Abstract
Vitamin D substantially influences sports performance and post-exercise recovery because it offers anti-inflammatory, antioxidant and cellular protective properties. However, deficient levels of 25-hydroxyvitamin D 25(OH)D (25(OH)D) (<30 ng/mL) could impact the health of individuals, lead to musculoskeletal disorders and decrease athletic performance. Therefore, it would be appropriate to know the interactions between genes and vitamin D. We evaluated whether 25(OH)D had a possible connection to the presence of certain SNPs in CYP2R1 (rs10741657), GC (rs2282679) and muscle VDR (rs2228570) genes, with serum 25(OH)D concentrations and the degree of WOD performance in highly trained CrossFit® practitioners. Knowing these relationships could be instrumental for personalized vitamin D supplementation and training strategies. Using a standardized commercial enzyme-linked immunosorbent assay procedure, the concentrations of 25(OH)D were determined and the genotyping procedures for each SNPs were carried out using specific assays with the KASpar® test. The 25(OH)DA performance level in grades was established based on the CrossFit® Total score (sum in kilograms of one Repetition Max Squat, Press and Deadlift). Significant differences (p < 0.05) in 25(OH)D concentration were found between each of the SNPs of CYP2R1 and GC with 25(OH)D. We discovered statistically significant weak positive correlations (p < 0.05) between 25(OH)D and AA-alleles of the CYP2R1 and VDR genes, and TT-alleles of the GC gene. Additionally, AA (rs10741657 and rs2228570) and TT (rs2282679) have a probability between 2 and 4 of having major concentrations of 25(OH)D and 25(OH)D25(OH)D. Conversely, GG alleles present a probability of suboptimum values of 25(OH)D of 69%, 34% and 24% for VDR, GC and CYP2R1, respectively, showing a strong moderate positive correlation (r = 0.41) between the degrees of sports performance and 25(OH)D25(OH)D plasma levels. The different polymorphisms of our three candidate genes CYP2R1 (rs10741657), GC (rs2282679) and VDR (rs2228570) disturb 25(OH)D concentration and play a critical role in the sports performance of elite CrossFit® practitioners. These results could highlight that the evaluation of genetic factors is key to designing a vitamin D supplementation strategy to improve sports performance.
1. Introduction
CrossFit® is a gimmick with very high-intensity-interval exercise (HIIT) [1], performed through the so-called “Workouts of the Day” (WODs) [1], that allow training with standardized routines [2]. The extreme physical and energetic demands of CrossFit® training necessitate high nutritional requirements. CrossFit® athletes seem to take their bodies to the physical limit and achieve maximum sports performance, requiring supplementation with nutrients, vitamins and minerals [3,4,5]. Consequently, nutritional practices must be implemented allowing the specific requirements of CrossFit® practitioners to be covered [3].
In this way, vitamin D is of particular importance to athletes [6] because of its multimodal role in the nervous, immune, muscular and skeletal systems [7]. Especially optimal levels of vitamin D seem to acquire a more relevant role in CrossFit® athletes by protecting bone, immune and muscle health [6]. Athletes who are deficient in vitamin D and perform high-intensity and -duration training are at high risk of musculoskeletal injuries, immunosuppression or arthritis [8,9]. In addition, optimal levels of serum 25-hydroxyvitamin D (25(OH)D) correlate positively with sports performance, including strength and power, running, endurance and aerobic abilities [10]. These physical capacities and physiological demands correspond to CrossFit® [11].
Genetic determinants that may influence circulating 25-(OH)D should be considered [12]. Recently, Fernández-Lázaro et al. described that single-nucleotide polymorphisms (SNPs) influence nutrients, including the behavior of vitamin D [12], and could specifically condition each athlete’s healthy state and sports performance [13]. Therefore, certain SNPs could modulate (increase or decrease) the concentration of bioactive nutrients in plasma [14]. Regarding vitamin D, SNPs in CYP2R1 gene have an impact on vitamin D metabolism. CYP2R1 codes for the hepatic 25-hydroxylase of the cytochrome P450 family [15], responsible for the first hydroxylation to the active form of vitamin D. In addition, SNPs in the GC gene coding for vitamin D-binding protein (VDBP) have an influence in vitamin D transport. VDBP is a protein belonging to the albumin family and is the main carrier of vitamin D in blood [16,17]. Finally, SNPs in the vitamin D-receptor (VDR) gene influence vitamin D biological activity in many tissues, with muscle being of particular interest in sport performance (25(OH)D) [16,17,18,19,20].
In view of the foregoing information, we conducted a pilot study to assess the possible connection between the presence of certain SNPs in CYP2R1 (rs10741657), GC (rs2282679) and muscle VDR (rs2228570) genes, with serum 25(OH)D concentrations and the degree of WOD performance in highly trained CrossFit® practitioners. Knowing these relationships could be instrumental for personalized vitamin D supplementation and training strategies.
2. Material and Methods
2.1. Study Design
A multicenter epidemiological, observational, longitudinal, pilot study was conducted in 2 CrossFit® Boxes and we report it here according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement [21]. This study’s participants were highly trained CrossFit® athletes (n = 126) training in CrossFit® Boxes in Spain (Figure 1). This study was approved by the Clinical Research Ethics Committee (CREC) of Valladolid Clinical Hospital (PI-19-1350) (Spain). Following the Declaration of Helsinki and the 2013 Fortaleza Revision [22], the informed consent document was drafted that all CrossFit® practitioners read, accepted and signed.
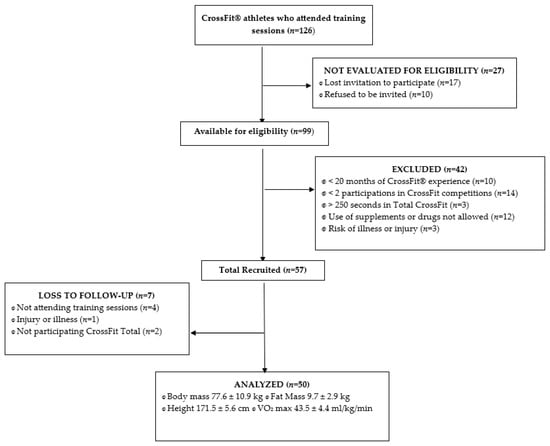
Figure 1.
STORBE flow diagram for recruitment.
2.2. Inclusion Criteria
Healthy adults of legal age made up the study sample of highly trained CrossFit® athletes in 2 CrossFit® Boxes in Salamanca (Spain) and Soria (Spain). Our athletes met the following criteria: (i) ≥20 months of experience training CrossFit®; (ii) ≥2 participations in CrossFit® competitions in the last season; (iii) completed “Fran” WODs < 250 s; (iv) passed a pre-study medical exam to rule out pre-existing illnesses or injuries; (v) do not use products or drugs from “The List of Prohibited Substances and Methods of 2023” established by the World Anti-Doping Agency (WADA), including vitamin D supplementation; (vi) knew and signed the informed consent where the potential benefit/risk of our pilot study was exhaustively explained.
2.3. Data Collection
Table 1 shows the sociodemographic data, anthropometric measurements, sports performance parameters and dietary evaluation of CrossFit® practitioners.

Table 1.
Characteristics of the study participants.
2.4. Sociodemographic and Anthropometrics
Gender, age, nationality (Spanish or other), body mass, fat mass, free fat mass and height were included as sociodemographic and anthropometric characteristics. Bioelectrical impedance (BC-730; Tanita, Japan) was used to assess body mass, fat mass and free fat mass [23]. The height was measured with a tape measure from the base of the floor to the measurement marked on the wall, read and recorded to the nearest millimeter.
2.5. Physical Performance
Fran and CrossFit® Total WODs, and maximum amount of oxygen (VO2max) were assessed as physical performance variables. Fran and CrossFit® Total tests were evaluated following the CrossFit® training guide by Glasman [1,2]. VO2max was determined using a modified Bruce treadmill protocol [24].
2.6. Dietary Assessment
The nutritional evaluation was carried out following our planned studies in elite athletes [25,26].
2.6.1. Quantification of Plasma 25(OH)D Concentration Level
Quantification of plasma 25(OH)D concentration level of DNA was carried out following the methodology of our previous studies [12]. According to Larson-Mayer et al. [8], which establishes 5 levels and reference ranges of based on 25(OH)D level for athletes: (i) <20 ng/mL deficiency; (ii) <30–32 ng/mL insufficient; (iii) >30–32 ng/mL sufficient; (iv) 40–100 ng/mL optimum; (v) >150 ng/mL + hypercalcemia toxic.
2.6.2. Single-Nucleotide Polymorphism (SNP) Determination by DNA Isolation and Genotyping
We evaluated 3 SNPs of the genes rs10741657 to CYP2R1, rs2282679 to GC and rs2228570 to VDR. The 3 candidate genes, CYP2R1, GC and VDR, with all their biallelic varieties have an influence on the bioactive concentration of 25(OH)D [16,18]. Isolation and genotyping of DNA were carried out following the methodology of our previous studies [12].
2.6.3. CrossFti® Total Level
CrossFit® Total degree was evaluated following the CrossFit® training guide level 1 by Glassman [1,2], which establishes the following levels: (i) <270 kg beginner; (ii) 271–360 kg intermediate; (iii) 361–450 kg advanced; (iv) ≥451 kg elite. The mandatory requirement to be able to compete locally and as an amateur in CrossFit® is ≥360 kg, which is the cut-off point in CrossFit® Total [27].
2.6.4. CrossFit Training
The training routine consisted of non-consecutive days of the week (Monday, Wednesday, Friday and Saturday). The 80 min CrossFit® training consisted of 4 parts: specific warm up of the working muscle groups, a technique part based on strength and skills, the main part of WODs and a cool down through muscle stretching. All training was planned and led by a certified CrossFit® trainer with a Grade I or II certificate.
2.6.5. Statistical Analysis
Statistical analyses were performed using StataCorp. 2023. Stata Statistical Software: Release 18 (StataCorp LLC: College Station, TX, USA). We calculated means and standard deviations (continuous variables) and frequencies and percentages (categorical variables) in the descriptive statistical analyses. A general univariate linear test of fixed factors was performed by comparing each SNP of the 3 genes and 25(OH)D. Subsequently, a Bonferroni post hoc test correction was applied to determine the differences between the polymorphisms. Spearman’s rank correlation coefficient was used to obtain correlations between polymorphisms and 25(OH)D.
Analyses were performed to determine odds ratios (ORs) and 95% confidence intervals (CIs) to quantify the association between the different variables, each SNP evaluated and 25(OH)D. We considered a two-sided p-value less than 0.05 to be considered statistically significant. Regression models were used and the Pearson correlation coefficient (r) was calculated according to the coefficient of determination (R2), for the different sports levels of CrossFit and 25(OH)D. p values less than 0.05 were considered statistically significant.
3. Results
3.1. CrossFit® Athlete’s Characteristics and Dietary Assessment
The sociodemographic and anthropometric characteristics, physical performance and nutritional characteristics are shown in Table 1. Table 2 records the energy and micronutrient consumption in CrossFit® athletes.

Table 2.
Macronutrients, energy and micronutrient consumption in CrossFit® athletes.
3.2. 25(OH)D Plasma Level
The 25(OH)D plasma level of highly trained CrossFit® males was 34.7 ± 5.2 ng/mL and 68.0% had 25(OH)D level sufficiency, as described in Table 3.

Table 3.
Distribution of CrossFit® athletes according to 25-hydroxy vitamin D ranges for sports population.
3.3. CrossFit® Total Degrees
The description of the SNPs, 25-OH/ and CrossFit® Total degrees is shown in Table 4. Seventeen athletes were classified as competitors according to Competition RuleBook CrossFit® Games 2023 [27].

Table 4.
CYP2R1, GC and VDR gene polymorphism; 25-hydroxy vitamin D plasma level; and sports performance levels in the CrossFit® Total.
3.4. Comparisons between 25-Hydroxy Vitamin D and Single-Nucleotide Polymorphisms of the CYP2R1, GC and VDR Genes
Table 5 shows the existence of significant differences (p < 0.05) in the plasma concentration of 25(OH)D between the three biallelic combinations of the SNPs r2228570 (VDR) and rs2282679 (GC). Furthermore, statistically significant differences (p < 0.05) were observed in the concentration of 25(OH)D between athletes carrying the GG genotype with respect to the homozygous bialleles TT (rs2282679 (GC)) and AA (r2228570 (VDR)). Also, the heterozygous biallele GA compared to AA for the SNP r2228570 (VDR) showed significant differences (p < 0.05) in 25OH/D plasma level.

Table 5.
Comparisons between 25-hydroxy vitamin D and single-nucleotide polymorphisms of the CYP2R1, GC and VDR genes.
3.5. Correlations between 25-Hydroxy Vitamin D and Single-Nucleotide Polymorphisms of the CYP2R1, GC and VDR Genes
For homozygous AA (rs1074165; rs2228570) and TT (rs2282679), bialleles showed remarkably weak positive correlations (p < 0.05). Moreover, mild negative correlations (p < 0.05) were obtained for all homozygous GGs of rs1074165 (r = −0.34; p = 0.016), rs2282679 (r = −0.33; p = 0.012) and rs2228570 (r = −0.43 (p < 0.001) (Table 6).

Table 6.
Correlations between 25-hydroxy vitamin D and single-nucleotide polymorphisms of the CYP2R1, GC and VDR genes.
3.6. Single-Nucleotide Polymorphisms of the CYP2R1, GC and VDR Genes Associated with 25-Hydroxy Vitamin D Plasma Level
Table 7 shows the set of results of the multivariate logistic regression analysis to generate a diagnostic/predictor model of the plasma concentration of 25(OH)D that consisted of three variables that were the three SNPs of our study: CYP2R1, GC and VDR. Athletes who include AA homozygous biallelic genotypes (rs10741657 [OR 2.01, 95% CI 0.77–5.48], rs2228570 [OR 2.88, 95% CI 1.43–5.92]) and TT (rs2282679[OR 3.67 95% CI 2.11–6.41]) could be more prone to having higher levels of 25(OH)D than other genotypes of these SNPs. On the contrary, our results have shown that athletes carrying the homozygous biallele GG (CYP2R1, GC and VDR) were associated with a lower concentration of 25(OH)D, being more relevant in VDR rs2228570 (OR 0.31, 95% CI 0.12–1.27).

Table 7.
Participant study characteristics and single-nucleotide polymorphisms of the CYP2R1, GC and VDR genes associated with 25-hydroxy vitamin D concentration. Odds ratio (OR) and 95% confidence intervals (95% CI).
3.7. Correlation of Sports Level Degree in CrossFit® Total and 25-Hydroxy Vitamin D (25(OH)D) Plasma Level
Figure 2 shows the correlation of sports level degree in CrossFit® Total and 25(OH)D plasma level. Figure 2 shows R2 = 0.23 indicating that at least 23% of the changes in the CrossFit® Total score are reliable for the 25(OH)D level. In addition, a positive correlation (r = 0.41) is shown between the sports level degree in CrossFit® Total and 25(OH)D concentration.
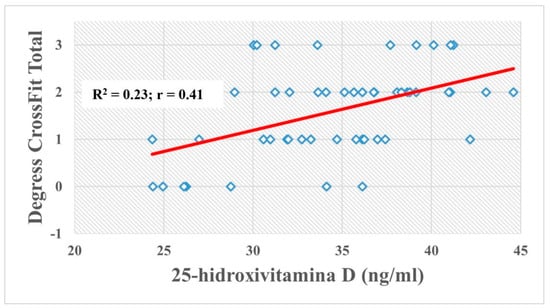
Figure 2.
Correlation of sports level degree in CrossFit® Total and 25-hydroxy vitamin D (25(OH)D) plasm levels.
4. Discussion
Vitamin D substantially influences sports performance and post-exercise recovery because it offers anti-inflammatory, antioxidant and cellular protective properties [28], on muscle cells [9]. The described pathways that reveal the effects of vitamin D in restoring and sustaining the optimal healthy condition of skeletal muscle are genomic and/or non-genomic [29], in the same way as other nuclear steroids [30], through the vitamin D receptor (VDR) based on myocytes [31]. Henceforth, adequate expression of VDR is essential, because vitamin D alone could not control or modulate the mass and/or functionality of skeletal muscle [32]. The loss or decrease in VDR expression is related to muscle pathologies and aging [33]. However, increases in VDR expression are related to regeneration after muscle damage [34]. Thus, the increased expression of VDR and the higher vitamin D plasma levels would favor this interaction (Vitamin D-VRD) [31]. In humans, rs2228570 is the only VDR polymorphism that has distinct structural consequences for the VDR protein [35]. Also, 25(OH)D ≥ 30 ng/mL induces the muscular-positive regulation of VDR [31,32] and exogenous vitamin D induces the upregulation of VDR in the systemic extracellular matrix in primary muscle cells [34,36].
We have identified that VDR genotype variants (rs2228570) have significant differences (p < 0.05) in blood levels of 25(OH)D. Our results showed a negative correlation (r = −0.43; p < 0.001) between the total concentration of 25(OH)D and the homozygous GG biallele. Furthermore, CrossFit® athletes carrying AA (rs2228570) were three times more likely (OR 2.88, 95% CI 1.43–5.92) to have higher levels of vitamin D. VDR with the homozygous FokI AA genotype results in increased VDR protein activity compared to GA or GG genotypes [37]. Thus, the VDR gene’s polymorphisms may condition VDR expression and protein stability [38]. In this sense, the A allele of VDR rs2228570 was associated with VDR mRNA copy number [18,39]. Also, VDR expression is elevated acutely (1 to 3 h) after resistance exercise [31], although low levels of VDR expression in skeletal muscle do not rule out direct actions on its physiological effects [31]. Our findings suggest that the A allele is an element that safeguards the achievement of optimal levels of 25(OH)D [12] and full VDR protein acquisition with optimal physiological functioning [35], which enables improved sports performance. In this sense, we identified 12 advanced athletes and 4 elite athletes’ carriers with the A allele of VDR (rs2228570), which means that 16 athletes were defined as level competitors in CrossFit® games, based on their sports performance.
The steps prior to the binding of 1-25OH/D and VDR require the hydroxylation of vitamin D by CYP2R1 [15] and 1-25OH/D transport to the target VDR, by DBP [16,17]. Both SNPs, GC and CYP2R1, substantially affect vitamin D status [13]. The GG genotype of CYP2R1 (rs10741657) was significantly more likely to have inadequate 25(OH)D levels [16] and GG and GT bialleles (GC rs2282679) were associated with lower 25(OH)D concentrations [17]. Consistent with these studies [16,17], in our CrossFit® athletes, the A allele of CYP2R1 rs10741657 and the T allele of GC rs2282679 were associated with a 2–3 times higher likelihood to present higher levels of 1-25OH/D, as in older adult patients [12]. In fact, we found that 16 and 14 “competitive” sports-grade athletes carried the A allele (rs10741657) and the T allele (rs2282679), respectively.
On the other hand, non-genomic VDR pathways should be considered due to the direct action of vitamin D that optimizes the contractile process of skeletal muscle through the greater mobilization of calcium towards the sarcoplasmic reticulum [40,41]. Plasma levels in physiological ranges (>30 ng/mL of 25(OH)D), as in the athletes in our study, will potentially improve skeletal muscle functionality [42] indirectly, which is key to athletic performance in CrossFit®.
Optimal 25(OH)D plasma levels act by promoting the improvement in neuromuscular function in older people [8,41]. In this way, increases in skeletal muscle strength and physical capacity are linked to levels in the adequate physiological range of 25(OHD) [12,43]. These results are consistent with those reported in our CrossFit® athletes in the strength performance test. The influence of the blood concentration of 25(OH)D in the skeletal muscle of our athletes will be responsible for 23% of the effects on the sports level, establishing a moderate positive correlation (r = 0.41) between the CrossFit® Total grade and 25(OH)D plasma levels. In our study, allelic variations in the SNPs GG (rs10741657), GC (rs2282679) and VDR (rs2228570) affect the plasma concentration of vitamin D in CrossFit® athletes, which may condition their level of sports performance, especially the effects induced by the 1,25OH/D-VDR complex, through genomic and non-genomic pathways, leading to progress in muscle health, muscle functionality, strength, muscle recovery and potentially physical work [8,41]. In fact, rs2228570 (FokI) is the only polymorphism that perturbs the length and functionality of the VDR protein. Furthermore, deficient (>10 ng/mL) or toxic (>150 mg) levels of 25(OH)D inactivate the biologically active form of VDR [44]. Our athletes maintained an adequate plasma concentration of 25(OH)D (34.7 ± 5.2 ng/mL), which would not structurally affect the VDR, maintaining its activity mediated by genomic and non-genomic pathways on skeletal muscle.
The possible practical applications of our findings, through SNPs, could allow athletes, coaches and/or sports nutritionists to recognize persons at potential risk of hypovitaminosis D and adjust possible nutritional actions by improving intake or supplementation. With the purpose of improving health, functionality and muscle performance in athletes, these tips are important for precision personalized nutrition and/or supplementation.
5. Conclusions
This research proved that those allelic variations in the CYP2R1 (rs10741657), GC (rs2282679) and VDR (rs2228570) SNPs disturb the (OH)D behavior in CrossFit® athletes. Thus, genetic polymorphisms of the genes (CYP2R1, GC and VDR) could be the key elements in the modulation of plasma 25(OH)D concentration. This will depend on its availability to modulate the expression of genes involved in skeletal muscle performance and/or health, such as VDR. Finally, we stated that the concentration of 25(OH)D has a moderate positive correlation (r = 0.41) with sports level degree in CrossFit® Total.
Author Contributions
Conceptualization, D.F.-L. and C.I.F.-L.; methodology, D.F.-L. and A.M.C.S.M.; software, C.I.F.-L.; validation, E.R. and J.S.-C.; formal analysis, C.I.F.-L.; investigation, D.F.-L., C.I.F.-L. and E.R.; resources, J.S.-C.; data curation, D.F.-L. and C.I.F.-L.; writing—original draft preparation, D.F.-L., C.I.F.-L. and E.R.; writing—review and editing, A.M.C.S.M. and J.S.-C.; visualization, A.M.C.S.M.; supervision, D.F.-L. and C.I.F.-L.; project administration, D.F.-L. and C.I.F.-L.; funding acquisition, D.F.-L. All authors have read and agreed to the published version of the manuscript.
Funding
The research was carried out in the Neurobiology laboratory of the Department of Cellular Biology, Genetics, Histology and Pharmacology directed by the author, was funded by the TCUE Plan 2021–2023, approved in agreement 134/2021, and has been selected within the framework of an operational program co-financed by the European Regional Development Fund (ERDF) and the Regional Government of Castilla-León (PI22/00008).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
This study was approved by the Clinical Research Ethics Committee (CREC) of Valladolid Clinical Hospital (PI-19-1350) (Spain). Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Glassman, G. What Is CrossFit? CrossFit J. 2004, 56, 1–7. Available online: http://journal.crossfit.com/2004/03/what-is-crossfitmar-04-cfj.tpl (accessed on 29 August 2023).
- Glassman, G. Understanding CrossFit. East Val. Crossfit Newsl. 2007, 1–115. Available online: http://journal.crossfit.com/2007/04/understanding-crossfit-by-greg.tpl (accessed on 29 August 2023).
- Gogojewicz, A.; Śliwicka, E.; Durkalec-Michalski, K. Assessment of Dietary Intake and Nutritional Status in CrossFit-Trained Individuals: A Descriptive Study. Int. J. Environ. Res. Public Health 2020, 17, 4772. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Quaresma, M.V.L.; Guazzelli Marques, C.; Nakamoto, F.P. Effects of diet interventions, dietary supplements, and performance-enhancing substances on the performance of CrossFit-trained individuals: A systematic review of clinical studies. Nutrition 2021, 82, 110994. [Google Scholar] [CrossRef] [PubMed]
- Brisebois, M.; Kramer, S.; Lindsay, K.G.; Wu, C.T.; Kamla, J. Dietary practices and supplement use among CrossFit® participants. J. Int. Soc. Sports Nutr. 2022, 19, 316–335. [Google Scholar] [CrossRef] [PubMed]
- Shuler, F.D.; Wingate, M.K.; Moore, G.H.; Giangarra, C. Sports Health Benefits of Vitamin D. Sports Health 2012, 4, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.J.; Pourshahidi, L.K.; McSorley, E.M.; Madigan, S.M.; Magee, P.J. Vitamin D: Recent Advances and Implications for Athletes. Sport Med. 2015, 45, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Larson-Meyer, D.E.; Willis, K.S. Vitamin D and athletes. Curr. Sports Med. Rep. 2010, 9, 220–226. [Google Scholar] [CrossRef]
- Yagüe, M.d.l.P.; Yurrita, L.C.; Cabañas, M.J.C.; Cenzual, M.A.C. Role of Vitamin D in Athletes and Their Performance: Current Concepts and New Trends. Nutrients 2020, 12, 579. [Google Scholar] [CrossRef]
- Mielgo-Ayuso, J.; Calleja-González, J.; Urdampilleta, A.; León-Guereño, P.; Córdova, A.; Caballero-García, A.; Fernández-Lázaro, D. Effects of Vitamin D Supplementation on Haematological Values and Muscle Recovery in Elite Male Traditional Rowers. Nutrients 2018, 10, 1968. [Google Scholar] [CrossRef]
- Oliver-Lopez, A.; Garcia-Valverde, A.; Sabido, R. Summary of the evidence on responses and adaptations derived from CrossFit training. A systematic review. Retos 2022, 46, 309–322. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Hernández, J.L.G.; Lumbreras, E.; Mielgo-Ayuso, J.; Seco-Calvo, J. 25-Hydroxyvitamin D Serum Levels Linked to Single Nucleotide Polymorphisms (SNPs) (rs2228570, rs2282679, rs10741657) in Skeletal Muscle Aging in Institutionalized Elderly Men Not Supplemented with Vitamin D. Int. J. Mol. Sci. 2022, 23, 11846. [Google Scholar] [CrossRef] [PubMed]
- Guest, N.S.; Horne, J.; Vanderhout, S.M.; El-Sohemy, A. Sport Nutrigenomics: Personalized Nutrition for Athletic Performance. Front. Nutr. 2019, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.; Adamski, M.M.; Twohig, C.; Murgia, C. Opportunities for training for nutritional professionals in nutritional genomics: What is out there? Nutr. Diet. 2018, 75, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.B.; Levine, M.A.; Bell, N.H.; Mangelsdorf, D.J.; Russell, D.W. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc. Natl. Acad. Sci. USA 2004, 101, 7711–7715. [Google Scholar] [CrossRef] [PubMed]
- Slater, N.A.; Rager, M.L.; Havrda, D.E.; Harralson, A.F. Genetic Variation in CYP2R1 and GC Genes Associated with Vitamin D Deficiency Status. J. Pharm. Pract. 2017, 30, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Zhang, F.; Richards, J.B.; Kestenbaum, B.; Van Meurs, J.B.; Berry, D.; Kiel, D.P.; Streeten, E.A.; Ohlsson, C.; Koller, D.L.; et al. Common genetic determinants of vitamin D insufficiency: A genome-wide association study. Lancet 2010, 376, 180–188. [Google Scholar] [CrossRef]
- Kamyshna, I.I.; Pavlovych, L.B.; Malyk, I.V.; Kamyshnyi, A.M. 25-OH Vitamin D blood serum linkage with VDR gene polymorphism (rs2228570) in thyroid pathology patients in the West-Ukrainian population. J. Med. Life 2021, 14, 549–556. [Google Scholar] [CrossRef]
- Redenšek, S.; Kristanc, T.; Blagus, T.; Trošt, M.; Dolžan, V. Genetic Variability of the Vitamin D Receptor Affects Susceptibility to Parkinson’s Disease and Dopaminergic Treatment Adverse Events. Front. Aging Neurosci. 2022, 14, 853277. [Google Scholar] [CrossRef]
- McGrath, J.J.; Saha, S.; Burne, T.H.J.; Eyles, D.W. A systematic review of the association between common single nucleotide polymorphisms and 25-hydroxyvitamin D concentrations. J. Steroid Biochem. Mol. Biol. 2010, 121, 471–477. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Soto, M.D.V.; Adams, D.P.; González-Bernal, J.J.; Seco-Calvo, J. The Effects of 6 Weeks of Tribulus terrestris L. Supplementation on Body Composition, Hormonal Response, Perceived Exertion, and CrossFit® Performance: A Randomized, Single-Blind, Placebo-Controlled Study. Nutrients 2021, 13, 3969. [Google Scholar] [CrossRef] [PubMed]
- Flouris, A.D.; Koutedakis, Y.; Nevill, A.; Metsios, G.S.; Tsiotra, G.; Parasiris, Y. Enhancing specificity in proxy-design for the assessment of bioenergetics. J. Sci. Med. Sport 2004, 7, 197–204. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fernández-Lázaro, D. Ergogenic Strategies for Optimizing Performance and Health in Regular Physical Activity Participants: Evaluation of the Efficacy of Compressive Cryotherapy, Exposure to Intermittent Hypoxia at Rest and Sectorized Training of the Inspiratory Muscles. Ph.D. Thesis, University of León, León, Spain, 2020. Available online: https://dialnet.unirioja.es/servlet/tesis?codigo=286163&info=resumen&idioma=SPA (accessed on 7 September 2023).
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; del Valle Soto, M.; Adams, D.P.; Gutiérrez-Abejón, E.; Seco-Calvo, J. Impact of Optimal Timing of Intake of Multi-Ingredient Performance Supplements on Sports Performance, Muscular Damage, and Hormonal Behavior across a Ten-Week Training Camp in Elite Cyclists: A Randomized Clinical Trial. Nutrients 2021, 13, 3746. [Google Scholar] [CrossRef] [PubMed]
- CrossFit Games. Competition Rulebook CrossFit 2023. Available online: https://games.crossfit.com/rules (accessed on 29 August 2023).
- Bello, H.J.; Caballero-García, A.; Pérez-Valdecantos, D.; Roche, E.; Noriega, D.C.; Córdova-Martínez, A. Effects of Vitamin D in Post-Exercise Muscle Recovery. A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 4013. [Google Scholar] [CrossRef] [PubMed]
- Bollen, S.E.; Atherton, P.J. Myogenic, genomic and non-genomic influences of the vitamin D axis in skeletal muscle. Cell Biochem. Funct. 2021, 39, 48–59. [Google Scholar] [CrossRef]
- Ksiażek, A.; Zagrodna, A.; Słowińska-Lisowska, M. Vitamin D, Skeletal Muscle Function and Athletic Performance in Athletes—A Narrative Review. Nutrients 2019, 11, 1800. [Google Scholar] [CrossRef]
- Girgis, C.M.; Mokbel, N.; Cha, K.M.; Houweling, P.J.; Abboud, M.; Fraser, D.R.; Mason, R.S.; Clifton-Bligh, R.J.; Gunton, J.E. The vitamin D receptor (VDR) is expressed in skeletal muscle of male mice and modulates 25-hydroxyvitamin D (25OHD) uptake in myofibers. Endocrinology 2014, 155, 3227–3237. [Google Scholar] [CrossRef]
- Bass, J.J.; Nakhuda, A.; Deane, C.S.; Brook, M.S.; Wilkinson, D.J.; Phillips, B.E.; Philp, A.; Tarum, J.; Kadi, F.; Andersen, D.; et al. Overexpression of the vitamin D receptor (VDR) induces skeletal muscle hypertrophy. Mol. Metab. 2020, 42, 101059. [Google Scholar] [CrossRef]
- Scimeca, M.; Centofanti, F.; Celi, M.; Gasbarra, E.; Novelli, G.; Botta, A.; Tarantino, U. Vitamin D Receptor in Muscle Atrophy of Elderly Patients: A Key Element of Osteoporosis-Sarcopenia Connection. Aging Dis. 2018, 9, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Srikuea, R.; Zhang, X.; Park-Sarge, O.K.; Esser, K.A. VDR and CYP27B1 are expressed in C2C12 cells and regenerating skeletal muscle: Potential role in suppression of myoblast proliferation. Am. J. Physiol. Cell Physiol. 2012, 303, C396–C405. [Google Scholar] [CrossRef] [PubMed]
- Lurie, G.; Wilkens, L.R.; Thompson, P.J.; Carney, M.E.; Palmieri, R.T.; Pharoah, P.D.P.; Song, H.; Hogdall, E.; Kjaer, S.K.; DiCioccio, R.A.; et al. Vitamin D receptor rs2228570 polymorphism and invasive ovarian carcinoma risk: Pooled analysis in five studies within the Ovarian Cancer Association Consortium. Int. J. Cancer 2011, 128, 936. [Google Scholar] [CrossRef] [PubMed]
- Olsson, K.; Saini, A.; Strömberg, A.; Alam, S.; Lilja, M.; Rullman, E.; Gustafsson, T. Evidence for Vitamin D Receptor Expression and Direct Effects of 1α,25(OH)2D3 in Human Skeletal Muscle Precursor Cells. Endocrinology 2016, 157, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Nunes, I.F.O.C.; Cavalcante, A.A.C.M.; Alencar, M.V.O.B.; Carvalho, M.D.F.; Sarmento, J.L.R.; Teixeira, N.S.C.C.A.; Paiva, A.A.; Carvalho, L.R.; Nascimento, L.F.M.; Cruz, M.S.P.; et al. Meta-Analysis of the Association Between the rs228570 Vitamin D Receptor Gene Polymorphism and Arterial Hypertension Risk. Adv. Nutr. 2020, 11, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Balta, B.; Gumus, H.; Bayramov, R.; Korkmaz Bayramov, K.; Erdogan, M.; Oztop, D.B.; Dogan, M.E.; Taheri, S.; Dundar, M. Increased vitamin D receptor gene expression and rs11568820 and rs4516035 promoter polymorphisms in autistic disorder. Mol. Biol. Rep. 2018, 45, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Vidigal, V.M.; Silva, T.D.; de Oliveira, J.; Pimenta, C.A.M.; Felipe, A.V.; Forones, N.M. Genetic polymorphisms of vitamin D receptor (VDR), CYP27B1 and CYP24A1 genes and the risk of colorectal cancer. Int. J. Biol. Markers 2017, 32, e224–e230. [Google Scholar] [CrossRef] [PubMed]
- Larson-Meyer, E. Vitamin D supplementation in athletes. Nestle Nutr. Inst. Workshop Ser. 2013, 75, 109–121. [Google Scholar]
- Caballero-García, A.; Córdova-Martínez, A.; Vicente-Salar, N.; Roche, E.; Pérez-Valdecantos, D. Vitamin D, Its Role in Recovery after Muscular Damage Following Exercise. Nutrients 2021, 13, 2336. [Google Scholar] [CrossRef]
- Orces, C.H. The association between 25-hydroxyvitamin D levels and muscle strength in adolescents. Nutr. Hosp. 2021, 38, 1169–1174. [Google Scholar]
- Remelli, F.; Vitali, A.; Zurlo, A.; Volpato, S. Vitamin D Deficiency and Sarcopenia in Older Persons. Nutrients 2019, 11, 2861. [Google Scholar] [CrossRef] [PubMed]
- Gussago, C.; Arosio, B.; Guerini, F.R.; Ferri, E.; Costa, A.S.; Casati, M.; Bollini, E.M.; Ronchetti, F.; Colombo, E.; Bernardelli, G.; et al. Impact of vitamin D receptor polymorphisms in centenarians. Endocrine 2016, 53, 558–564. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).