Monitoring of a Calcium Biofortification Workflow for Tubers of Solanum tuberosum L. cv. Picasso Using Smart Farming Technology †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biofortification Itinerary
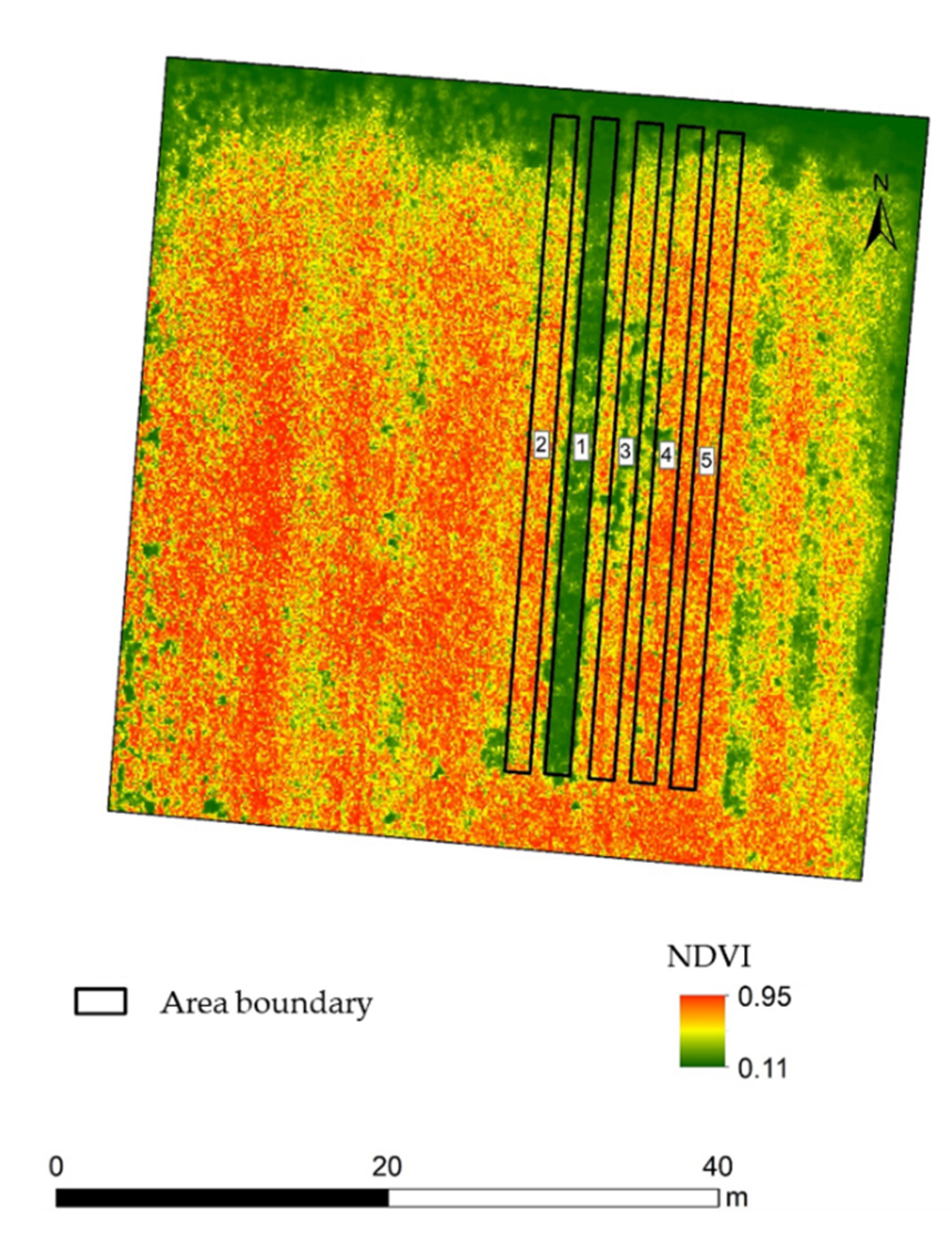
2.2. NDVI (Normalized Difference Vegetation Index) in the Experimental Field
2.3. Calcium Content in Leaves
2.4. Colorimetric Parameters
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Radoglou-Grammatikis, P.; Sarigiannidis, P.; Lagkas, T.; Moscholios, I. A compilation of UAV applications for precision agriculture. Comput. Netw. 2020, 172, 107148. [Google Scholar] [CrossRef]
- Sylvester, G. E-Agriculture in Action: Drones for Agriculture; Food and Agriculture Organization on the United Nations and International Telecommunication Union: Rome, Italy, 2018. [Google Scholar]
- Masud, M.M.; Azam, M.N.; Mohiuddin, M.; Banna, H.; Akhtar, R.; Alam, A.F.; Begum, H. Adaptation barriers and strategies towards climate change: Challenges in the agricultural sector. J. Clean. Prod. 2017, 156, 698–706. [Google Scholar] [CrossRef]
- Xue, J.; Su, B. Significant remote sensing vegetation indices: A review of developments and applications. J. Sens. 2017, 2017, 1353691. [Google Scholar] [CrossRef]
- Karnieli, A.; Agam, N.; Pinker, R.; Anderson, M.; lmhoff, M.; Gutman, G.; Panov, N.; Goldberg, A. Use of NDVI and land surface temperature for drought assessment: Merits and limitations. J. Clim. 2010, 23, 618–633. [Google Scholar] [CrossRef]
- Zaeen, A.A.; Sharma, L.; Jasim, A.; Bali, S.; Buzza, A.; Alyokhin, A. In-season potato yield prediction with active optical sensors. Agrosystems Geosci. Environ. 2020, 3, e20024. [Google Scholar] [CrossRef]
- Hunt, E.R.; Rondon, S.I. Detection of potato beet-le damage using remote sensing from small unmanned aircraft systems. J. Appl Remote Sens. 2017, 11, 026013. [Google Scholar] [CrossRef]
- Fernández, C.I.; Leblon, B.; Haddadi, A.; Wang, J.; Wang, K. Potato late blight detection at the leaf and canopy level using hyperspectral data. Can. J. Remote Sens. 2020, 46, 390–413. [Google Scholar] [CrossRef]
- Liu, C.; Hu, Z.; Islam, A.T.; Kong, R.; Yu, L.; Wang, Y.; Chen, S.; Zhang, X. Hyperspectral characteristics and inversion model estimation of winter wheat under different elevated CO2 concentrations. Int. J. Remote Sens. 2021, 42, 1035–1053. [Google Scholar] [CrossRef]
- CIP—International Potato Center. Potato Facts and Figures. Available online: https://cipotato.org/crops/po-tato/potato-facts-and-figures/ (accessed on 9 March 2021).
- FAO (Food and Agriculture Organization). Potato world: Production and Consumption—International Year of the Potato 2008. Available online: http://www.fao.org/potato-2008/en/world/ (accessed on 10 March 2021).
- Muthoni, J.; Mbiyu, M.; Nyamongo, D. A review of potato seed systems and germplasm conservation in Kenya. J. Agric. Food Inf. 2010, 11, 157–167. [Google Scholar] [CrossRef]
- Poggi, V.; Arcioni, A.; Filippini, P.; Pifferi, P. Foliar application of selenite and selenate to potato (Solanum tuberosum): Effect of a ligand agent on selenium content of tubers. J. Agric. Food Chem. 2000, 48, 4749–4751. [Google Scholar] [CrossRef]
- Oliveira, V.; Faquin, V.; Andrade, F.; Carneiro, J.; Júnior, E.; Souza, K.; Pereira, J.; Guil-herme, L. Physiological and physicochemical responses of potato to selenium biofortification in tropical soil. Potato Res. 2019, 62, 315–331. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Z.; Zhang, X.; Zhang, W.; Huang, L.; Zhang, Z.; Yuan, L.; Liu, X. Effects of foliar application of selenate and selenite at different growth stages on Selenium accumulation and speciation in potato (Solanum tuberosum L.). Food Chem. 2019, 286, 550–556. [Google Scholar] [CrossRef]
- White, P.; Thompson, J.; Wright, G.; Rasmussen, S. Biofortifying scottish potatoes with zinc. Plant Soil 2016, 411, 151–165. [Google Scholar] [CrossRef]
- Kromann, P.; Valverde, F.; Alvarado, S.; Vélez, R.; Pisuña, J.; Potosí, B.; Taipe, A.; Caballero, D.; Cabezas, A.; Devaux, A. Can Andean potatoes be agronomically biofortified with iron and zinc fertilizers? Plant Soil 2017, 411, 121–138. [Google Scholar] [CrossRef]
- Coelho, A.R.F.; Lidon, F.C.; Pessoa, C.C.; Marques, A.C.; Luís, I.C.; Caleiro, J.; Simões, M.; Kullberg, J.; Legoinha, P.; Brito, M.; et al. Can foliar pulverization with CaCl2 and Ca(NO3)2 trigger Ca enrichment in Solanum tuberosum L. tubers? Plants 2021, 10, 245. [Google Scholar] [CrossRef]
- Siró, I.; Kápolna, E.; Kápolna, B.; Lugasi, A. Functional food. Product development, marketing and consumer acceptance—A review. Appetite 2008, 51, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Marques, E.; Darby, H.M.; Kraft, J. Benefits and limitations of non-transgenic micronutrient biofortification approaches. Agronomy 2021, 11, 464. [Google Scholar] [CrossRef]
- Galanakis, C.M. The food systems in the era of the coronavirus (COVID-19) pandemic crisis. Foods 2020, 9, 523. [Google Scholar] [CrossRef]
- Precedence Research. Functional Food Market Size, Share, Growth, Trends, Consumption, Regional Insights and Forecast 2020 to 2027. Available online: https://www.precedenceresearch.com/functional-food-market (accessed on 12 March 2021).
- NIH (National Institutes of Health). Available online: https://pubchem.ncbi.nlm.nih.gov/compound/calcium (accessed on 12 March 2021).
- Luís, I.C.; Lidon, F.C.; Pessoa, C.C.; Marques, A.C.; Coelho, A.R.F.; Simões, M.; Patanita, M.; Dôres, J.; Ramalho, J.C.; Silva, M.M.; et al. Zinc enrichment in two contrasting genotypes of Triticum aestivum L grains: Interactions between Edaphic Conditions and Foliar Fertilizers. Plants 2021, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.; Pessoa, C.; Marques, A.; Luís, I.; Daccak, D.; Silva, M.M.; Simões, M.; Reboredo, F.; Pessoa, M.; Legoinha, P.; et al. Natural mineral enrichment in Solanum tuberosum L. cv. Agria: Accumulation of Ca and interaction with other nutrients by XRF analysis. In Proceedings of the 1st International Electronic Conference on Plant Science, Online, 1–15 December 2020; Volume 1, p. 15. [Google Scholar] [CrossRef]
- Ramalho, J.C.; Rebelo, M.C.; Santos, M.E.; Antunes, M.L.; Nunes, M.A. Effects of calcium deficiency on Coffea arabica. Nutrient changes and correlation of calcium levels with some photosynthetic parameters. Plant Soil 1995, 172, 87–96. [Google Scholar] [CrossRef]
- Hochmal, A.K.; Schulze, S.; Trompelt, K.; Hippler, M. Calcium-dependent regulation of photosynthesis. Biochim. Biophys. Acta 2015, 1847, 993–1003. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, S.; Wan, S.; Li, X. The Significance of calcium in photosynthesis. Int. J. Mol. Sci. 2019, 20, 1353. [Google Scholar] [CrossRef] [PubMed]
- de Souza Alonso, T.A.; Ferreira Barreto, R.; de Mello Prado, R.; Pereira de Souza, J.; Falleiros Carvalho, R. Silicon spraying alleviates calcium deficiency in tomato plants, but Ca-EDTA is toxic. J. Plant Nutr. Soil Sci. 2020, 183, 659–664. [Google Scholar] [CrossRef]
- El-Yazied, A.; Ragab, M.E.; Ibrahim, R.E.; El-Wafa, A. Effect of nitrogen fertigation levels and chelated Calcium foliar application on the productivity of sweet corn. AJS 2007, 15, 131–139. [Google Scholar] [CrossRef]
- Rasouli-Sadaghiani, M.; Moghaddas Gerani, M.; Ashrafi Saeidlou, S.; Sepehr, E. Effect of different calcium sources application on antioxidant, enzymatic activity and qualitative characteristics of Apple (Malus domestic). J. Crop Prod. Processing 2017, 7, 73–87. [Google Scholar] [CrossRef]

| Treatments | Ca (%) |
|---|---|
| Control | 4.29 d ± 0.17 |
| CaCl2 (12 kg ha−1) | 6.05 b ± 0.00 |
| CaCl2 (24 kg ha−1) | 7.94 a ± 0.01 |
| Ca-EDTA (12 kg ha−1) | 8.14 a ± 0.02 |
| Ca-EDTA (24 kg ha−1) | 5.57 c ± 0.01 |
| Treatments | L | Chroma | Hue |
|---|---|---|---|
| Control | 42.61 a ± 0.03 | 28.77 a ± 0.01 | 112.2 a ± 0.02 |
| CaCl2 (12 kg ha−1) | 33.25 d ± 0.00 | 16.66 d ± 0.00 | 95.66 c ± 0.05 |
| CaCl2 (24 kg ha−1) | 32.62 e ± 0.00 | 14.85 e ± 0.00 | 85.05 d ± 0.01 |
| Ca-EDTA (12 kg ha−1) | 33.50 c ± 0.01 | 17.68 c ± 0.01 | 83.19 e ± 0.04 |
| Ca-EDTA (24 kg ha−1) | 38.22 b ± 0.01 | 22.80 b ± 0.00 | 106.3 b ± 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, A.R.F.; Luís, I.C.; Marques, A.C.; Pessoa, C.C.; Daccak, D.; Caleiro, J.; Brito, M.; Kullberg, J.; Silva, M.M.; Simões, M.; et al. Monitoring of a Calcium Biofortification Workflow for Tubers of Solanum tuberosum L. cv. Picasso Using Smart Farming Technology. Biol. Life Sci. Forum 2021, 3, 18. https://doi.org/10.3390/IECAG2021-09660
Coelho ARF, Luís IC, Marques AC, Pessoa CC, Daccak D, Caleiro J, Brito M, Kullberg J, Silva MM, Simões M, et al. Monitoring of a Calcium Biofortification Workflow for Tubers of Solanum tuberosum L. cv. Picasso Using Smart Farming Technology. Biology and Life Sciences Forum. 2021; 3(1):18. https://doi.org/10.3390/IECAG2021-09660
Chicago/Turabian StyleCoelho, Ana Rita F., Inês Carmo Luís, Ana Coelho Marques, Cláudia Campos Pessoa, Diana Daccak, João Caleiro, Maria Brito, José Kullberg, Maria Manuela Silva, Manuela Simões, and et al. 2021. "Monitoring of a Calcium Biofortification Workflow for Tubers of Solanum tuberosum L. cv. Picasso Using Smart Farming Technology" Biology and Life Sciences Forum 3, no. 1: 18. https://doi.org/10.3390/IECAG2021-09660
APA StyleCoelho, A. R. F., Luís, I. C., Marques, A. C., Pessoa, C. C., Daccak, D., Caleiro, J., Brito, M., Kullberg, J., Silva, M. M., Simões, M., Reboredo, F. H., Pessoa, M. F., Legoinha, P., Silva, M. J., Rodrigues, A. P., Ramalho, J. C., Scotti-Campos, P., Semedo, J. N., Pais, I. P., & Lidon, F. C. (2021). Monitoring of a Calcium Biofortification Workflow for Tubers of Solanum tuberosum L. cv. Picasso Using Smart Farming Technology. Biology and Life Sciences Forum, 3(1), 18. https://doi.org/10.3390/IECAG2021-09660

















