Abstract
The European Rapid Alert System for Food and Feed (RASFF) has shown 1133 notifications for spices and herbs in the last 10 years (2013–2023). The analysis of these notifications indicated that 58.7% (665 alerts) of the alerts corresponded to chemical hazards. Mycotoxins corresponding to aflatoxin B1 (24 alerts) and ochratoxin A (39 alerts) were found in 19.4% of the samples. Due to the presence of these biological hazards in foodstuffs, comprehensive knowledge of their molecular mechanisms of action is required as part of the risk assessment strategy. Aflatoxin B1 (AFB1) is a known potent carcinogen that has been linked to liver cancer in humans and animals. Its toxic effects consist of forming DNA adducts, causing mutations, and interfering with cellular processes. On the other hand, ochratoxin A (OTA) is known to be nephrotoxic, hepatotoxic, carcinogenic, and immunosuppressive in both humans and animals. OTA targets the kidneys and liver, exerting its toxic effects similarly to AFB1, i.e., through DNA damage, oxidative stress, and interference with cellular processes. This communication reviews the molecular mechanism of action underlying the toxicity of AFB1 and OTA found in herbs and spices in Europe, focusing on their biosynthesis, toxicodynamics, interaction with cellular components, and the resulting biochemical pathways leading to adverse health effects. Moreover, it discusses potential strategies for mitigating their presence in spices, emphasizing the importance of hazard characterization for effective risk management and regulation.
1. Introduction
Herbs and spices cover a broad range of plant materials, with herbs usually referring to the green parts of plants and spices, including other parts such as bulbs, roots, bark, flowers, and seeds. This diversity is illustrated by resources such as the List of Culinary Herbs and Spices offered by the European Spices Association (ESA) [1]. The use of herbs and spices to improve quality of life is widespread worldwide, both in developing countries as an important source of medicine and in highly developed countries due to an increasing trend towards self-medication and acceptance of natural products. These products take many forms, including their aromatic, flavoring, and coloring properties, dietary supplements, homeopathic remedies, herbal teas, and cosmetic preparations [1,2]. Spices and herbs are consumed in small amounts, typically distributed in dry, low-water activity forms, and are part of complex distribution chains [3]. Besides their culinary application, spices have a wide range of phytochemicals, which justifies their medicinal use [4]. As the global demand for spices rises, so do safety concerns, particularly regarding mycotoxin contamination, as research indicates that many herbal products and spices may surpass regulatory limits [5]. The presence of mycotoxins in these products is mainly due to poor storage practices; aflatoxins, ochratoxin A, chlorpyriphos, and triazophos are the ones with the highest priority risk management in both herbs and spices [4,5]. The European Rapid Alert System for Food and Feed (RASFF) has shown 1133 notifications for spices and herbs in the last 10 years (2013–2023), with mycotoxins present in 19.4% of the samples. These toxins are produced by fungi. Their negative impact on consumer health has been demonstrated by showing mutagenic, carcinogenic, nephrotoxic, neurotoxic, and teratogenic activities [4]. Therefore, it is essential to understand the mechanisms of action of these toxins and to develop mitigation strategies, thus ensuring consumer safety and reducing economic losses [6,7].
Mycotoxins are secondary metabolites that could be produced by filamentous fungi (such as Aspergillum, Alternaria, and Penicillium) during the harvesting, processing, storage, and/or handling of spices [4,8]. These metabolites are usually produced under temperatures ranging from 25–30 °C, with moisture content above 16% and water activity (Aw) of 0.7 [4]. Aflatoxins (AF) and ochratoxins (OT) are considered the most hazardous mycotoxins [9,10]. Since these toxins are prone to develop in herbs and spices and because of their negative effects on human health, it is crucial to monitor their occurrence. For example, 130 samples of spices were analyzed for AF and OT, showing that 15.4% and 23.8% of the samples were contaminated by AF and OT, respectively [11]. In another study, 14 spice samples were analyzed for aflatoxins, detected in chili, black pepper, tandoori masala, garam masala, and turmeric [12]. Black pepper, red pepper, garam masala, and turmeric samples (120) of branded and non-branded spices were collected and analyzed in another study, showing higher aflatoxin contamination in those that were non-branded [8]. The adverse effects of AF and OT on humans range from liver necrosis and hemorrhage to neurological disorders and, in extreme cases, death [6,13].
AF are mycotoxins generally produced by Aspergillus spp. and Penicillium spp., which have been linked to several detrimental effects on both human and animal health, such as immunosuppressive, mutagenic, carcinogenic, and teratogenic effects [12]. Aflatoxins can naturally occur and are characterized by their heat resistance, distinguishing aflatoxin B1, B2, G1, and G2 [12]. Aflatoxin B1 (AFB1) is the most prevalent aflatoxin, representing 75% of all aflatoxins produced in fungal-contaminated food products [8]. Toxic levels of AFB1 are typically regulated at 2 µg/kg in foods such as nuts and dried fruits. Some limits allow up to 8 µg/kg for nuts intended for further processing. For OTA, toxic levels are regulated at 3 µg/kg for cereals, 5 µg/kg for dried vine fruit, and 2 µg/kg for coffee and wine [14,15,16].
OTs are mycotoxins mainly produced by Aspergillus and Penicillium species and can be classified into A, B, and C. The ingestion of these secondary metabolites has been linked to several health issues, including kidney diseases, immunotoxicity, neurotoxicity, or mutagenicity [17]. The presence of mycotoxins in many spices and herbs, especially those grown in countries with tropical climates characterized by significant fluctuations in temperature, humidity, and rainfall, has been noted due to inadequate storage conditions, prolonged drying times, and high levels of humidity [18]. The limit for ochratoxin A (OTA) in these spices ranges from 15 to 20 μg/kg. The European Food Safety Authority (EFSA) has set a tolerable daily intake (TDI) of 17 ng/kg-b.w/d for OTA and 1000 ng/kg-b.w/d for AFB1. Currently, the Margin of Exposure is used for assessing the safety of aflatoxin exposure [9].
This review focuses on the presence of AFB1 and OTA in herbs and spices consumed in Europe, showing the mechanism of action of these secondary metabolites, their adverse health effects, and strategies to detect and mitigate their presence in these products.
2. Material and Methods
In this study, data from the European RASFF database from 2013 to 2023 were analyzed to assess the occurrence and impact of AFB1 and OTA in herbs and spices in Europe. A comprehensive literature search focused on the biosynthesis, toxicodynamics, and toxicological effects of AFB1 and OTA was performed using databases such as PubMed, Scopus, ScienceDirect, Google Scholar, and Web of Science. To ensure reliable and up-to-date data, only high-quality first (Q1) and second (Q2) quartile articles were included, prioritizing the most recent articles. Studies were evaluated to characterize hazards to support effective risk management and regulation. Furthermore, data collected by the EFSA played a critical role in providing comprehensive and up-to-date information on the prevalence and risks of these chemical hazards, thereby increasing the robustness of the study findings.
3. Molecular Mechanisms of Action of Aflatoxin B1 and Ochratoxin A
3.1. Aflatoxin B1
AFB1 is the most toxic aflatoxin produced by Aspergillus fungi [19], with immunotoxic, carcinogenic, hepatotoxic, cardiotoxicity, teratogenicity, and neurotoxicity properties in humans and animals after acute and chronic exposure, with the liver being the primary target organ [20,21]. This toxin is characterized by its high resilience to physical, chemical, and heat processes, being difficult to remove from food products [20]. Since AFB1 is widely distributed, it has become a public health concern, and various studies regarding its toxicity and ability to contaminate food products have been developed, including spices [20].
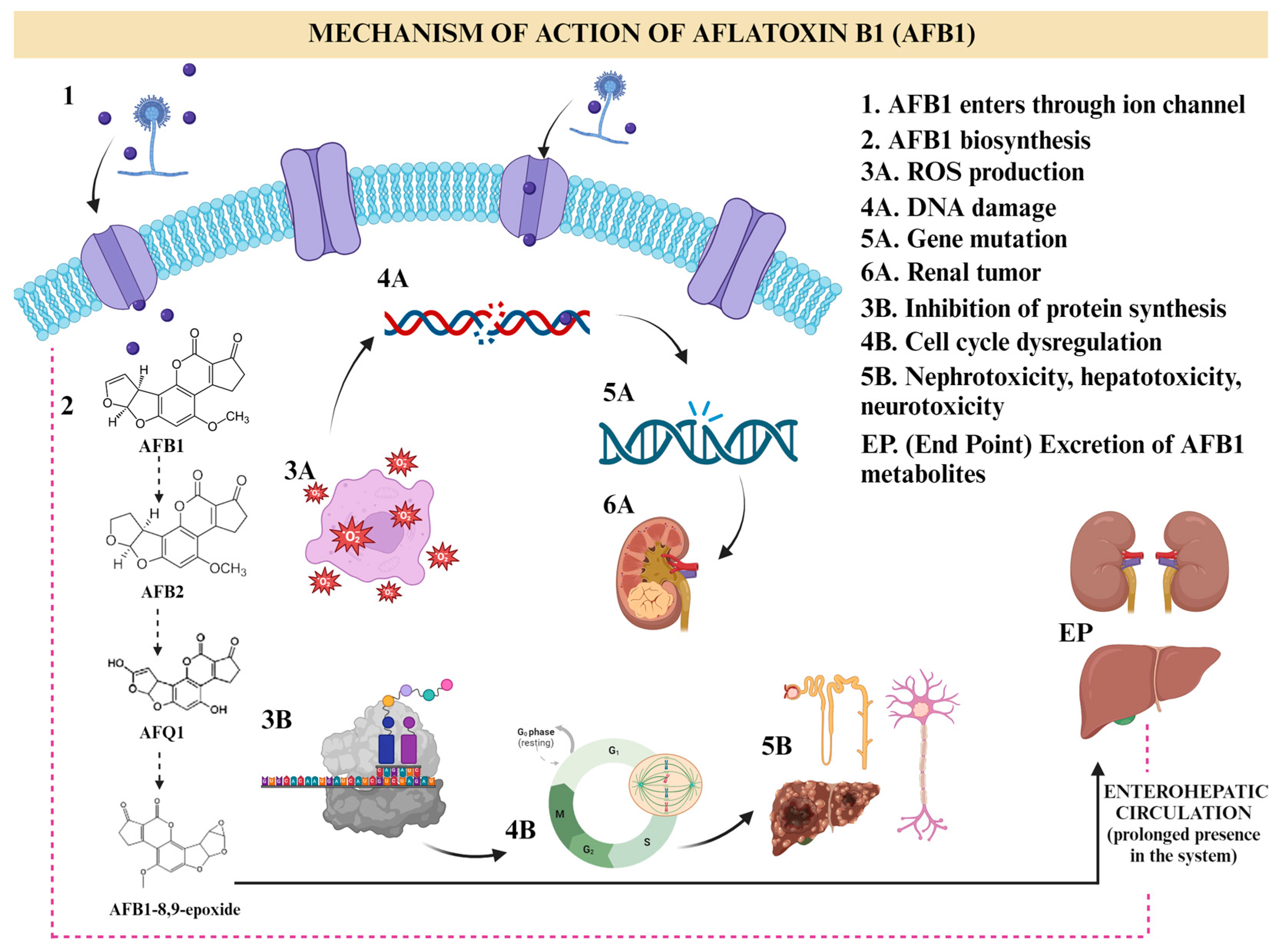
The AFB1 hepatotoxicity mechanism was studied, showing a cascade of biochemical pathways by the hepatic microsomal enzymes (cytochrome P450 or CYP450). By a hydrolyzation process, AFB1 is metabolized to AFB2 and AFQ1, which is hydrated to AFB2, demethylated to AFP1, and epoxidated to AFB1-8,9-exo-epoxide and 8,9-endo-epoxide [20]. Phosphatidylinositol-3-kinase/Protein Kinase B/Forkhead box O1 or PI3K/AKT1/FOXO1 (the pathway that regulates cell survival) and Epidermal Growth Factor Receptor/Extracellular Signal-regulated Kinase (EGFR/ERK) are the signaling pathways; EGFR (epidermal growth factor receptor) was suggested as the transmitter of the extracellular stimulus signals to forkhead box FOXO1, and FOXO1 initiates the transcriptional activity that induces apoptosis, leading to liver damage and dysfunction [21]. Apart from liver toxicity, this secondary metabolite has shown neurotoxic activity in several epidemiological studies, affecting the central nervous system by crossing the blood-brain barrier and bioaccumulating in the brain [20]. The neurotoxic effect of AFB1 can be explained by multiple mechanisms involving increased Reactive Oxygen Species (ROS) generation, S-phase arrest, DNA damage, and IMR-32cells apoptosis [20]. For instance, oral administration of AFB1 for 30, 60, and 90 days demonstrated the neurotoxic activity on astrocytes and microglia cells as well as in the brain enzymes associated with neurodegeneration [17]. The neurodevelopment effects of this mycotoxin were studied in pregnant rats, showing delayed learning ability, reflex response development, and locomotor performance after subcutaneous injections of AFB1 on 11–14 or 15–18 days of gestation [20]. In another study, adult male rats were exposed to AFB1 for 4 weeks, exhibiting sciatic nerve damage and degeneration of myelin sheath, confirming the neurotoxicity and induction of oxidative injury and mastocytosis in rats [22]. Figure 1 shows the mechanism of action.
Figure 1.
Illustration of the mechanism of action of aflatoxin B1 (AFB1). The mechanism is mediated through cell cycle arrest, apoptosis, oxidative stress, ER stress, and autophagy via multiple genes, non-coding RNAs, and signaling pathways. Created with BioRender.com.
3.2. Ochratoxin A
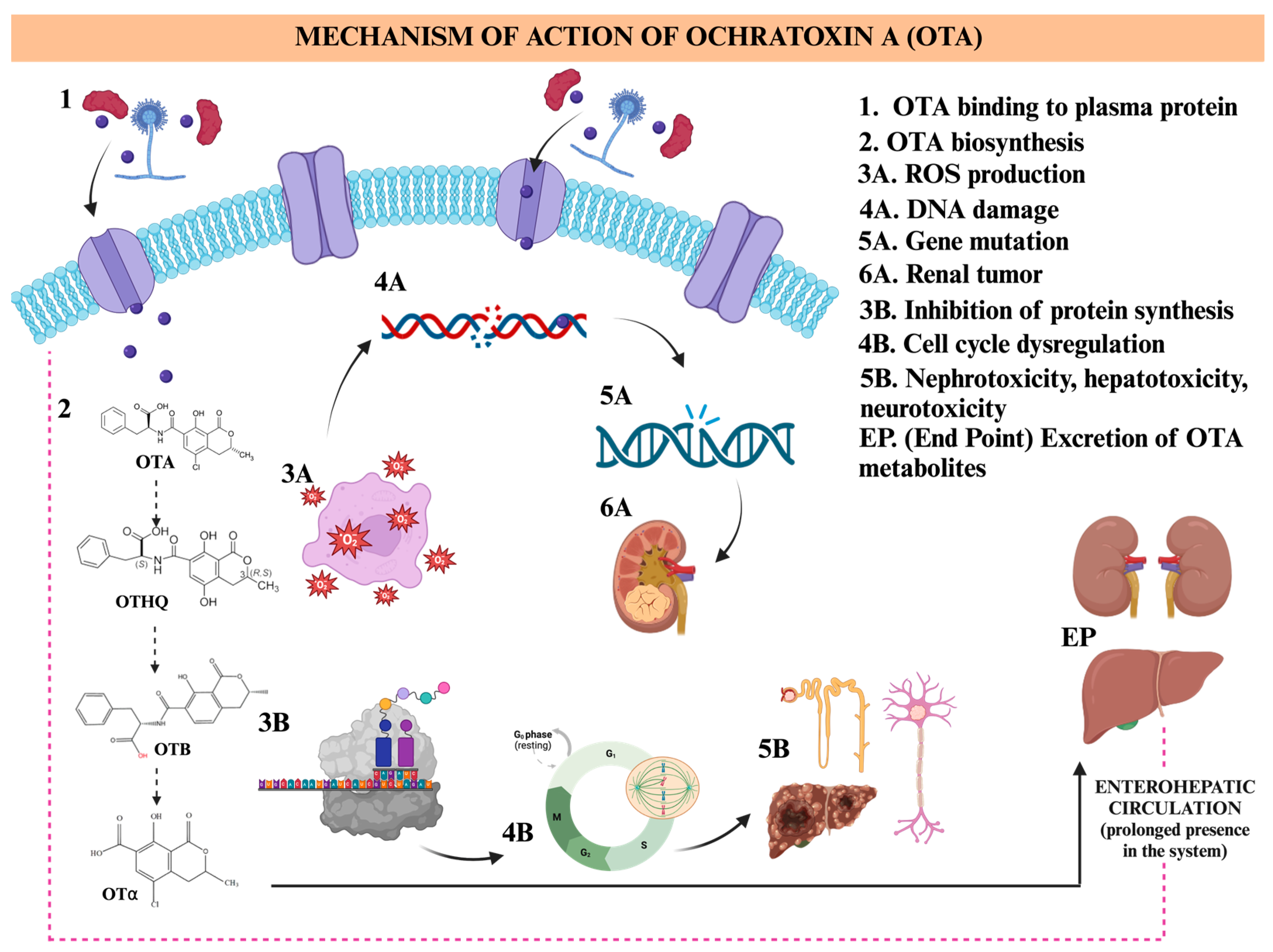
OTA is the most ubiquitous and detrimental ochratoxin. It is a low molecular weight toxin with a high heat stability, making it persistent in food processing and, therefore, prevails in food [23]. The consumption of this toxin is linked to hepatotoxic, nephrotoxic, immunotoxic, genotoxic, teratogenic, neurotoxic, and carcinogenic activities [11,23]. Humans are exposed to OTA by ingestion of contaminated food products. Cereals, wine, beer, coffee, or cocoa and their derivatives are among the main products contributing to OTA dietary exposure. Also, herbs and spices are susceptible to the presence of OTA [24]. By ingesting OTA, the toxin enters the circulatory system through gastrointestinal adsorption and accumulates in organs thanks to its affinity with proteins like serum albumin, the most abundant plasma protein in the human circulatory system [23,25]. The regulatory mechanism of OTA involves several enzymes, including polyketide synthase, non-ribosomal peptide synthase, CYP450 monooxygenase, halogenase, and esterase. The biosynthesis of this toxin starts with the formation of isocoumarin. Then, phenylalanine molecules are linked to chlorinated dihydroisocoumarin, and finally, the halogenase enzyme adds a chlorine atom to the isocoumarin molecule [25]. The toxic mechanism of OTA was also studied in zebrafish embryos, with a 10-day LC50 of 0.16 mg/L. Results showed changes in the activity of detoxification enzymes Glutathione S-transferase (GST) and CYP450. Moreover, the apoptosis-associated enzyme caspase 3 and expression of nine genes (including apoptosis, antioxidant, immunosuppression, and endocrine genes) showed significant variations [26]. OTA’s neurotoxicity is of special interest due to its high affinity for the brain, being able to cross the blood-brain barrier and enter the brain [27,28]. OTA neurotoxicity was assessed in an in vitro study using normal human astrocyte cells immortalized with the Simian Virus 40 Large T antigen (SV40LT) (NHA-SV40LT). Results showed reduced cellular proliferative activity by inhibiting Cyclin D1 (CCND1), Cyclin E1 (CCNE1), Cyclin-Dependent Kinase 4 (CDK4), and c-Myc expression. NHA-SV40LT apoptosis occurred through mitochondrial membrane potential loss and up-regulation of Bcl-2-associated X (BAX) and Tumor Protein 53 (TP53) pro-apoptotic proteins, while the cytosolic and mitochondrial calcium levels increased [28]. In another in vitro study, OTA’s neurotoxicity was assessed using immortalized human microglia-SV40 cells (which are involved in neuroinflammation and neurodegeneration processes). Neurotensin and lipopolysaccharide were used as positive triggers, and OTA in 1, 10, and 100 mM was applied to microglia cells for 24 h. Results showed the ability of OTA to stimulate microglia cells to release IL-1β and IL-18 (which are pro-inflammatory cytokines that have been involved in brain diseases) and interleukin-8 (IL-8) (CXCL8, a receptor of inflammatory diseases) in a dose-dependent manner [27,29,30,31]. Figure 2 shows the mechanism of action.
Figure 2.
Illustration of the mechanism of action of Ochratoxin A (OTA). The hydrolysis process of OTA leads to a cascade of reactions (OTA, OTHQ, OTB, and OTα) and the production of free radicals. Created with BioRender.com.
4. Strategies for Detection and Mitigation of Aflatoxin B1 and Ochratoxin A Presence in Spices
Since the consumption of AFB1 and OTA is linked to several negative health effects, it is necessary to develop accurate strategies for the detection and mitigation of these mycotoxins. The classical methods for the identification and detection of these mycotoxins include cultivation and micro/macro-morphological analysis [32]. These methods are time consuming as well as labor and cost ineffective, and sometimes are not sensitive enough [32]. Moreover, mycotoxin content analyses in spices are especially complex due to high levels of essential oil (EO) compounds and pigmentation, interfering in the process [13]. In this way, molecular methods (such as Polymerase Chain Reaction (PCR)) have been established as alternatives to traditional procedures. The main drawbacks of PCR methodologies are the necessity of trained personnel, the specific equipment needed, and the clean-up and concentration for prior analysis required [32]. HPLC (High-Performance Liquid Chromatography), GC (Gas Chromatography), and LC-MS/MS (Liquid Chromatography-Tandem Mass Spectrometry) are commonly used equipment for the determination of mycotoxins. These procedures require an extraction step with an adequate solvent and a clean-up step for the elimination of interfering compounds [13]. Loop-mediated isothermal amplification (LAMP) has been introduced for the identification of fungi, and it is characterized by its high sensitivity and specificity, and results can be seen within 30–60 min. An assay using LAMP in the detection of AF was performed in a study, showing that a nor1 gene-specific LAMP assay detects all major aflatoxin-producing species of Aspergillus within 60 min [32]. The simultaneous determination of aflatoxins and OTA in spices was assessed by using High-Performance Liquid Chromatography with Fluorescence Detection (HPLC-FLD), and results showed a suitable method that could be applied in both mixed and single spices [13]. In another study, a comparison between the optical waveguide light mode spectroscopy (OWLS) immunosensor technique, HPLC, and ELISA for the determination of AFB1 in 60 paprika samples from different countries was performed. The results showed an excellent correlation of OWLS with the other commonly used methods, suggesting this is an excellent and quick method for AFB1 determination in paprika samples [33]. The application of a multifunctional column as a rapid and simple method for the determination of AFB1 in various spices was also carried out. In this study, results showed the quickness (2 h), safety, and reproducibility of the method compared to those using chloroform extraction steps [34].
Mitigation of the occurrence of these secondary metabolites includes correct procedures during agricultural steps and storage. Regarding storage conditions, the detection and quantification of aflatoxins in spices were assessed in a study. In a study, spices were stored for 120 days in different packages (jute bags and high-density polythene (HDPE) bags), and the conditions (85, 75, and 65% of relative humidity (RH)) were analyzed by HPLC. The results showed that spice storage in 65% RH and HDPE packages had less AF contamination [35]. Apart from the application of suitable packaging and storing conditions, mycotoxin-mitigating agents have also been studied as a possible tool. TOXO-XL (XL) is a mitigating agent containing bentonite, inactive yeast cell wall fractions, and exposed β-1,3/1,6-glucans. Through mycotoxin binding, TOXO-XL mitigates mycotoxin action, and this study shows the AFB1-induced cytotoxicity in Leghorn male hepatomas (LMHs), porcine jejunum epithelial cell lines (IPEC-J2), and porcine alveolar macrophages cells (3D4/21) [36]. Another study used vasicine from Adhatoda vasica for the management of aflatoxicosis and ochratoxicosis using an in silico molecular docking. The results showed the great binding ability of vasicine to both AFB1 and OTA, although both in vitro and in vivo studies are required to confirm these results [37]. The encapsulation of EOs has also been presented as a mitigation strategy against mycotoxins. A nanoformulation using chitosan and EOs from Pinus roxburghii, Juniperus communis, and Cupressus sempervirens was developed for AFB1 mitigation in a model food system. The results showed in-situ fungi toxicity, AFB1 mitigation efficacy, lipid peroxidation suppressing potential, and the maintenance of both macro and micronutrients, suggesting the potential application of these encapsulations for mycotoxin mitigation [38]. Pulsed electric fields were also applied in red pepper flakes, inactivating AF production in Aspergillus parasiticus and reducing AF mutagenicity without affecting the physical properties of the spice [39].
Many factors should be considered when choosing the best method, including the specific mycotoxin, the food matrix, regulatory requirements, and practical considerations such as cost and scalability. Currently, physical adsorption methods using activated carbon or clays are widely used because they are simple and efficient. They can be applied to most food matrices, are considered relatively safe in almost all cases, and have a high degree of universality. However, further developments aimed at increasing the sorbent specificity are needed.
All in all, studies are showing different mitigating strategies to avoid mycotoxin proliferation, including the use of EOs, suitable packaging materials, or the use of mycotoxin-mitigating agents. However, there is still a necessity to develop rapid and accurate detection methodologies of mycotoxins in both spices and herbs matrixes in case the mitigation strategies fail.
5. Conclusions
In summary, herbs and spices are essential to global culinary, medicinal, and cultural practices, providing diverse flavors, aromas, and health benefits. However, their increasing use worldwide is raising concerns about mycotoxin contamination, particularly AFB1 and OTA, due to poor storage practices. These toxins, produced by various fungi, pose serious health risks, including liver and kidney damage, neurotoxicity, and carcinogenicity. Regulatory bodies such as the EFSA have set maximum limits for mycotoxins, but compliance remains a challenge.
Detection methods such as PCR and HPLC are used, but faster and more accurate techniques are needed. Current research focuses on mycotoxin-binding agents, encapsulation of essential oils, and proper storage to reduce mycotoxin levels. The molecular mechanisms of AFB1 and OTA highlight their toxic effects and emphasize the importance of understanding them for effective mitigation. Strategies for detection and mitigation include advanced techniques such as LAMP and OWLS, as well as proper storage conditions and mycotoxin mitigants. However, further development of rapid and accurate detection methods is needed. By combining regulatory efforts with ongoing research and innovative strategies, we can ensure the safety of spices and minimize health risks from mycotoxin contamination.
Author Contributions
Conceptualization, M.C., A.P.-V., and P.B.; methodology, M.C., A.P.-V., P.B., J.T., and K.N.; investigation, M.C., A.P.-V., and P.B., resources, M.T. and M.A.P.; data curation, M.C., A.P.-V., and P.B.; writing—original draft preparation, M.C., A.P.-V., and P.B.; writing—review and editing, M.C., M.T., and M.A.P.; visualization, M.C., M.T., and M.A.P.; supervision, M.T. and M.A.P.; project administration, M.C.; funding acquisition, M.C. and M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
The research leading to these results was supported by Xunta de Galicia for supporting the pre-doctoral grant of M. Carpena (ED481A 2021/313) and by the University of Vigo for supporting the pre-doctoral grant of P. Barciela (PREUVIGO-23). This work has been carried out in the frame of the European Food Risk Assessment (EU-FORA) Fellowship Program (EUBA-EFSA-2022-ENREL-02) cycle 2023–2024 funded by EFSA.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Velázquez, R.; Rodríguez, A.; Hernández, A.; Casquete, R.; Benito, M.J.; Martín, A. Spice and Herb Frauds: Types, Incidence, and Detection: The State of the Art. Foods 2023, 12, 3373. [Google Scholar] [CrossRef] [PubMed]
- Pallarés, N.; Berrada, H.; Font, G.; Ferrer, E. Mycotoxins occurrence in medicinal herbs dietary supplements and exposure assessment. J. Food Sci. Technol. 2022, 59, 2830–2841. [Google Scholar] [CrossRef]
- Székács, A.; Wilkinson, M.G.; Mader, A.; Appel, B. Environmental and food safety of spices and herbs along global food chains. Food Control 2018, 83, 1–6. [Google Scholar] [CrossRef]
- Thanushree, M.P.; Sailendri, D.; Yoha, K.S.; Moses, J.A.; Anandharamakrishnan, C. Mycotoxin contamination in food: An exposition on spices. Trends Food Sci. Technol. 2019, 93, 69–80. [Google Scholar] [CrossRef]
- Russo, K.; Lucchetti, D.; Triolone, D.; Di Giustino, P.; Mancuso, M.; Delfino, D.; Neri, B. Pesticides and mycotoxins evaluation in medicinal herbs and spices from EU and non-EU countries. Phytochem. Lett. 2021, 46, 153–161. [Google Scholar] [CrossRef]
- Mukhtar, K.; Nabi, B.G.; Ansar, S.; Bhat, Z.F.; Aadil, R.M.; Mousavi Khaneghah, A. Mycotoxins and consumers’ awareness: Recent progress and future challenges. Toxicon 2023, 232, 107227. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, S.F.; Ahmadpourmir, H.; Hayes, A.W.; Rezaee, R.; Karimi, G. Probabilistic risk assessment of exposure to multiple mycotoxins in consumers of packaged and unpackaged spices in Iran. Toxicon 2023, 232, 107222. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.; Riaz, M.; Naeem, I.; Gong, Y.Y.; Ismail, A.; Hussain, M.; Akram, K. Risk assessment of aflatoxins and selected heavy metals through intake of branded and non-branded spices collected from the markets of Multan city of Pakistan. Food Control 2020, 112, 107132. [Google Scholar] [CrossRef]
- Wang, Y.; Su, B.; Yan, X.; Geng, C.; Lian, T.; Li, X.; Xu, Y.; Li, Y. Studies of Mycotoxins in Medicinal Plants Conducted Worldwide over the Last Decade: A Systematic Review, Meta-Analysis, and Exposure Risk Assessment. Phytomedicine 2024, 128, 155367. [Google Scholar] [CrossRef]
- Hamad, G.M.; Mehany, T.; Simal-Gandara, J.; Abou-Alella, S.; Esua, O.J.; Abdel-Wahhab, M.A.; Hafez, E.E. A review of recent innovative strategies for controlling mycotoxins in foods. Food Control 2023, 144, 109350. [Google Scholar] [CrossRef]
- Prelle, A.; Spadaro, D.; Garibaldi, A.; Gullino, M.L. Co-occurrence of aflatoxins and ochratoxin A in spices commercialized in Italy. Food Control 2014, 39, 192–197. [Google Scholar] [CrossRef]
- Hammami, W.; Fiori, S.; Al Thani, R.; Ali Kali, N.; Balmas, V.; Migheli, Q.; Jaoua, S. Fungal and aflatoxin contamination of marketed spices. Food Control 2014, 37, 177–181. [Google Scholar] [CrossRef]
- Wan Ainiza, W.M.; Jinap, S.; Sanny, M. Simultaneous determination of aflatoxins and ochratoxin A in single and mixed spices. Food Control 2015, 50, 913–918. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Q.; Wang, S.; Cai, R.; Yuan, Y.; Yue, T.; Wang, Z. Bio-control on the contamination of Ochratoxin A in food: Current research and future prospects. Curr. Res. Food Sci. 2022, 5, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Mahato, D.K.; Lee, K.E.; Kamle, M.; Devi, S.; Dewangan, K.N.; Kumar, P.; Kang, S.G. Aflatoxins in Food and Feed: An Overview on Prevalence, Detection and Control Strategies. Front. Microbiol. 2019, 10, 2266. [Google Scholar] [CrossRef] [PubMed]
- Pfohl-Leszkowicz, A.; Manderville, R.A. Ochratoxin A: An overview on toxicity and carcinogenicity in animals and humans. Mol. Nutr. Food Res. 2007, 51, 61–99. [Google Scholar] [CrossRef] [PubMed]
- Almusa, M.A.; Al-Otibi, F.O.; Alreshoodi, F.M.; Alsalman, S.A.; Mukhtar, L.E.; Alharbi, A.L.; Aldosari, Z.M.; Alkaleeb, M.A.; Alarjani, K.M.; Alkhanani, M.F.; et al. Detection and molecular characterization of aflatoxin and ochratoxin produce aspergillus species in capsicum spices in Saudi Arabia. Food Control 2024, 162, 110377. [Google Scholar] [CrossRef]
- Tosun, H.; Arslan, R. Determination of aflatoxin B1 levels in organic spices and herbs. Sci. World J. 2013, 2013, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Li, Q. Aflatoxin B1 in poultry liver: Toxic mechanism. Toxicon 2023, 233, 107262. [Google Scholar] [CrossRef]
- Adedara, I.A.; Atanda, O.E.; Sant’Anna Monteiro, C.; Rosemberg, D.B.; Aschner, M.; Farombi, E.O.; Rocha, J.B.T.; Furian, A.F.; Emanuelli, T. Cellular and molecular mechanisms of aflatoxin B1-mediated neurotoxicity: The therapeutic role of natural bioactive compounds. Environ. Res. 2023, 237, 116869. [Google Scholar] [CrossRef]
- Ge, B.; Yan, K.; Sang, R.; Wang, W.; Liu, X.; Yu, M.; Liu, X.; Qiu, Q.; Zhang, X. Integrated network toxicology, molecular docking, and in vivo experiments to elucidate molecular mechanism of aflatoxin B1 hepatotoxicity. Ecotoxicol. Environ. Saf. 2024, 275, 116278. [Google Scholar] [CrossRef] [PubMed]
- Makhlouf, M.M.M. Histological and ultrastructural study of AflatoxinB1 induced neurotoxicity in Sciatic nerve of adult male Albino rats. Ultrastruct. Pathol. 2020, 44, 52–60. [Google Scholar] [CrossRef]
- Obafemi, B.A.; Adedara, I.A.; Rocha, J.B.T. Neurotoxicity of ochratoxin A: Molecular mechanisms and neurotherapeutic strategies. Toxicology 2023, 497–498, 153630. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.C.; Nebbia, C.S.; et al. Risk assessment of ochratoxin A in food. EFSA J. 2020, 18, e06113. [Google Scholar] [CrossRef]
- Yang, Q.; Dhanasekaran, S.; Ngea, G.L.N.; Tian, S.; Li, B.; Zhang, H. Unveiling ochratoxin a controlling and biodetoxification molecular mechanisms: Opportunities to secure foodstuffs from OTA contamination. Food Chem. Toxicol. 2022, 169, 113437. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Deng, M.; Chen, C.; Lv, L.; Zhu, H.; Chen, L.; Weng, H. Interacted toxic mechanisms of ochratoxin A and tricyclazole on the zebrafish (Danio rerio). Chemosphere 2023, 326, 138429. [Google Scholar] [CrossRef]
- Tsilioni, I.; Theoharides, T.C. Ochratoxin A stimulates release of IL-1β, IL–18 and CXCL8 from cultured human microglia. Toxicology 2024, 502, 153738. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lim, W.; You, S.; Song, G. Ochratoxin A exerts neurotoxicity in human astrocytes through mitochondria-dependent apoptosis and intracellular calcium overload. Toxicol. Lett. 2019, 313, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Ihim, S.A.; Abubakar, S.D.; Zian, Z.; Sasaki, T.; Saffarioun, M.; Maleknia, S.; Azizi, G. Interleukin-18 cytokine in immunity, inflammation, and autoimmunity: Biological role in induction, regulation, and treatment. Front. Immunol. 2022, 13, 919973. [Google Scholar] [CrossRef]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011, 22, 189. [Google Scholar] [CrossRef]
- Russo, R.C.; Garcia, C.C.; Teixeira, M.M.; Amaral, F.A. The CXCL8/IL-8 chemokine family and its receptors in inflammatory diseases. Expert Rev. Clin. Immunol. 2014, 10, 593–619. [Google Scholar] [CrossRef] [PubMed]
- Niessen, L.; Bechtner, J.; Fodil, S.; Taniwaki, M.H.; Vogel, R.F. LAMP-based group specific detection of aflatoxin producers within Aspergillus section Flavi in food raw materials, spices, and dried fruit using neutral red for visible-light signal detection. Int. J. Food Microbiol. 2018, 266, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Majer-Baranyi, K.; Zalán, Z.; Mörtl, M.; Juracsek, J.; Szendro, I.; Székács, A.; Adányi, N. Optical waveguide lightmode spectroscopy technique-based immunosensor development for aflatoxin B1 determination in spice paprika samples. Food Chem. 2016, 211, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Goda, Y.; Tanaka, T.; Toyoda, M. Determination of aflatoxins B1, B2, G1 and G2 in spices using a multifunctional column clean-up. J. Chromatogr. A 2001, 932, 153–157. [Google Scholar] [CrossRef]
- Abrar, M.; Ahsan, S.; Nadeem, M.; Liaqat, A.; Chughtai, M.F.J.; Farooq, M.A.; Mehmood, T.; Khaliq, A.; Siddiqa, A. Detection and quantification of aflatoxins in spices stored in different food packaging materials. J. Stored Prod. Res. 2023, 101, 102081. [Google Scholar] [CrossRef]
- Mo, Y.X.; Ruan, M.L.; Wang, J.; Liu, Y.; Wu, Y.Y.; Wang, G.L.; Han, Y.M.; Wan, H.F.; Lamesgen, D.; Kuča, K.; et al. Mitigating the adverse effects of Aflatoxin B1 in LMH, IPEC-J2 and 3D4/21 cells by a novel integrated agent. Food Chem. Toxicol. 2023, 178, 113907. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, S.P.; Jagadeeswaran, A.; Natarajan, A. Pharmacokinetics, dynamics, toxicology and molecular docking of bioactive alkaloid vasicine from Adhatoda vasica: A promising toxin binder against aflatoxin B1 and ochratoxin A. Poult. Sci. 2024, 103, 103272. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Chaudhari, A.K.; Singh, V.K.; Singh, B.K.; Dubey, N.K. High speed homogenization assisted encapsulation of synergistic essential oils formulation: Characterization, in vitro release study, safety profile, and efficacy towards mitigation of aflatoxin B1 induced deterioration in rice samples. Food Chem. Toxicol. 2022, 169, 113443. [Google Scholar] [CrossRef]
- Akdemir Evrendilek, G.; Bulut, N.; Atmaca, B.; Uzuner, S. Prediction of Aspergillus parasiticus inhibition and aflatoxin mitigation in red pepper flakes treated by pulsed electric field treatment using machine learning and neural networks. Food Res. Int. 2022, 162, 111954. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).