Abstract
In acidic soils with Mn toxicity, the development of an intact arbuscular mycorrhiza extraradical mycelium (ERM) by stress-adapted native plants can promote increased growth and protection against metal toxicity, in subsequent crops. In a recent study, the growth of Ornithopus compressus (ORN) in acidic soil doubled shoot weight, increased P contents and decreased shoot Mn in successive wheat crops. The biochemical mechanisms involved in this beneficial effect may include the subcellular redistribution of nutrients and of excess Mn. In the present work, shoot Ca, Fe, Mg, Mn, P, K, Si, Na and Zn were mapped through Laser Ablation-Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS), in wheat grown for 3 weeks in undisturbed soil, where ORN previously developed an extensive ERM network. Element mapping allowed for the detection of higher levels of Fe, Mg, Mn, P, K, Na and Zn in the interveinal sections of wheat leaves while Ca and Si showed higher levels in vascular zones of the adaxial side. This preliminary work is part of an ongoing project which aims at identifying biochemical mechanisms responsible for the protective properties of an early AM colonization of crops, by ERM previously developed in association with native plants under Mn toxicity. Future research will determine the subcellular redistribution of these elements and excess Mn.
1. Introduction
Arbuscular mycorrhizal fungi (AMF) are obligate biotrophic soil microorganisms of the phylum Glomeromycota that establish mutualistic relationships with about 80% of terrestrial plants [1]. Benefits to plant hosts include (a) improved access to water and nutrients, such as phosphorus (P), nitrogen (N) and other elements, (b) higher protection against abiotic and biotic stresses and (c) improvement of soil structure [2]. In acidic soils that generally promote an increase in levels of bioavailable manganese (Mn), AMF can both buffer metal toxicity and increase plant growth, allowing hosts to thrive in inhospitable soils. Stress adapted microbiota can be used to improve growth and productivity of agricultural species in acidic soils, by growing local native plants and using the intact extraradical mycelium (ERM) they develop in the soil as main inoculum source for the subsequent crop [3]. This sustainable agricultural practice can lead to an almost 3-fold decrease in Mn levels and a 1.5-fold increase in plant growth [4,5]. In the Montado ecosystem, in the south-east of Portugal, acidic soils, commonly Cambisols and Arenosols, can promote Mn toxicity, being considered one of the main constraints to plant production [6]. In wheat, the previous growth of native Ornithopus compressus (ORN) showed noteworthy beneficial effects granted by the earlier and faster colonization by its associated intact AMF extraradical mycelium [5]. The presence of ERM was important for the AMF diversity that subsequently colonized wheat [7]. The use of this AMF inoculum source was seen to promote the transcription of genes related to cellular division and growth in wheat, but also to manage the transcription of genes that code for metal transporters responsible for translocation and distribution of Mn in the plant [8]. In the present work, Mn and other plant nutrients and elements were quantified and mapped in the leaves of wheat grown in undisturbed acidic soil where stress adapted AMF were developed by the previous growth of ORN. Element abundance heat maps were obtained by laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS). The present preliminary work was performed as part of an ongoing larger project which aims at identifying the beneficial effects of the early AM colonization, by ERM previously developed in association with native plants, on the element transport dynamics of wheat under Mn toxicity in acidic soil.
2. Material and Methods
2.1. Experimental Setup and Plant Material
Plants were grown on acidic soil collected from the top 20 cm of a granitic Cambisol reported to induce Mn toxicity symptoms in wheat [9,10], which was chemically [11] and biologically characterized [7]. The experimental setup followed was previously reported [5]. Briefly, 8 L pots filled with acidic Cambisol were used to grow ORN, a strongly mycotrophic endemic plant. After seven weeks, plants were eliminated leaving the soil undisturbed (intact ERM). After seven days, ten wheat (Triticum aestivum L. cv. Ardila) seedlings were planted and after 21 days of growth, shoots were excised and immediately frozen intact in liquid nitrogen and stored at −80 °C until analysis.
2.2. Wheat Shoot Element Mapping
Wheat leaf anatomical distribution of Ca, Fe, Mg, Mn, P, K, Si, Na and Zn was determined by a Laser Ablation LSX-213 G2+ system, CETAC technologies, connected to an Agilent 8800 Triple Quadrupole ICP-MS [11]. Intact frozen sections of leaves of wheat grown in undisturbed soil, from previously grown ORN, were mounted and fixed on a glass microscope slide with carbon tape, with abaxial or adaxial sides facing upwards. Laser ablation was performed, immediately, in 2 mm length transversal sections of the whole leaf width. Determination of multi-elemental distribution was performed by line scan with 30 µm diameter size, 60% laser energy, 20 Hz laser shot frequency, 110 µm/s scan speed and 1 L/min of helium (He) carrier gas flow. Conversion of obtained counts per second (cps) to images was performed using iQuant2 software, developed by Institute of Technology of Tokyo (Tokyo, Japan) and University of Kyoto.
2.3. Wheat Shoot Element Quantification
Element levels were quantified in wheat shoot tissues after acid digestion, as described in [12]. The concentrations of Ca, Fe, Mg, Mn, P, K, Si, Na and Zn were determined in 2% HNO3 aqueous solutions of the fully digested shoot tissue with an Agilent 8800 Triple Quadrupole ICP-MS, according to [11]. The triple quadrupole mass spectrometer collision/reaction cell was set to “no-gas mode” for the quantification of Mg, Mn, Na and K, “O2 mode” for the quantification of P and Si, “NH3 mode” for the quantification of Ca, Fe and “He mode” for the quantification of Zn. Results were presented as average and standard error of 4 replicates.
3. Results
In mature wheat, the leaf lamina is composed of a well-marked midrib, enclosing the major vascular bundle. This central vein divides the lamina into two sections containing parallel veins with smaller vascular bundles, in strips alternating with the interveinal portions composed of mesophyll tissue. In the adaxial side, the tissue covering the main vascular bundle is ridged while the abaxial side is flatter. Strips of abundant stomata are disposed along the lamina (examples in [13,14]). Element mapping allowed for the identification of macro and micronutrients in transversal sections of the abaxial and adaxial sides of wheat leaves, following their distribution and abundance in heat maps.
The distribution of Fe, Mg, Mn, P, K, Na and Zn was higher and more homogenous in the interveinal sections of wheat leaves, while parallel veins showed lower levels of these elements (Figure 1c–j or Figure 1m–r).
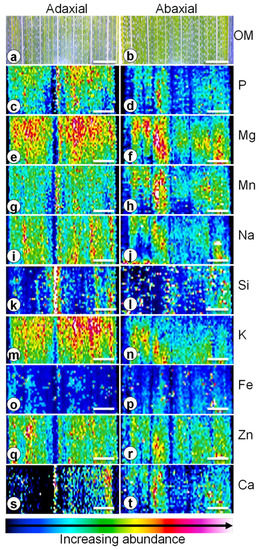
Figure 1.
Light micrograph (a,b) and laser ablation coupled to inductively coupled plasma mass spectrometry heat maps of phosphorus (c,d), magnesium (e,f), manganese (g,h), sodium (i,j), silicon (k,l), potassium (m,n), iron (o,p), zinc (q,r) and calcium (s,t) of the adaxial (a,c,e,g,i,k,m,o,q,s) and abaxial (b,d,f,h,j,l,n,p,r,t) sides of leaves of 3-week-old wheat plants grown in undisturbed soil with previously grown Ornithopus compressus L. associated arbuscular mycorrhizal fungi. Bar = 1 mm.
The ridged midrib in the adaxial side appeared to show lower levels of these elements which made the remaining tissue show a higher response (warmer colours). In the abaxial side, differences were not so marked, but a clear difference could still be seen between the interveinal zones and veins. Whole tissue element analysis quantified 136 ± 14 mg/kg for Fe, 1707 ± 115 mg/kg for Mg, 61 ± 6 mg/kg for Mn, well below the 100 to 200 mg/kg threshold considered toxic for most cereals [5,15], 2280 ± 55 mg/kg for P, 31,652 ± 938 mg/kg for K, 169 ± 38 mg/kg for Na and 80 ± 7 mg/kg for Zn (Table 1).

Table 1.
Concentration of Ca, Fe, Mg, Mn, P, K, Si, Na and Zn (mg/kg shoot dry weight) in shoots of wheat grown for 21 days in acidic soil containing intact extraradical mycelium of arbuscular mycorrhiza associated to Ornithopus compressus L.
Silicon and Ca showed a different pattern in the adaxial side, and heat maps displayed a higher abundance of these elements in the midrib in comparison to the interveinal zones, which can indicate a higher translocation of these elements. In the abaxial side, their distribution pattern closely resembles that of the other elements (Figure 1k,l,s,t). Additionally, Si in high proportions appeared in punctuated areas on both sides, which may be attributed to areas with trichomes. Shoot tissue concentrations for Ca and Si were 2253 ± 129 and 1132 ± 148 mg/kg, respectively (Table 1).
4. Discussion
Colonization of plant roots with AMF can lead to extensive changes in chemical and biochemical processes in the plant hosts. Its most notable effect on element concentration is the increase in concentrations of P and N in the host, nevertheless other elements can also be influenced. In a study using LA-ICP-MS to compare the effect of AMF colonization between grain element concentration in wheat and barley, the localizations of the metals Zn, Fe, Mn, Cu, Co and Ni appeared more deeply influenced in wheat than in barley while those of the macronutrients P, Mg, K and S were more influenced in barley grains, after AMF colonization [16]. This indicates that AMF influence on the mechanisms of element redistribution can be extremely dependent on species. The preliminary work herewith presented allows establishing a basis of comparison for the analysis of the intensity in which previous growth of different mycotrophic plants can affect element distribution in succeeding wheat grown under Mn toxicity associated to acidic soil. For this effect, the methodologies established in this work will be applied, in future studies, to leaves of wheat grown in soil with the previous growth of native plants with diverse levels of mycothrophy, such as Silene gallica L., Rumex bucephalophorus L. or Lolium rigidum L., and where no native plant was planted. Agronomic practices that include the pre-establishment of native stress adapted ERM with low to no tillage techniques can benefit from this knowledge in the selection of native AMF Developers that favor crop growth while contributing to the biofortification of host nutritional composition.
Author Contributions
Conceptualization, J.M.S.F., P.B., D.M.T., A.P.P., I.B. and M.C.; methodology, J.M.S.F., P.B., D.M.T., A.P.P., I.B. and M.C.; software, J.M.S.F. and P.B.; investigation, J.M.S.F. and P.B.; resources, D.M.T., A.P.P., I.B. and M.C.; writing—original draft preparation, J.M.S.F.; writing—review and editing, J.M.S.F., P.B., D.M.T., A.P.P., I.B. and M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partially funded by Fundo Europeu de Desenvolvimento Regional (FEDER), Programa Operacional Regional Alentejo 2020 under research contract ALT20-03-0145-FEDER-000039.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the findings of this study are available from the corresponding author (Jorge M. S. Faria) upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Panneerselvam, P.; Kumar, U.; Sugitha, T.C.K.; Parameswaran, C.; Sahoo, S.; Binodh, A.K.; Jahan, A.; Anandan, A. Arbuscular Mycorrhizal Fungi (AMF) for Sustainable Rice Production. In Advances in Soil Microbiology: Recent Trends and Future Prospects, Microorganisms for Sustainability; Adhya, T.K., Mishra, B.B., Annapurna, K., Verma, D.K., Kumar, U., Eds.; Springer: Singapore, 2017; pp. 99–126. ISBN 9789811073809. [Google Scholar]
- Rintoul, N. Arbuscular mycorrhizal associations in plant nutrition and health. CAB Rev. 2016, 11, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Brito, I.; Goss, M.J.; Alho, L.; Brígido, C.; van Tuinen, D.; Félix, M.R.; Carvalho, M. Agronomic management of AMF functional diversity to overcome biotic and abiotic stresses—The role of plant sequence and intact extraradical mycelium. Fungal Ecol. 2019, 40, 72–81. [Google Scholar] [CrossRef]
- Alho, L.; Carvalho, M.; Brito, I.; Goss, M.J. The effect of arbuscular mycorrhiza fungal propagules on the growth of subterranean clover (Trifolium subterraneum L.) under Mn toxicity in ex situ experiments. Soil Use Manag. 2015, 31, 337–344. [Google Scholar] [CrossRef]
- Brito, I.; Carvalho, M.; Alho, L.; Goss, M.J. Managing arbuscular mycorrhizal fungi for bioprotection: Mn toxicity. Soil Biol. Biochem. 2014, 68, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Goss, M.J.; Carvalho, M.J.G.P.R. Manganese toxicity: The significance of magnesium for the sensitivity of wheat plants. Plant Soil 1992, 139, 91–98. [Google Scholar] [CrossRef]
- Brígido, C.; van Tuinen, D.; Brito, I.; Alho, L.; Goss, M.J.; Carvalho, M. Management of the biological diversity of AM fungi by combination of host plant succession and integrity of extraradical mycelium. Soil Biol. Biochem. 2017, 112, 237–247. [Google Scholar] [CrossRef] [Green Version]
- Campos, C.; Nobre, T.; Goss, M.J.; Faria, J.; Barrulas, P.; Carvalho, M. Transcriptome Analysis of Wheat Roots Reveals a Differential Regulation of Stress Responses Related to Arbuscular Mycorrhizal Fungi and Soil Disturbance. Biology 2019, 8, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goss, M.J.; Carvalho, M.J.G.P.R.; Cosimini, V.; Fearnhead, M.L. An approach to the identification of potentially toxic concentrations of manganese in soils. Soil Use Manag. 1992, 8, 40–43. [Google Scholar] [CrossRef]
- Carvalho, M.; Goss, M.J.; Teixeira, D. Manganese toxicity in Portuguese Cambisols derived from granitic rocks: Causes, limitations of soil analyses and possible solutions. Rev. Ciências Agrárias 2015, 38, 518–527. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Teixeira, D.M.; Pinto, A.P.; Brito, I.; Barrulas, P.; Alho, L.; Carvalho, M. Toxic levels of manganese in an acidic Cambisol alters antioxidant enzymes activity, element uptake and subcellular distribution in Triticum aestivum. Ecotoxicol. Environ. Saf. 2020, 193, 110355. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.M.S.; Teixeira, D.M.; Pinto, A.P.; Brito, I.; Barrulas, P.; Carvalho, M. The Protective Biochemical Properties of Arbuscular Mycorrhiza Extraradical Mycelium in Acidic Soils Are Maintained throughout the Mediterranean Summer Conditions. Agronomy 2021, 11, 748. [Google Scholar] [CrossRef]
- Evert, R.F. Esau’s Plant Anatomy; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; ISBN 9780470047385. [Google Scholar]
- Parker, M.L.; Ford, M.A. The structure of the mesophyll of flag leaves in three Triticum species. Ann. Bot. 1982, 49, 165–176. [Google Scholar] [CrossRef]
- Pinto, E.; Aguiar, A.A.R.M.; Ferreira, I.M.P.L.V.O. Influence of soil chemistry and plant physiology in the phytoremediation of Cu, Mn, and Zn. CRC. Crit. Rev. Plant Sci. 2014, 33, 351–373. [Google Scholar] [CrossRef] [Green Version]
- Watts-Williams, S.J.; Gilbert, S.E. Arbuscular mycorrhizal fungi affect the concentration and distribution of nutrients in the grain differently in barley compared with wheat. Plants People Planet 2021, 3, 567–577. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).