Recent Advances in Understanding the Keys Factors Influencing Pressurized Liquid Extraction of Secondary Metabolites: A Comprehensive Review †
Abstract
1. Introduction
2. Methodology
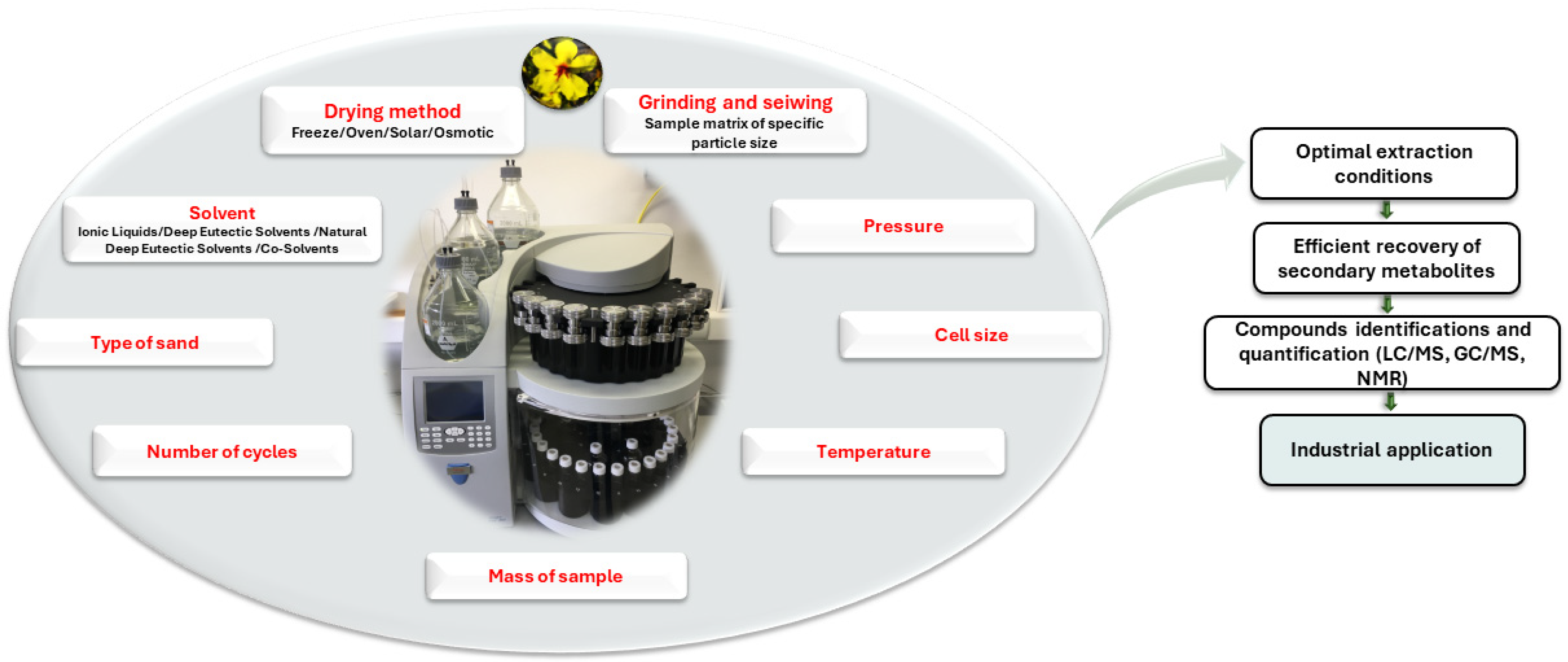
3. Key Factors Influencing PLE
3.1. Sample Drying Methods
3.2. Matrix Pre-Treatment and Characteristics
3.3. Solvent Selection
3.4. Pressure and Temperature
3.5. Instrumentation
- Automation and integration;
- High-pressure capabilities;
- Temperature control;
- Pressure monitoring and regulation;
- Enhanced safety features;
- Compatibility with a variety of matrices;
- Reduced solvent consumption;
- Improved sample throughput;
- User-friendly interfaces and software;
- Miniaturization and portability.
4. Optimal Extraction Parameters for Specific Secondary Metabolites from Different Plant Sources
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basholli-Salihu, M.; Schuster, R.; Hajdari, A.; Mulla, D.; Viernstein, H.; Mustafa, B.; Mueller, M. Phytochemical Composition, Anti-Inflammatory Activity and Cytotoxic Effects of Essential Oils from Three Pinus spp. Pharm. Biol. 2017, 55, 1553–1560. [Google Scholar] [CrossRef]
- Alvarez-Rivera, G.; Bueno, M.; Ballesteros-Vivas, D.; Mendiola, J.A.; Ibañez, E. Pressurized Liquid Extraction. In Liquid-Phase Extraction; Elsevier: Amsterdam, The Netherlands, 2019; pp. 375–398. ISBN 9780128169117. [Google Scholar]
- Wijngaard, H.; Brunton, N. The Optimization of Extraction of Antioxidants from Apple Pomace by Pressurized Liquids. J. Agric. Food Chem. 2009, 57, 10625–10631. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Lin, S.; Wang, Z.; Wang, C.; Wang, E.; Zhang, Y. Supercritical Fluid Extraction of Flavonoids from Maydis Stigma and Its Nitrite-Scavenging Ability. Food Bioprod. Process. 2011, 89, 333–339. [Google Scholar] [CrossRef]
- Herrero, M.; Castro-Puyana, M.; Mendiola, J.A.; Ibañez, E. Compressed Fluids for the Extraction of Bioactive Compounds. TrAC-Trends Anal. Chem. 2013, 43, 67–83. [Google Scholar] [CrossRef]
- Da Silva, L.C.; Vigano, J.; Souza, L.M.D.; Bai, A.L.; De Souza, M.C.; Sanches, V.L.; Chaves, J.O.; Pizani, R.S.; Contieri, L.S.; Rostagno, M.A. Recent Advances and Trends in Extraction Techniques to Recover Polyphenols Compounds from Apple By-Products. Food Chem. X 2021, 12, 100133. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.C.; Viganó, J.; Sanches, V.L.; De Souza Mesquita, L.M.; Pizani, R.; Rostagno, M.A. Simultaneous Extraction and Analysis of Apple Pomace by Gradient Pressurized Liquid Extraction Coupled In-Line with Solid-Phase Extraction and on-Line with HPLC. Food Chem. 2023, 407, 135117. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.E.d; Aguiar, G.P.S.; Magro, C.D.; Lacowicz, R.A.; Fedrigo, I.M.T.; Bordignon-Luiz, M.T.; Oliveira, J.V.; Lanza, M. Impact of Drying Method as Pretreatment for Extraction of Bioactive Compounds from Jambolan (Syzygium cumini (L.) Skeels). Braz. J. Food Technol. 2022, 25, e2021055. [Google Scholar] [CrossRef]
- Belwal, T.; Cravotto, C.; Prieto, M.A.; Venskutonis, P.R.; Daglia, M.; Devkota, H.P.; Baldi, A.; Ezzat, S.M.; Gómez-Gómez, L.; Salama, M.M.; et al. Effects of Different Drying Techniques on the Quality and Bioactive Compounds of Plant-Based Products: A Critical Review on Current Trends. Dry. Technol. 2022, 40, 1539–1561. [Google Scholar] [CrossRef]
- Awad, A.M.; Kumar, P.; Ismail-Fitry, M.R.; Jusoh, S.; Ab Aziz, M.F.; Sazili, A.Q. Green Extraction of Bioactive Compounds from Plant Biomass and Their Application in Meat as Natural Antioxidant. Antioxidants 2021, 10, 1465. [Google Scholar] [CrossRef]
- Fuente-Ballesteros, A.; Priovolos, I.; Ares, A.M.; Samanidou, V.; Bernal, J. Green Sample Preparation Methods for the Analysis of Bioactive Compounds in Bee Products: A Review. Adv. Sample Prep. 2023, 6, 100060. [Google Scholar] [CrossRef]
- Barp, L.; Višnjevec, A.M.; Moret, S. Pressurized Liquid Extraction: A Powerful Tool to Implement Extraction and Purification of Food Contaminants. Foods 2023, 12, 2017. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; E Silva, F.A.; Quental, M.V.; Mondal, D.; Freire, M.G.; Coutinho, J.A.P. Ionic-Liquid-Mediated Extraction and Separation Processes for Bioactive Compounds: Past, Present, and Future Trends. Chem. Rev. 2017, 117, 6984–7052. [Google Scholar] [CrossRef]
- Feng, J.; Loussala, H.M.; Han, S.; Ji, X.; Li, C.; Sun, M. Recent Advances of Ionic Liquids in Sample Preparation. TrAC-Trends Anal. Chem. 2020, 125, 115833. [Google Scholar] [CrossRef]
- Lim, J.R.; Chua, L.S.; Mustaffa, A.A. Ionic Liquids as Green Solvent and Their Applications in Bioactive Compounds Extraction from Plants. Process Biochem. 2022, 122, 292–306. [Google Scholar] [CrossRef]
- Huamán-Castilla, N.L.; Gajardo-Parra, N.; Pérez-Correa, J.R.; Canales, R.I.; Martínez-Cifuentes, M.; Contreras-Contreras, G.; Mariotti-Celis, M.S. Enhanced Polyphenols Recovery from Grape Pomace: A Comparison of Pressurized and Atmospheric Extractions with Deep Eutectic Solvent Aqueous Mixtures. Antioxidants 2023, 12, 1446. [Google Scholar] [CrossRef]
- García-Roldán, A.; Piriou, L.; Jauregi, P. Natural Deep Eutectic Solvents as a Green Extraction of Polyphenols from Spent Coffee Ground with Enhanced Bioactivities. Front. Plant Sci. 2023, 13, 1072592. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chang, H.; Jiang, S.; Chen, J.; Wang, J.; Liang, H.; Li, G.; Tang, X. An Efficient Co-Solvent Tailoring Interfacial Polymerization for Nanofiltration: Enhanced Selectivity and Mechanism. J. Memb. Sci. 2023, 677, 121615. [Google Scholar] [CrossRef]
- Raj, T.; Morya, R.; Chandrasekhar, K.; Kumar, D.; Soam, S.; Kumar, R.; Patel, A.K.; Kim, S.H. Microalgae Biomass Deconstruction Using Green Solvents: Challenges and Future Opportunities. Bioresour. Technol. 2023, 369, 128429. [Google Scholar] [CrossRef]
- García, P.; Fredes, C.; Cea, I.; Lozano-Sánchez, J.; Leyva-Jiménez, F.J.; Robert, P.; Vergara, C.; Jimenez, P. Recovery of Bioactive Compounds from Pomegranate (Punica granatum L.) Peel Using Pressurized Liquid Extraction. Foods 2021, 10, 203. [Google Scholar] [CrossRef]
- Huamán-Castilla, N.L.; Campos, D.; García-Ríos, D.; Parada, J.; Martínez-Cifuentes, M.; Mariotti-Celis, M.S.; Pérez-Correa, J.R. Chemical Properties of Vitis Vinifera Carménère Pomace Extracts Obtained by Hot Pressurized Liquid Extraction, and Their Inhibitory Effect on Type 2 Diabetes Mellitus Related Enzymes. Antioxidants 2021, 10, 472. [Google Scholar] [CrossRef]
- Nagybákay, N.E.; Sarapinaitė, L.; Syrpas, M.; Venskutonis, P.R.; Kitrytė-Syrpa, V. Optimization of Pressurized Ethanol Extraction for Efficient Recovery of Hyperoside and Other Valuable Polar Antioxidant-Rich Extracts from Betula Pendula Roth Leaves. Ind. Crops Prod. 2023, 205, 117565. [Google Scholar] [CrossRef]
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R.S. Pressurized Aqueous Solutions of Deep Eutectic Solvent (DES): A Green Emergent Extraction of Anthocyanins from a Brazilian Berry Processing by-Product. Food Chem. X 2022, 13, 100236. [Google Scholar] [CrossRef] [PubMed]
- Chamali, S.; Bendaoud, H.; Bouajila, J.; Camy, S.; Saadaoui, E.; Condoret, J.S.; Romdhane, M. Optimization of Accelerated Solvent Extraction of Bioactive Compounds from Eucalyptus Intertexta Using Response Surface Methodology and Evaluation of Its Phenolic Composition and Biological Activities. J. Appl. Res. Med. Aromat. Plants 2023, 35, 100464. [Google Scholar] [CrossRef]
- Myers, C.; Herrington, J.S.; Hamrah, P.; Anderson, K. Accelerated Solvent Extraction of Terpenes in Cannabis Coupled with Various Injection Techniques for GC-MS Analysis. Front. Chem. 2021, 9, 619770. [Google Scholar] [CrossRef] [PubMed]
| Matrix | Target Compounds | Key Extraction Factors | Yield | Ref. | |||
|---|---|---|---|---|---|---|---|
| Solvent (Category) | Temperature (°C) | Pressure (MPa) | Time (min) | ||||
| Punica granatum peels | Phenolic acids, flavonoids, hydrolysable tannins | Ethanol/water 50:50 (v/v) (co-solvents) | 200 | 10 | 20 | Punicalagin: 22.0 ± 0.3 mg/g DW | [20] |
| Vitis vinifera L pomace | Monomers of flavanols, flavanols, phenolic acids | Glycerol/water 15:85 (v/v) (co-solvents) | 150 | 10 | 5 | Catechin: 203.60 ± 12.22 µg/g DW | [21] |
| Betulapendula Roth leaves | Hyperoside, Betuloside | Ethanol 100% (polar solvent) | 86 | 10 | 39 | 32% w/w; Hyperoside (54.1 mg/g), Betuloside (18.3 mg/g) | [22] |
| Myrciaria cauliflora peels | Anthocyanin | ChCl: Pro at 1:2 M ratio 47% (DES) | 90 | 10 | 12 | AR = 69.08 ± 2.62% w/w | [23] |
| Eucalyptus intertexta leaves | Flavonoids, phenolic acids | Ethanol 26.6% (polar solvent) | 179 | 36 | 52.3 g extract/100 g DW | [24] | |
| Cannabis sativa L flowers | Terpenoids | Isopropanol (polar solvent) | 75 | 10 | 5 | Β-pinene, Giraniol; ~higher vs. conventional hand shakeout | [25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donn, P.; Seyyedi-Mansour, S.; Chamorro, F.; Garcia-Oliveira, P.; Echave, J.; Perez-Vazquez, A.; Barciela, P.; Cassani, L.; Prieto, M.A. Recent Advances in Understanding the Keys Factors Influencing Pressurized Liquid Extraction of Secondary Metabolites: A Comprehensive Review. Biol. Life Sci. Forum 2024, 35, 1. https://doi.org/10.3390/blsf2024035001
Donn P, Seyyedi-Mansour S, Chamorro F, Garcia-Oliveira P, Echave J, Perez-Vazquez A, Barciela P, Cassani L, Prieto MA. Recent Advances in Understanding the Keys Factors Influencing Pressurized Liquid Extraction of Secondary Metabolites: A Comprehensive Review. Biology and Life Sciences Forum. 2024; 35(1):1. https://doi.org/10.3390/blsf2024035001
Chicago/Turabian StyleDonn, Pauline, Sepidar Seyyedi-Mansour, Franklin Chamorro, Paula Garcia-Oliveira, Javier Echave, Ana Perez-Vazquez, Paula Barciela, Lucia Cassani, and Miguel Angel Prieto. 2024. "Recent Advances in Understanding the Keys Factors Influencing Pressurized Liquid Extraction of Secondary Metabolites: A Comprehensive Review" Biology and Life Sciences Forum 35, no. 1: 1. https://doi.org/10.3390/blsf2024035001
APA StyleDonn, P., Seyyedi-Mansour, S., Chamorro, F., Garcia-Oliveira, P., Echave, J., Perez-Vazquez, A., Barciela, P., Cassani, L., & Prieto, M. A. (2024). Recent Advances in Understanding the Keys Factors Influencing Pressurized Liquid Extraction of Secondary Metabolites: A Comprehensive Review. Biology and Life Sciences Forum, 35(1), 1. https://doi.org/10.3390/blsf2024035001











