Protection of Selenium Against Methylmercury in the Human Body: A Comprehensive Review of Biomolecular Interactions †
Abstract
1. Introduction
2. Methodology
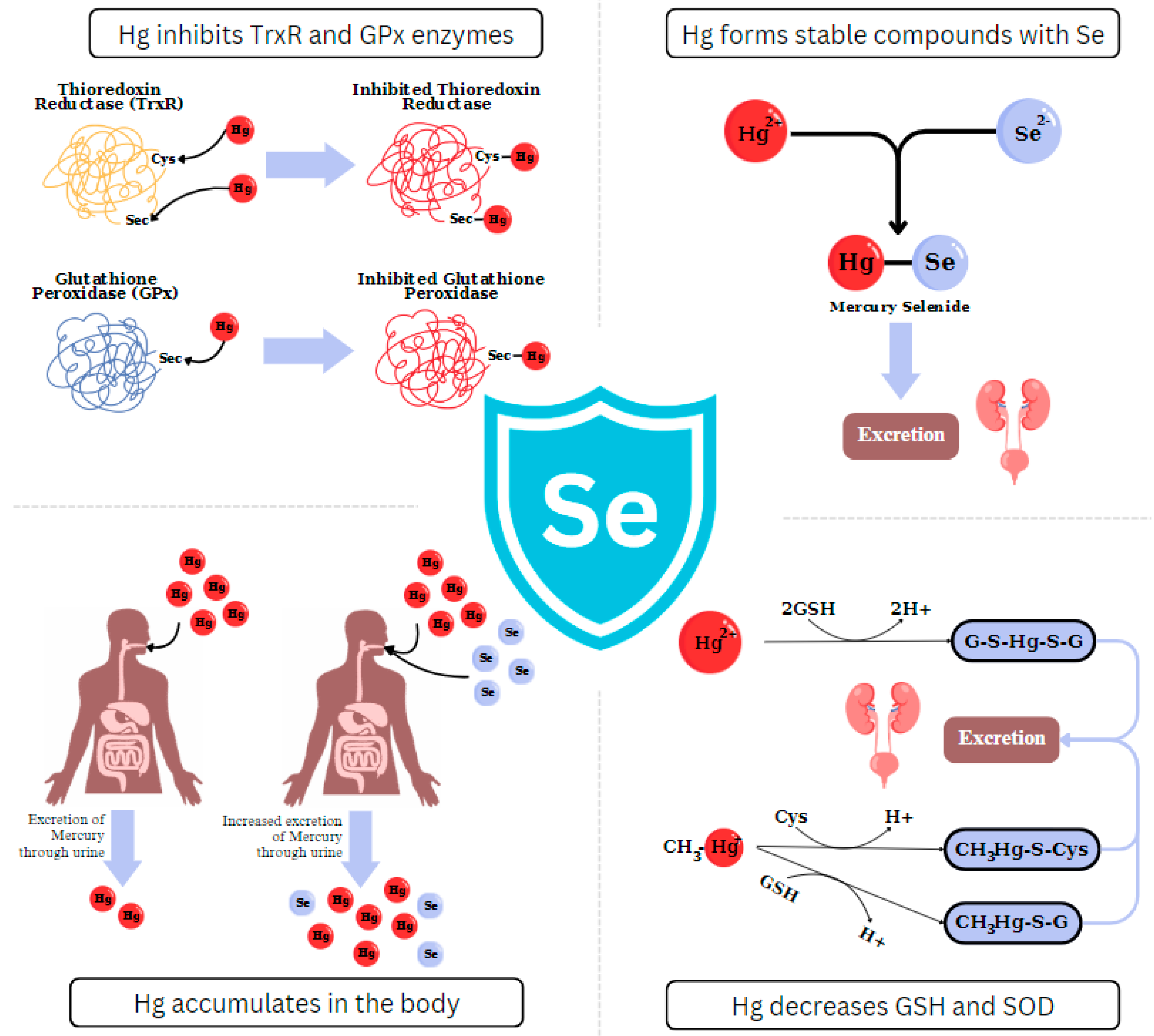
3. Biochemical Interactions between Selenium and Mercury
4. Impact of Cooking and Food Processing
5. Epidemiological Evidence and Health Assessments
6. Health Criteria and Public Health Implications
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karimi, R.; Fitzgerald, T.P.; Fisher, N.S. A Quantitative Synthesis of Mercury in Commercial Seafood and Implications for Exposure in the United States. Environ. Health Perspect. 2012, 120, 1512–1519. [Google Scholar] [CrossRef]
- Cuvin-Aralar, M.L.A.; Furness, R.W. Mercury and selenium interaction: A review. Ecotoxicol. Environ. Saf. 1991, 21, 348–364. [Google Scholar] [CrossRef] [PubMed]
- Branco, V.; Canário, J.; Lu, J.; Holmgren, A.; Carvalho, C. Mercury and selenium interaction in vivo: Effects on thioredoxin reductase and glutathione peroxidase. Free Radic. Biol. Med. 2012, 52, 781–793. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Wang, M.; Yin, S.-T.; Wang, H.-L.; Chen, L.; Sun, L.-G.; Ruan, D.-Y. The interaction of selenium and mercury in the accumulations and oxidative stress of rat tissues. Ecotoxicol. Environ. Saf. 2008, 70, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Hajeb, P.; Sloth, J.J.; Shakibazadeh, S.; Mahyudin, N.A.; Afsah-Hejri, L. Toxic Elements in Food: Occurrence, Binding, and Reduction Approaches. Compr. Rev. Food Sci. Food Saf. 2014, 13, 457–472. [Google Scholar] [CrossRef]
- Ouédraogo, O.; Amyot, M. Effects of various cooking methods and food components on bioaccessibility of mercury from fish. Environ. Res. 2011, 111, 1064–1069. [Google Scholar] [CrossRef]
- Milea, Ș.-A.; Lazăr, N.-N.; Simionov, I.-A.; Petrea, Ș.-M.; Călmuc, M.; Călmuc, V.; Georgescu, P.-L.; Iticescu, C. Effects of cooking methods and co-ingested foods on mercury bioaccessibility in pontic shad (Alosa immaculata). Curr. Res. Food Sci. 2023, 7, 100599. [Google Scholar] [CrossRef]
- Maulvault, A.L.; Machado, R.; Afonso, C.; Lourenço, H.M.; Nunes, M.L.; Coelho, I.; Langerholc, T.; Marques, A. Bioaccessibility of Hg, Cd and As in cooked black scabbard fish and edible crab. Food Chem. Toxicol. 2011, 49, 2808–2815. [Google Scholar] [CrossRef]
- Houlbrèque, F.; Hervé-Fernández, P.; Teyssié, J.-L.; Oberhaënsli, F.; Boisson, F.; Jeffree, R. Cooking makes cadmium contained in Chilean mussels less bioaccessible to humans. Food Chem. 2011, 126, 917–921. [Google Scholar] [CrossRef]
- Laparra, J.M.; Vélez, D.; Montoro, R.; Barberá, R.; Farré, R. Bioaccessibility of inorganic arsenic species in raw and cooked Hizikia fusiforme seaweed. Appl. Organomet. Chem. 2004, 18, 662–669. [Google Scholar] [CrossRef]
- Ichikawa, S.; Kamoshida, M.; Hanaoka, K.; Hamano, M.; Maitani, T.; Kaise, T. Decrease of arsenic in edible brown algae Hijikia fusiforme by the cooking process. Appl. Organomet. Chem. 2006, 20, 585–590. [Google Scholar] [CrossRef]
- Sengupta, M.; Hossain, M.; Mukherjee, A.; Ahamed, S.; Das, B.; Nayak, B.; Pal, A.; Chakraborti, D. Arsenic burden of cooked rice: Traditional and modern methods. Food Chem. Toxicol. 2006, 44, 1823–1829. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Liu, Y.; Dong, G.; Wu, H. Effect of boiling and frying on the selenium content, speciation, and in vitro bioaccessibility of selenium-biofortified potato (Solanum tuberosum L.). Food Chem. 2021, 348, 129150. [Google Scholar] [CrossRef]
- Lu, X.; He, Z.; Lin, Z.; Zhu, Y.; Yuan, L.; Liu, Y.; Yin, X.; Lu, X.; He, Z.; Lin, Z.; et al. Effects of Chinese cooking methods on the content and speciation of selenium in selenium bio-fortified cereals and soybeans. Nutrients 2018, 10, 317. [Google Scholar] [CrossRef]
- Singhato, A.; Judprasong, K.; Sridonpai, P.; Laitip, N.; Ornthai, N.; Yafa, C.; Chimkerd, C. Effect of different cooking methods on selenium content of fish commonly consumed in Thailand. Foods 2022, 11, 1808. [Google Scholar] [CrossRef]
- Martins, C.T.; Almeida, C.M.M.; Alvito, P.C. Selenium content of raw and cooked marine species consumed in Portugal. Food Anal. Methods 2009, 4, 77–83. [Google Scholar] [CrossRef]
- Vicente-Zurdo, D.; Gómez-Gómez, B.; Pérez-Corona, M.T.; Madrid, Y. Impact of fish growing conditions and cooking methods on selenium species in swordfish and salmon fillets. J. Food Compos. Anal. 2019, 84, 103275. [Google Scholar] [CrossRef]
- Solan, T.D.; Lindow, S.W. Mercury exposure in pregnancy: A review. J. Perinat. Med. 2014, 42, 725–729. [Google Scholar] [CrossRef]
- Kajiwara, Y.; Yasutake, A.; Adachi, T.; Hirayama, K. Methylmercury transport across the placenta via neutral amino acid carrier. Arch. Toxicol. 1996, 70, 310–314. [Google Scholar] [CrossRef]
- Baldewsingh, G.K. Prenatal Mercury Exposure in Pregnant Women from Suriname’s Interior and Its Effects on Birth Outcomes. Int. J. Environ. Res. Public Health 2020, 17, 4032. [Google Scholar] [CrossRef]
- Debes, F.; Weihe, P.; Grandjean, P. Cognitive deficits at age 22 years associated with prenatal exposure to methylmercury. Cortex 2016, 74, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Cace, I.B.; Milardovic, A.; Prpic, I.; Krajina, R.; Petrovic, O.; Vukelic, P.; Spiric, Z.; Horvat, M.; Mazej, D.; Snoj, J. Relationship between the prenatal exposure to low-level of mercury and the size of a newborn’s cerebellum. Med. Hypotheses 2011, 76, 514–516. [Google Scholar] [CrossRef]
- Davidson, P.W.; Cory-Slechta, D.A.; Thurston, S.W.; Huang, L.-S.; Shamlaye, C.F.; Gunzler, D.; Watson, G.; van Wijngaarden, E.; Zareba, G.; Klein, J.D.; et al. Fish consumption and prenatal methylmercury exposure: Cognitive and behavioral outcomes in the main cohort at 17 years from the Seychelles child development study. Neurotoxicology 2011, 32, 711–717. [Google Scholar] [CrossRef]
- Crump, K.S.; Kjellström, T.; Shipp, A.M.; Silvers, A.; Stewart, A. Influence of Prenatal Mercury Exposure Upon Scholastic and Psychological Test Performance: Benchmark Analysis of a New Zealand Cohort. Risk Anal. 1998, 18, 701–713. [Google Scholar] [CrossRef]
- Di Bella, G.; Potortì, A.G.; Turco, V.L.; Bua, D.; Licata, P.; Cicero, N.; Dugo, G. Trace elements in Thunnus thynnus from Mediterranean Sea and benefit–risk assessment for consumers. Food Addit. Contam. Part B 2015, 8, 175–181. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, X.; Chan, H.M.; Larssen, T. New Insights into Traditional Health Risk Assessments of Mercury Exposure: Implications of Selenium. Environ. Sci. Technol. 2014, 48, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Technical Standard Orders (TSO). Advice on Fish Consumption: Benefits & Risks. TSO: London, UK, 2004. [Google Scholar]
- World Health Organization (WHO); Food and Agriculture Organization of the United Nations (FAO). Report of the Joint FAO/WHO Expert Consultation on the Risks and Benefits of Fish Consumption, 25–29 January 2010, Rome, Italy. In FAO Fisheries and Aquaculture Report; World Health Organization: Geneva, Switzerland, 2011. Available online: https://iris.who.int/handle/10665/44666 (accessed on 1 October 2024).
- Health Canada. Human Health Risk Assessment of Mercury in Fish and Health Benefits of Fish Consumption; Health Canada: Ottawa, ON, Canada, 2008.
- Food Standards Australia New Zealand (FSANZ). Mercury in fish: Advice on fish consumption; FSANZ: Kingston, Australia; Wellington, New Zealand, 2020.
- Ralston, N.V.C.; Ralston, C.R.; Raymond, L.J. Selenium Health Benefit Values: Updated Criteria for Mercury Risk Assessments. Biol. Trace Elem. Res. 2016, 171, 262–269. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on Dietetic products, nutrition and allergies [NDA] related to the Tolerable Upper Intake Level of Vanadium. EFSA J. 2004, 2, 33. [Google Scholar] [CrossRef]
- Melgar, M.J.; Núñez, R.; García, M.Á. Selenium intake from tuna in Galicia (Spain): Health risk assessment and protective role against exposure to mercury and inorganic arsenic. Sci. Total Environ. 2019, 694, 133716. [Google Scholar] [CrossRef]
- Yamashita, Y. Discovery of the strong antioxidant selenoneine in tuna and selenium redox metabolism. World J. Biol. Chem. 2010, 1, 144. [Google Scholar] [CrossRef]
- Chamorro, F.; Cassani, L.; Garcia-Oliveira, P.; Barral-Martinez, M.; Jorge, A.O.S.; Pereira, A.G.; Otero, P.; Fraga-Corral, M.; Oliveira, M.B.P.P.; Prieto, M.A. Health benefits of bluefin tuna consumption: (Thunnus thynnus) as a case study. Front. Nutr. 2024, 11, 1340121. [Google Scholar] [CrossRef] [PubMed]
| Recommendation | Fish Type | Organization | Reference |
|---|---|---|---|
| 125 g per week for healthy adults | Oily fish | EFSA | [25] |
| 50 g per week for children aged 3–12 (total of 120 g per month). | |||
| Up to four medium-sized cans or two tuna steaks per week for pregnant women and women intending to become pregnant. | Tuna | FSA | [26] |
| Children and other adults do not need to restrict their tuna amount. | |||
| No more than 85 g (3 ounces) per week for children. | Canned light or white (albacore) tuna, cod, perch, black sea bass | Dietary Guidelines for Americans | [27] |
| Up to 340 g (12 ounces) per week for pregnant or nursing women or women who might become pregnant, with a maximum of 170 g (6 ounces) of albacore tuna. | All fish | US EPA | [28] |
| Up to a total of 150 g per week for adults. | Tuna, shark, swordfish, escolar, marlin, and orange roughie | Bureau of Chemical Safety (Canada) | [29] |
| 150 g per month for women who are or may become pregnant and breastfeeding mothers. | |||
| 1 portion of 150 g per week for the general population. | Shark, swordfish, and marlin | Food standards Australia New Zealand | [30] |
| 1 portion of 150 g per fortnight for women who are pregnant or planning pregnancy (and no other fish during that fortnight). | |||
| 1 portion of 75 g per fortnight for children up to 6 years old (and no other fish during that fortnight). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jorge, A.O.S.; Chamorro, F.; Carpena, M.; Echave, J.; Pereira, A.G.; Oliveira, M.B.P.P.; Prieto, M.A. Protection of Selenium Against Methylmercury in the Human Body: A Comprehensive Review of Biomolecular Interactions. Biol. Life Sci. Forum 2024, 35, 8. https://doi.org/10.3390/blsf2024035008
Jorge AOS, Chamorro F, Carpena M, Echave J, Pereira AG, Oliveira MBPP, Prieto MA. Protection of Selenium Against Methylmercury in the Human Body: A Comprehensive Review of Biomolecular Interactions. Biology and Life Sciences Forum. 2024; 35(1):8. https://doi.org/10.3390/blsf2024035008
Chicago/Turabian StyleJorge, A. O. S., F. Chamorro, M. Carpena, J. Echave, A. G. Pereira, M. Beatriz P. P. Oliveira, and M. A. Prieto. 2024. "Protection of Selenium Against Methylmercury in the Human Body: A Comprehensive Review of Biomolecular Interactions" Biology and Life Sciences Forum 35, no. 1: 8. https://doi.org/10.3390/blsf2024035008
APA StyleJorge, A. O. S., Chamorro, F., Carpena, M., Echave, J., Pereira, A. G., Oliveira, M. B. P. P., & Prieto, M. A. (2024). Protection of Selenium Against Methylmercury in the Human Body: A Comprehensive Review of Biomolecular Interactions. Biology and Life Sciences Forum, 35(1), 8. https://doi.org/10.3390/blsf2024035008












