools for Syphilis: Current Status and Future Prospects. Front. Cell. Infect. Microbiol. 2021, 10, 574806. [Google Scholar] [CrossRef]
- Henao-Martínez, A.F.; Johnson, S.C. Diagnostic tests for syphilis: New tests and new algorithms. Neurol. Clin. Pr. 2014, 4, 114–122. [Google Scholar] [CrossRef] [Green Version]
- Wolgemuth, C.W. Flagellar motility of the pathogenic spirochetes. Semin. Cell Dev. Biol. 2015, 46, 104–112. [Google Scholar] [CrossRef] [Green Version]
- Ito, F.; Hunter, E.F.; George, R.W.; Pope, V.; A Larsen, S. Specific immunofluorescent staining of pathogenic treponemes with a monoclonal antibody. J. Clin. Microbiol. 1992, 30, 831–838. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Xie, Y.; Xu, M.; Liu, S.; Jiang, C.; Zhao, F.; Zeng, T.; Liu, Z.; Yu, J.; Wu, Y. Development and Evaluation of a Loop-Mediated Isothermal Amplification Assay for the Detection of Treponema pallidum DNA in the Peripheral Blood of Secondary Syphilis Patients. Am. J. Trop. Med. Hyg. 2017, 97, 1673–1678. [Google Scholar] [CrossRef] [Green Version]
- Grange, P.A.; Jary, A.; Isnard, C.; Burrel, S.; Boutolleau, D.; Touati, A.; Bébéar, C.; Saule, J.; Martinet, P.; Robert, J.-L.; et al. Use of a Multiplex PCR Assay To Assess the Presence of Treponema pallidum in Mucocutaneous Ulcerations in Patients with Suspected Syphilis. J. Clin. Microbiol. 2021, 59, e01994-20. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, Y.; Liu, B.; Wang, Y.; Gong, W.; Qian, Y.; Zhou, P. Sensitive detection of Treponema pallidum DNA from the whole blood of patients with syphilis by the nested PCR assay. Emerg. Microbes Infect. 2018, 7, 83. [Google Scholar] [CrossRef] [Green Version]
- Satyaputra, F.; Hendry, S.; Braddick, M.; Sivabalan, P.; Norton, R. The Laboratory Diagnosis of Syphilis. J. Clin. Microbiol. 2021, 59, e00100-21. [Google Scholar] [CrossRef]
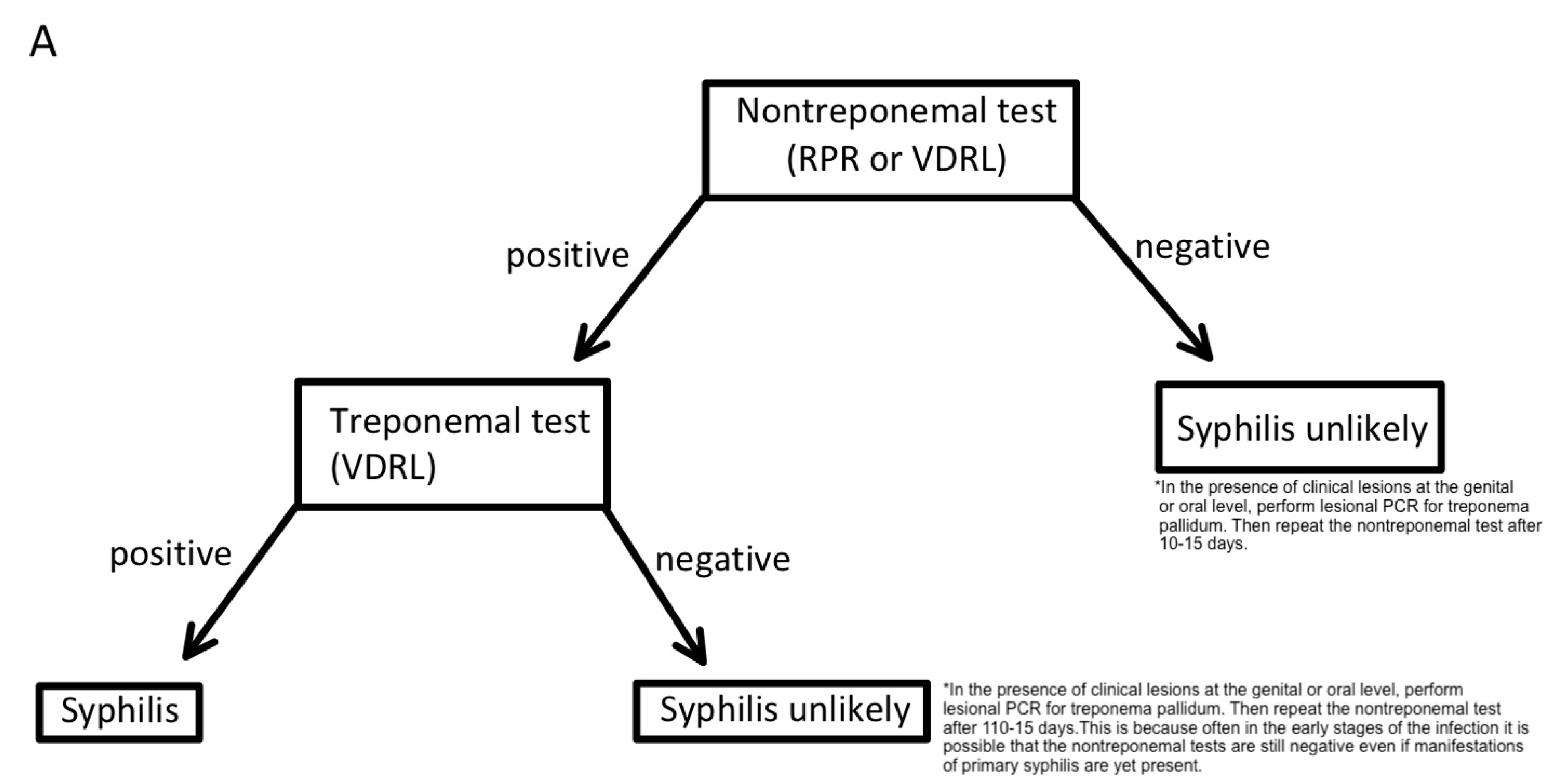
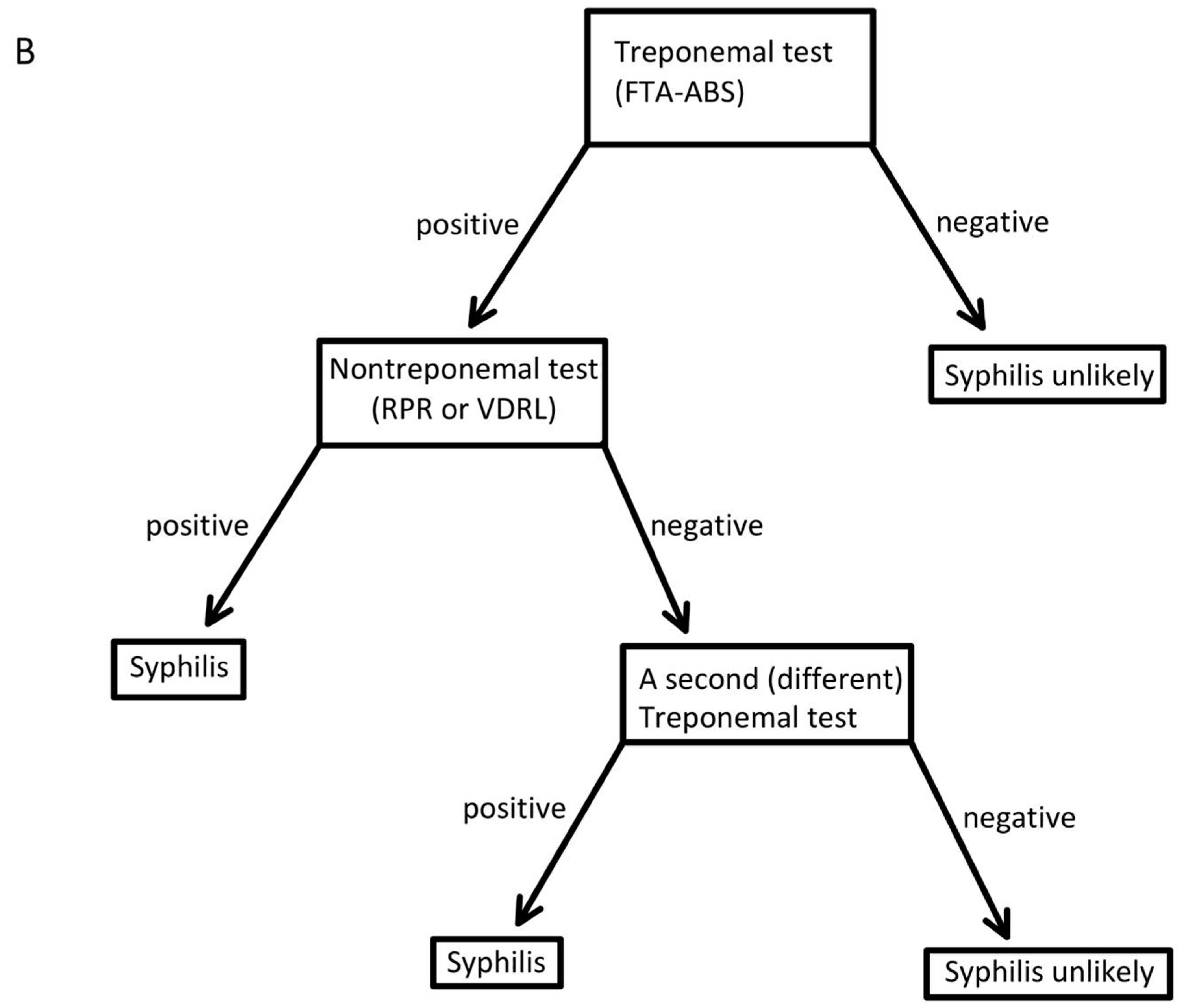
| Test | Sensitivity | Specificity | Reference |
|---|---|---|---|
| Nontreponemal tests | |||
| VDRL | 100% | 96–99% | [67] |
| RPR | 100% | 98% | [67] |
| Treponemal tests | |||
| FTA-ABS | 92.8–100% | 87.0–100% | [58] |
| MHA-TP | 90–100% | 90–100% | [58] |
| TP-PA | 100% | 99.6–100% | [58] |
| EIA | 96.9% | 94.7% | [59] |
| Nucleic acid amplification test from Genital swabs | |||
| mPCR | 79% | 99.2% | [65] |
| nPCR | 88% | 100% | [22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaudhry, S.; Akinlusi, I.; Shi, T.; Cervantes, J. Secondary Syphilis: Pathophysiology, Clinical Manifestations, and Diagnostic Testing. Venereology 2023, 2, 65-75. https://doi.org/10.3390/venereology2020006
Chaudhry S, Akinlusi I, Shi T, Cervantes J. Secondary Syphilis: Pathophysiology, Clinical Manifestations, and Diagnostic Testing. Venereology. 2023; 2(2):65-75. https://doi.org/10.3390/venereology2020006
Chicago/Turabian StyleChaudhry, Shahrukh, Idris Akinlusi, Ted Shi, and Jorge Cervantes. 2023. "Secondary Syphilis: Pathophysiology, Clinical Manifestations, and Diagnostic Testing" Venereology 2, no. 2: 65-75. https://doi.org/10.3390/venereology2020006
APA StyleChaudhry, S., Akinlusi, I., Shi, T., & Cervantes, J. (2023). Secondary Syphilis: Pathophysiology, Clinical Manifestations, and Diagnostic Testing. Venereology, 2(2), 65-75. https://doi.org/10.3390/venereology2020006






