In Vitro Digestion of Chia Seed Oil Nanoemulsions †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Emulsion Preparation
2.3. Emulsion Characterization
2.3.1. Particle Size and ζ-Potential
2.3.2. Physical Stability
2.3.3. Microscopic Analysis
2.4. In Vitro Digestion
2.4.1. Particle Size and Microscopic Analysis
2.4.2. Extent of Lipolysis
2.4.3. Fatty Acid Composition
2.5. Statistical Analysis
3. Results and Discussion
3.1. Emulsion Characterization
3.2. In Vitro Digestion
3.2.1. Particle Size and Microscopic Analysis
3.2.2. Extent of Lipolysis and Fatty Acid Composition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahidi, F.; Ambigaipalan, P. Omega-3 polyunsaturated fatty acids and their health benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Ixtaina, V.Y.; Martínez, M.L.; Spotorno, V.; Mateo, C.M.; Maestri, D.M.; Diehl, B.W.; Nolasco, S.M.; Tomás, M.C. Characterization of chia seed oils obtained by pressing and solvent extraction. J. Food Compos. Anal. 2011, 24, 166–174. [Google Scholar] [CrossRef]
- Julio, L.M.; Copado, C.N.; Diehl, B.W.; Tomás, M.C.; Ixtaina, V.Y. Development of chia oil-in-water nanoemulsions using different homogenization technologies and the layer-by-layer technique. Explor. Foods Foodomics 2024, 2, 107–124. [Google Scholar] [CrossRef]
- Ruan, R.; Wang, J. In vitro digestion models for the study of dietary lipids: A review. J. Food Sci. 2020, 85, 3555–3567. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção RBallance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; Clemente, A.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Prot. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.C.; Lad, M.; Silva, H.D.; Coimbra, M.A.; Boland, M.; Vicente, A. Unravelling the behaviour of curcumin nanoemulsions during in vitro digestion: Effect of the surface charge. Soft Matter 2013, 9, 3147–3154. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Adhikari, R.; Barrow, C.J.; Adhikari, B. Digestion behaviour of chia seed oil encapsulated in chia seed protein-gum complex coacervates. Food Hydrocoll. 2017, 66, 71–81. [Google Scholar] [CrossRef]
- Okuro, P.K.; Viau, M.; Marze, S.; Laurent, S.; Cunha, R.L.; Berton-Carabin, C.; Meynier, A. In vitro digestion of high-lipid emulsions: Towards a critical interpretation of lipolysis. Food Funct. 2023, 14, 10868–10881. [Google Scholar] [CrossRef] [PubMed]
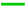 ), after gastric (
), after gastric ( ), and intestinal (
), and intestinal ( ) stages of the in vitro digestion assay. CO: chia oil; Cas1000: chia O/W nanoemulsions.
) stages of the in vitro digestion assay. CO: chia oil; Cas1000: chia O/W nanoemulsions.
 ), after gastric (
), after gastric ( ), and intestinal (
), and intestinal ( ) stages of the in vitro digestion assay. CO: chia oil; Cas1000: chia O/W nanoemulsions.
) stages of the in vitro digestion assay. CO: chia oil; Cas1000: chia O/W nanoemulsions.

| Fatty Acid | Initial Chia Oil | Intestinal Phase | |
|---|---|---|---|
| CO | Cas1000 | ||
| Linoleic acid (%) | 18.30 ± 0.50 a | 20.88 ± 0.08 b | 21.67 ± 0.01 c |
| α-linolenic acid (%) | 67.00 ± 1.30 b | 58.28 ± 0.09 a | 58.48 ± 0.30 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Julio, L.; Quintero-Gamero, G.; Guiotto, E.; Ixtaina, V. In Vitro Digestion of Chia Seed Oil Nanoemulsions. Biol. Life Sci. Forum 2024, 37, 3. https://doi.org/10.3390/blsf2024037003
Julio L, Quintero-Gamero G, Guiotto E, Ixtaina V. In Vitro Digestion of Chia Seed Oil Nanoemulsions. Biology and Life Sciences Forum. 2024; 37(1):3. https://doi.org/10.3390/blsf2024037003
Chicago/Turabian StyleJulio, Luciana, Greilis Quintero-Gamero, Estefanía Guiotto, and Vanesa Ixtaina. 2024. "In Vitro Digestion of Chia Seed Oil Nanoemulsions" Biology and Life Sciences Forum 37, no. 1: 3. https://doi.org/10.3390/blsf2024037003
APA StyleJulio, L., Quintero-Gamero, G., Guiotto, E., & Ixtaina, V. (2024). In Vitro Digestion of Chia Seed Oil Nanoemulsions. Biology and Life Sciences Forum, 37(1), 3. https://doi.org/10.3390/blsf2024037003






