Abstract
Mangrove ecosystems are renowned for their rich fungal diversity, housing a plethora of multicellular fungi and yeasts. In this investigation, we examined the yeast diversity associated with various compartments (rhizospheric soil, stems, roots, leaves, barks, and flowers) of the widely distributed mangrove tree, Avicennia officinalis, from the Kumbalam and Puthuvype mangroves in central Kerala, India. Our study revealed that the yeast strains were not uniformly distributed in various compartments. The highest abundance of yeasts was found in leaves (42%), followed by sediment (21%), and the lowest in flowers (5%). Among the 45 isolates, 27% comprised red yeasts. Dominant genera included Rhodotorula (27.5%), Debaryomyces (17.6%), Kluyveromyces (5.9%), Cryptococcus (9.8%), and Candida (7.8%), while genera such as Geotrichum, Lodderomyces, Ogataea, Galactomyces, and Saitozyma were represented by single isolates. Certain yeast species, such as C. tropicalis and Rhodotorula paludegina, exhibited a cosmopolitan distribution in various plant compartments of A. officinalis. An analysis of the proximate composition of different plant compartments of A. officinalis revealed variations in C, N, S, H, Ca, K, and the C/N ratio. Interestingly, these variations were positively correlated with the yeast community composition, suggesting a potential role of the elemental composition of plants in shaping the yeast biome of A. officinalis. However, our understanding of the inter-relationships among yeast communities in different plant compartments remains limited, highlighting the need for further comprehensive investigations in this field.
1. Introduction
Plants coexist with a wide variety of microorganisms, collectively referred to as the plant microbiome [1]. Studies from the last decade have unveiled highly intricate microbial communities linked with various plants and specific plant parts, and they have important roles in plant growth and health [2,3]. Plant microbiota comprise different types of organisms including archaea, bacteria, and fungi [2]. Most of the studies have focused on bacterial and fungal biomes because of the abundance of information on these organisms and the industry’s interest in them.
The composition of plant microbiomes is determined by various plant-related factors, such as the genotype, organ type, species, and health, as well as environmental factors such as land use, climate, and nutrient availability [4]. Additionally, the distinct microbial communities found in plant organs are influenced by plant genotype, nutrient availability, and prevailing environmental conditions in the above and underground plant parts [5,6]. Plant and soil carbon (C) and nitrogen (N) levels were influential in altering bacterial and fungal communities [7]. Similarly, factors like the carbon-to-nitrogen ratio (C/N) and the availability of phosphorus (P) and potassium (K) are key in shaping the root microbial community. Thus, the elemental composition of plant–soil systems, along with their potential threshold ratios, plays a crucial role in determining the functioning and dynamics of microorganisms [4].
Understanding the diversity of mangrove fungi, including manglicolous yeasts, is essential for comprehending their ecological roles and interactions (saprophytic, mutualistic, commensal, or parasitic) with mangrove plants [8,9]. Mangroves provide yeast species with a promising space for colonization and survival. Yeasts can associate with the plant phyllosphere and rhizosphere [10]. The immigration of the phylloplane yeasts depends upon the leaf characteristics as well as the compounds present in it [11]. Community assembly in the stem/bark is based on stochastic colonization (e.g., due to wind, rain, or insects), selection by the host plant, and several factors, such as nutrients, temperature, and the metabolic activity of yeasts [12].
However, there is very limited information on the association and influence of the composition of mangrove plant parts on the diversity of microbes, especially yeasts it harbors. Thus, the inter-relationships of yeast communities in different plant compartments are still poorly understood. To improve this understanding, this paper focuses on identifying the plant elemental composition’s influence on the diversity of yeasts associated with the mangrove tree species A. officinalis found in the Puthuvype and Kumbalam mangrove forests.
2. Materials and Methods
2.1. Yeast Species Used for Study
The yeast strains were isolated from plant parts, including leaves, roots, stems, bark, and flowers, and rhizospheric sediments of the mangrove species Avicennia officinalis found in Puthuvype and Kumbalam using the spread plate technique [13]. The morphological distinct strains were identified, and details of the isolated strains are available in the paper [13].
2.2. Sample Preparation
The representative samples of the plant parts, such as sediment, roots, leaves, stems, bark, and flowers of both A. officinalis were collected in triplicates. The samples were washed with deionized water to remove any surface impurities. Then, the samples were air-dried thoroughly to remove excess moisture. The samples were air-dried at 30 °C (to avoid loss of N) and ground into a fine powder using a mortar and pestle and a mechanical grinder (Figure 1). It was ensured that the grinding equipment was clean and free from any residues that could contaminate the samples. Clean spatulas or scoops were used to transfer the samples into appropriate containers for analysis, avoiding cross-contamination between samples. To prevent moisture adsorption, these samples were kept in screw-capped bottles. The containers were labeled clearly with relevant sample information to ensure proper identification during analysis.
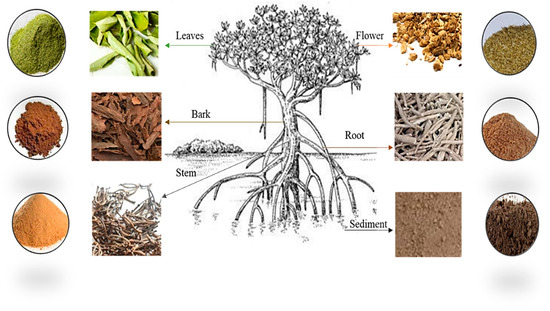
Figure 1.
Sample preparation.
2.2.1. Elemental Composition of Plant Parts
The samples were powdered to talc grade and were subsequently analyzed using a CHNS analyzer (VarioEL III- Sophisticated Test and Instrumentation Centre (STIC)-CUSAT-Kochi, India). The elemental composition (i.e., carbon (C), hydrogen (H), nitrogen (N), and sulfur (S)) was expressed in percentages (%).
2.2.2. Determination of Sodium, Potassium, and Calcium
Standard solutions containing known sodium, potassium, and calcium concentrations were prepared. The instrument was calibrated using the prepared standard solutions. The sample solution was introduced into the flame of the flame photometer (Systronics—Model 1027, Ahmedabad, India). The emitted light intensity was measured at 589.0 nm, 766.5 nm, and 422.7 nm for sodium (Control—NaCl), potassium (Control—KCl), and calcium (Control—CaCO3) ions, respectively, using a flame photometer. The concentration of test ions in the sample was determined using the calibration curve.
2.3. Statistical Analysis
Diversity indices, such as the Shannon–Weiner diversity index, of yeast species were analyzed using PRIMER 7 [14]. Principal Component Analysis (PCA) was used to investigate the relationship between yeast diversity and the elemental composition of plant parts using PRIMER 7.
3. Results and Discussion
3.1. Diversity of Yeast Associated with A. officinalis
The knowledge about the influence of plant elemental composition on the diversity of yeasts associated with it is limited. This study aimed to elucidate the potential roles of plant elemental composition and its impact on the distribution of yeasts within mangrove vegetation. From Avicennia officinalis found in the Kumbalam mangrove, 19 yeast strains were isolated, whereas from the Puthuvype station, 26 strains were isolated. These yeasts have been previously identified, and accession numbers were obtained from the GenBank [13].
In both the stations a significant variation in the distribution of yeast species in the different plant parts of A. officinalis was observed. In the Kumbalam station, 40% of the yeast isolates were from leaves. Among the isolates from the leaves 40% were C. tropicalis followed by Rhodosporidiobolus sp. (12.5%), R. paludegina (12.5%), K. natalensis (12.5%), and K. siamensis (12.5%). In the root and sediment only C. tropicalis was present, whereas in the stem, 67% of the isolates were C. tropicalis which was followed by 33% of R. mucilaginosa. In the bark, only Rhodosporidiobolus sp., and in flower only R. mucilaginosa, were noted (Figure 2A).
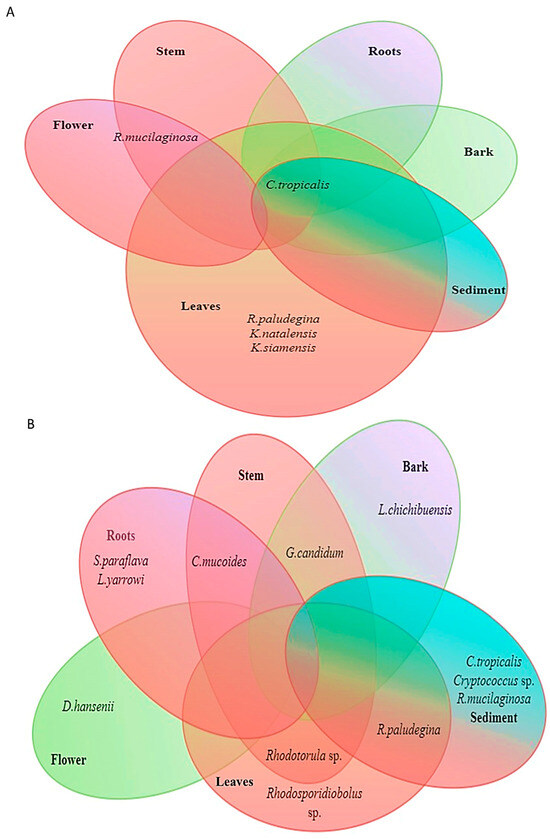
Figure 2.
Diversity of yeasts associated with mangrove plant parts of A. officinalis in two stations: (A) Kumbalam and (B) Puthuvype.
In the case of the yeast isolated from the leaves of A. officinalis from Puthuvype, 50% of the isolates were R. paludegina followed by Rhodotorula sp. (33%) and Rhodosporidiobolus sp. (17%). In the roots, isolates observed were S. paraflava (33%), L. yarrowi (33%), and C. mucoides (33%). In the stem, 50% of the isolates were G. candidum followed by Rhodotorula sp. (25%) and C. mucoides (25%). In bark, 50% of the yeast isolates were G. candidum and the other 50% were L. chichibuensis. From the flower, only D. hansenii was isolated. Among the yeast isolates from the rhizospheric sediment 44% belonged to C. tropicalis which was followed by Cryptococcus sp. (22%), R. mucilaginosa (22%), and R. paludegina (11%) (Figure 2B).
The Shannon–Wiener diversity index (H′ (log2) = 1.49) was highest in the Avicennia officinalis from the Puthuvype station. This study also revealed that among the plant parts the highest Shannon–Wiener diversity index (H′ (log2) = 2.0) was observed in the leaves of the Avicennia officinalis sampled from Kumbalam, while a diversity index of 1.84 was recorded in the sediment from Puthuvype (Table 1).

Table 1.
Diversity indices of manglicolous yeasts associated with A. officinalis.
3.2. Variability of Plant Elemental Composition Among Mangrove Plant Compartments
The analysis of the elemental content in different plant parts of Avicennia officinalis across the Kumbalam and Puthuvype stations revealed considerable spatial and compositional variability (Figure 3). The carbon content ranged from 1.14% to 29.25%, with leaves generally exhibiting the highest concentrations, while flowers showed the lowest in both stations.
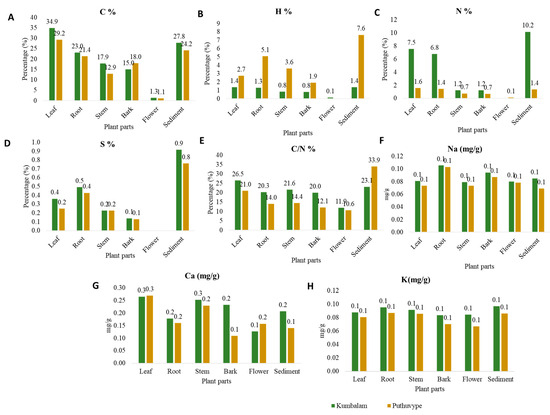
Figure 3.
Variation in elemental composition of various mangrove plant compartments of A. officinalis found in two stations (A) carbon, (B) hydrogen, (C) nitrogen, (D) sulfur, (E) C/N, (F) Na, (G) Ca, and (H) K.
Compared to the Kumbalam station, the hydrogen content was higher at the Puthuvype station, with sediments showing the highest values, while it was undetectable in flower samples. In contrast, the nitrogen content was highest at the Kumbalam station, ranging from 1.21% to 10.18%, with sediments exhibiting the highest values, while it remained undetectable in flower samples. These variations in elemental composition (C and N) were further emphasized by the C/N ratio analysis, which revealed significant differences among plant parts, with sediments frequently displaying the highest ratios. The sulfur content was predominantly high in sediments, while bark exhibited the lowest. However, the levels of sulfur were undetectable in flowers from both stations.
In the case of sodium (p = 0.215) and potassium (p = 0.154) ions, they did not significantly vary between the plant parts of the Avicennia officinalis sampled from both Kumbalam and Puthuvype. It also reveals that the mangrove species maintains ionic homeostasis across its tissues despite external salinity fluctuations. This stable ion regulation is crucial for mangrove-associated microbial communities, including osmotolerant yeasts, which must adapt to saline conditions. Glycerol-3-phosphate dehydrogenase (Gpd1) in osmotolerant yeasts facilitates glycerol biosynthesis, a key osmo-protectant that helps maintain cellular turgor and enzyme function under high-salinity stress [15,16]. The calcium content was significantly higher in leaves, followed by stems in both stations. These findings collectively highlight the adaptive strategies of A. officinalis in elemental uptake and distribution, as influenced by environmental conditions and plant physiology. The values of the limit of detection (LOD) and limit of quantification (LOQ) of the different analytes are as follows: sodium (0.001 LOD, 0.003 LOQ), potassium (0.01 LOD, 0.03 LOQ), and calcium (0.02 LOD, 0.06 LOQ). For all of these ions, the relationship between the peak area (y) and concentration of analyte (x) was found to be linear with determination coefficients above 0.999.
Multivariate Principal Component Analysis (PCA) of the relationship between the plant elemental composition and diversity of yeasts associated with A. officinalis found in Kumbalam and Puthuvype revealed that 93% of the total variance within the data set could be accounted for by the first two principal components, PC1 and PC2 (Figure 4). The proportion of the total variance captured by PC1 and PC2 is indicated on the axes. For example, PC1 explained 82% of the variance, while PC2 explained 11%, capturing 93% of the total variance. The direction and length of vectors show the influence of specific environmental parameters. Parameters pointing in the same direction are positively correlated, while those pointing in opposite directions are negatively correlated. Clusters of points may reveal patterns of diversity in relation to environmental gradients. For instance, samples with similar environmental parameters may exhibit similar levels of diversity. In the present figure (Figure 4), in both samples flowers clustered together, whereas the sediment in both sets of samples showed some dissimilarities. Points that are distant from others might be outliers, indicating unique environmental conditions or diversity profiles. In the present figure, we can consider flowers to be the outliers. It was clear that C/N% and C% are the major plant parameters that influence the diversity and distribution of yeasts associated with the mangrove species A. officinalis. It was also clear that the diversity found in the flowers of A. officinalis was least influenced by the elemental composition, as they were outliers.
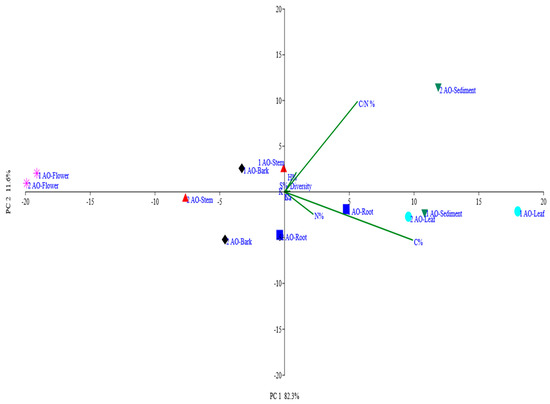
Figure 4.
Principal Component Analysis plot showing relationship between elemental composition of A. officinalis in 2 stations and yeast diversity.
4. Conclusions
This paper highlights the significant influence of the plant elemental composition on yeast establishment within the different compartments of mangrove plants, namely Avicennia officinalis. Significant variations were observed in the content of carbon (C), hydrogen (H), nitrogen (N), sulfur (S), calcium (Ca), and potassium (K) across the plant compartments of A. officinalis found in both stations. However, the sodium (Na) and potassium (K) content showed no significant variations. The PCA further highlighted the role of macronutrients in determining yeast diversity, in all plant compartments except the flower and bark. These findings suggest that the elemental composition of mangrove plant species plays a crucial role in shaping the yeast communities associated with them.
Author Contributions
Study conception and design: K.A.S.N., K.M. Material preparation and analysis: K.A.S.N. Data collection: K.A.S.N. PCA plot: J.V., K.A.S.N. wrote the draft of the manuscript. K.M. edited and approved the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by a grant from the Kerala University of Fisheries and Ocean Studies. KUFOS KARP, DoR (1)/5587/2021.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data underlying this article are available in the article.
Acknowledgments
This work was supported by the Kerala University of Fisheries and Ocean Studies aided research project (KARP) scheme, Order No: DoR (1)/5587/2021. The authors K Nimsi and K Manjusha, would also like to acknowledge the support rendered by S. Suresh Kumar, The Dean of the Faculty of Ocean Science and Technology, Kerala University of Fisheries and Ocean Studies. Jasna Vijayan grateful to the UGC Kothari Post Doctoral fellowship (DSKPDF—UGC-BL/20-21/0310).
Conflicts of Interest
No conflicts of interest.
References
- Dastogeer, K.M.; Tumpa, F.H.; Sultana, A.; Akter, M.A.; Chakraborty, A. Plant microbiome—An account of the factors that shape community composition and diversity. Curr. Plant Biol. 2020, 23, 100161. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Vorholt, J.A. Microbial life in the phyllosphere. Nat. Rev. Microbiol. 2012, 10, 828–840. [Google Scholar] [CrossRef] [PubMed]
- Purahong, W.; Kapturska, D.; Pecyna, M.J.; Jariyavidyanont, K.; Kaunzner, J.; Juncheed, K.; Uengwetwanit, T.; Rudloff, R.; Schulz, E.; Hofrichter, M.; et al. Effects of forest management practices in temperate beech forests on bacterial and fungal communities involved in leaf litter degradation. Microb. Ecol. 2015, 69, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Coleman-Derr, D.; Desgarennes, D.; Fonseca-Garcia, C.; Gross, S.; Clingenpeel, S.; Woyke, T.; North, G.; Visel, A.; Partida-Martinez, L.P.; Tringe, S.G. Plant compartment and biogeography affect microbiome composition in cultivated and native Agave species. New Phytol. 2016, 209, 798–811. [Google Scholar] [CrossRef] [PubMed]
- Cregger, M.A.; Veach, A.M.; Yang, Z.K.; Crouch, M.J.; Vilgalys, R.; Tuskan, G.A.; Schadt, C.W. The Populus holobiont: Dissecting the effects of plant niches and genotype on the microbiome. Microbiome 2018, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Peng, Z.; Qi, J.; Gao, J.; Wei, G. Linking bacterial-fungal relationships to microbial diversity and soil nutrient cycling. mSystems 2021, 6, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Nimsi, K.A.; Manjusha, K.; Hatha, A.A.M.; Kathiresan, K. Diversity, distribution, and bioprospecting potentials of manglicolous yeasts: A review. FEMS Microbiol. Ecol. 2023, 99, fiad044. [Google Scholar] [CrossRef] [PubMed]
- Thatoi, H.; Behera, B.C.; Mishra, R.R.; Dutta, S.K. Biodiversity and biotechnological potential of microorganisms from mangrove ecosystems: A review. Ann. Microbiol. 2012, 63, 1–19. [Google Scholar] [CrossRef]
- Lee, G.; Lee, S.H.; Kim, K.M.; Ryu, C.M. Foliar application of the leaf-colonizing yeast Pseudozymachurashimaensis elicits systemic defense of pepper against bacterial and viral pathogens. Sci. Rep. 2017, 7, 39432. [Google Scholar]
- SlÁviková, E.; Vadkertiová, R.; Vránová, D. Yeasts colonizing the leaves of fruit trees. Ann. Microbiol. 2009, 59, 419–424. [Google Scholar] [CrossRef]
- Bordet, F.; Joran, A.; Klein, G.; Roullier-Gall, C.; Alexandre, H. Yeast–yeast interactions: Mechanisms, methodologies and impact on composition. Microorganisms 2020, 8, 600. [Google Scholar] [CrossRef] [PubMed]
- Nimsi, K.A.; Manjusha, K.; Krupesh, C.K. Diversity and hydrolytic potentials of manglicolous yeasts associated with mangrove trees Rhizophora and Avicennia found in Kerala, India. J. Aquat. Biol. Fish. 2023, 11 (Suppl. S1), 1–7. [Google Scholar]
- Clarke, K.; Gorley, R. User Manual/Tutorial, Primer Version 5; Primer-E: Plymouth, UK, 2001. [Google Scholar]
- Pallapati, A.R.; Sirigiri, S.D.; Jain, S.; Ratnala, V.; Roy, I. Lysine245 plays a crucial role in stability and function of glycerol 3-phosphate dehydrogenase (Gpd1) in Saccharomyces cerevisiae. J. Cell. Biochem. 2021, 122, 1726–1736. [Google Scholar] [CrossRef] [PubMed]
- Pallapati, A.R.; Prasad, S.; Roy, I. Glycerol 3-phosphate dehydrogenase regulates heat shock response in Saccharomyces cerevisiae. Biochim. Et Biophys. Acta. Mol. Cell Res. 2022, 1869, 119238. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).