Assessment of Nutritional Profile of Sargassum muticum Alga from the Spanish Coastline †
Abstract
1. Introduction
2. Materials and Methods
2.1. Alga Material
2.2. Proximate Analysis
2.3. Proteins and Carbohydrates
2.4. Lipids
2.5. Fibers
2.6. Macro- and Micro-Nutrients and Other Elements
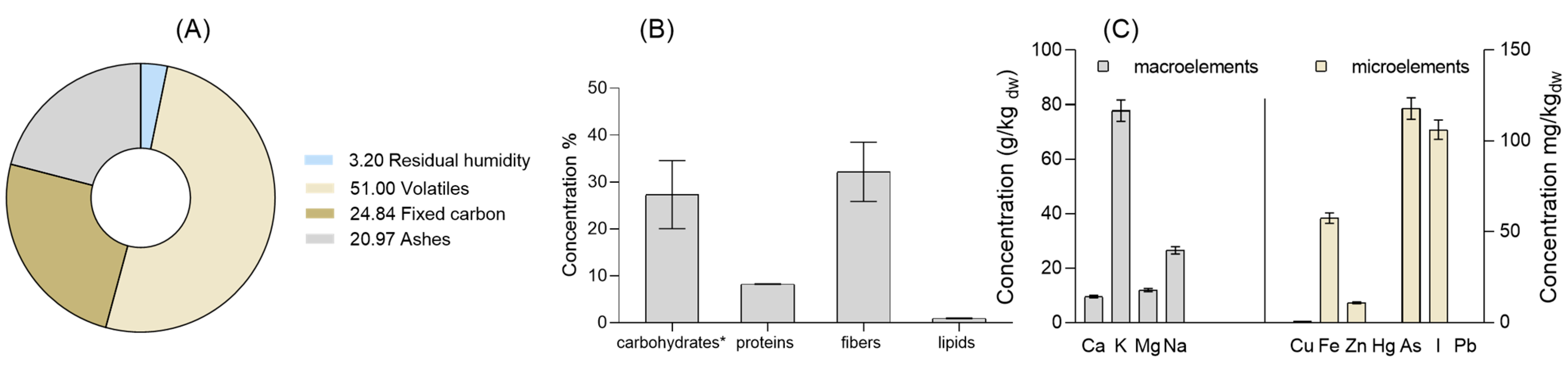
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tanniou, A.; Vandanjon, L.; Incera, M.; Serrano Leon, E.; Husa, V.; Le Grand, J.; Nicolas, J.L.; Poupart, N.; Kervarec, N.; Engelen, A.; et al. Assessment of the Spatial Variability of Phenolic Contents and Associated Bioactivities in the Invasive Alga Sargassum muticum Sampled along Its European Range from Norway to Portugal. J. Appl. Phycol. 2014, 26, 1215–1230. [Google Scholar] [CrossRef]
- Pinteus, S.; Lemos, M.F.L.; Alves, C.; Neugebauer, A.; Silva, J.; Thomas, O.P.; Botana, L.M.; Gaspar, H.; Pedrosa, R. Marine Invasive Macroalgae: Turning a Real Threat into a Major Opportunity—The Biotechnological Potential of Sargassum muticum and Asparagopsis armata. Algal Res. 2018, 34, 217–234. [Google Scholar] [CrossRef]
- Silva, A.; Cassani, L.; Grosso, C.; Garcia-Oliveira, P.; Morais, S.L.; Echave, J.; Carpena, M.; Xiao, J.; Barroso, M.F.; Simal-Gandara, J.; et al. Recent Advances in Biological Properties of Brown Algae-Derived Compounds for Nutraceutical Applications. Crit. Rev. Food Sci. Nutr. 2022, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.D.; Bahcevandziev, K.; Pereira, L. Production of Bio-Fertilizer from Ascophyllum nodosum and Sargassum muticum (Phaeophyceae). J. Oceanol. Limnol. 2019, 37, 918–927. [Google Scholar] [CrossRef]
- Young Park, S.; Su Seo, I.; Joo Lee, S.; Pyung Lee, S. Study on the Health Benefits of Brown Algae (Sargassum muticum) in Volunteers. J. Food Nutr. Res. 2015, 3, 126–130. [Google Scholar] [CrossRef][Green Version]
- Rodrigues, D.; Freitas, A.C.; Pereira, L.; Rocha-Santos, T.A.P.; Vasconcelos, M.W.; Roriz, M.; Rodríguez-Alcalá, L.M.; Gomes, A.M.P.; Duarte, A.C. Chemical Composition of Red, Brown and Green Macroalgae from Buarcos Bay in Central West Coast of Portugal. Food Chem. 2015, 183, 197–207. [Google Scholar] [CrossRef]
- Pacheco, D.; Araújo, G.S.; Cotas, J.; Gaspar, R.; Neto, J.M.; Pereira, L. Invasive Seaweeds in the Iberian Peninsula: A Contribution for Food Supply. Mar. Drugs 2020, 18, 560. [Google Scholar] [CrossRef]
- Amanto, B.S.; Umanailo, M.C.B.; Wulandari, R.S.; Taufik, T.; Susiati, S. Local Consumption Diversification. Int. J. Sci. Technol. Res. 2019, 8, 1865–1869. [Google Scholar]
- Lourenço-Lopes, C.; Silva, A.; Garcia-Oliveira, P.; Soria-Lopez, A.; Echave, J.; Grosso, C.; Cassani, L.; Barroso, M.F.; Simal-Gandara, J.; Fraga-Corral, M.; et al. Kinetic Extraction of Fucoxanthin from Undaria pinnatifida Using Ethanol as a Solvent. Mar. Drugs 2023, 21, 414. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication; National University of Ireland: Galway, Ireland, 2023. [Google Scholar]
- García, R.; Pizarro, C.; Lavín, A.G.; Bueno, J.L. Biomass Proximate Analysis Using Thermogravimetry. Bioresour. Technol. 2013, 139, 1–4. [Google Scholar] [CrossRef]
- Cassani, L.; Lourenço-Lopes, C.; Barral-Martinez, M.; Chamorro, F.; Garcia-Perez, P.; Simal-Gandara, J.; Prieto, M.A. Thermochemical Characterization of Eight Seaweed Species and Evaluation of Their Potential Use as an Alternative for Biofuel Production and Source of Bioactive Compounds. Int. J. Mol. Sci. 2022, 23, 2355. [Google Scholar] [CrossRef] [PubMed]
- Angell, A.R.; Mata, L.; de Nys, R.; Paul, N.A. The Protein Content of Seaweeds: A Universal Nitrogen-to-Protein Conversion Factor of Five. J. Appl. Phycol. 2016, 28, 511–524. [Google Scholar] [CrossRef]
- Soares, C.; Tenreiro Machado, J.A.; Lopes, A.M.; Vieira, E.; Delerue-Matos, C. Electrochemical Impedance Spectroscopy Characterization of Beverages. Food Chem. 2020, 302, 125345. [Google Scholar] [CrossRef] [PubMed]
- Rowland, A.P.; Roberts, J.D. Lignin and Cellulose Fractionation in Decomposition Studies Using Acid-Detergent Fibre Methods. Commun. Soil Sci. Plant Anal. 1994, 25, 269–277. [Google Scholar] [CrossRef]
- Millos, J.; Costas Rodriguez, M.; Lavilla, I.; Bendicho, C. Multiple Small Volume Microwave-Assisted Digestions Using Conventional Equipment for Multielemental Analysis of Human Breast Biopsies by Inductively Coupled Plasma Optical Emission Spectrometry. Talanta 2009, 77, 1490–1496. [Google Scholar] [CrossRef] [PubMed]
- Balboa, E.M.; Gallego-Fábrega, C.; Moure, A.; Domínguez, H. Study of the Seasonal Variation on Proximate Composition of Oven-Dried Sargassum muticum Biomass Collected in Vigo Ria, Spain. J. Appl. Phycol. 2016, 28, 1943–1953. [Google Scholar] [CrossRef]
- VANDANJON, L.; Maureen, D.; Maya, P.; Philippe, D.; Valarie, S.-P.; Gilles, B.; Nathalie, B. Seasonal Variation of Sargassum muticum Biochemical Composition Determined by Fourier Transform Infra-Red Spectroscopy. J. Anal. Bioanal. Sep. Tech. 2017, 2, 75–84. [Google Scholar] [CrossRef]
- Quitério, E.; Soares, C.; Ferraz, R.; Delerue-Matos, C.; Grosso, C. Marine Health-Promoting Compounds: Recent Trends for Their Characterization and Human Applications. Foods 2021, 10, 3100. [Google Scholar] [CrossRef]
- Dewinta, A.F.; Susetya, I.E.; Suriani, M. Nutritional Profile of Sargassum sp. from Pane Island, Tapanuli Tengah as a Component of Functional Food. J. Phys. Conf. Ser. 2020, 1542, 012040. [Google Scholar] [CrossRef]
- Jiménez-Escrig, A.; Sánchez-Muniz, F.J. Dietary Fibre from Edible Seaweeds: Chemical Structure, Physicochemical Properties and Effects on Cholesterol Metabolism. Nutr. Res. 2000, 20, 585–598. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive Compounds in Seaweed: Functional Food Applications and Legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- DeLuccia, R.; Cheung, M.; Ng, T.; Ramadoss, R.; Altasan, A.; Sukumar, D. Calcium to Magnesium Ratio Higher Than Optimal Across Age Groups (P10-100-19). Curr. Dev. Nutr. 2019, 3, nzz034.P10-100-19. [Google Scholar] [CrossRef]
- Al Alawi, A.M.; Majoni, S.W.; Falhammar, H. Magnesium and Human Health: Perspectives and Research Directions. Int. J. Endocrinol. 2018, 2018, 9041694. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, E.; Giltinan, M.; Kehoe, L.; Nugent, A.P.; McNulty, B.A.; Flynn, A.; Walton, J. Sodium and Potassium Intakes and Their Ratio in Adults (18–90 y): Findings from the Irish National Adult Nutrition Survey. Nutrients 2020, 12, 938. [Google Scholar] [CrossRef]
- World Health Organization. Guideline: Sodium Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Cipolloni, O.-A.; Gigault, J.; Dassié, É.P.; Baudrimont, M.; Gourves, P.-Y.; Amaral-Zettler, L.; Pascal, P.-Y. Metals and Metalloids Concentrations in Three Genotypes of Pelagic Sargassum from the Atlantic Ocean Basin-Scale. Mar. Pollut. Bull. 2022, 178, 113564. [Google Scholar] [CrossRef]
- Park, G.; Kang, D.; Davaatseren, M.; Shin, C.; Kang, G.-J.; Chung, M.-S. Reduction of Total, Organic, and Inorganic Arsenic Content in Hizikia fusiforme (Hijiki). Food Sci. Biotechnol. 2019, 28, 615–622. [Google Scholar] [CrossRef]
| Ca | Cu | Fe | K | Mg | Na | Zn | Hg | As | I | Pb | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Analytical technique | ICP-OES | ICP-OES | ICP-OES | ICP-OES | ICP-OES | ICP-OES | ICP-OES | CVAAS | ICP-MS | ICP-MS | ICP-MS |
| LOQ mg/kg | 10.00 | 0.60 | 1.00 | 20.00 | 10.00 | 20.00 | 0.20 | 0.040 | 2.50 | 5.00 | 1.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.; Soares, C.; Carpena, M.; Oliveira, P.G.; Echave, J.; Chamorro, F.; Donn, P.; Mansour, S.S.; Barroso, M.F.; Prieto, M.A. Assessment of Nutritional Profile of Sargassum muticum Alga from the Spanish Coastline. Biol. Life Sci. Forum 2023, 26, 94. https://doi.org/10.3390/Foods2023-15028
Silva A, Soares C, Carpena M, Oliveira PG, Echave J, Chamorro F, Donn P, Mansour SS, Barroso MF, Prieto MA. Assessment of Nutritional Profile of Sargassum muticum Alga from the Spanish Coastline. Biology and Life Sciences Forum. 2023; 26(1):94. https://doi.org/10.3390/Foods2023-15028
Chicago/Turabian StyleSilva, Aurora, Cristina Soares, Maria Carpena, Paula Garcia Oliveira, Javier Echave, Franklin Chamorro, Pauline Donn, Sepidar S. Mansour, Maria Fátima Barroso, and Miguel A. Prieto. 2023. "Assessment of Nutritional Profile of Sargassum muticum Alga from the Spanish Coastline" Biology and Life Sciences Forum 26, no. 1: 94. https://doi.org/10.3390/Foods2023-15028
APA StyleSilva, A., Soares, C., Carpena, M., Oliveira, P. G., Echave, J., Chamorro, F., Donn, P., Mansour, S. S., Barroso, M. F., & Prieto, M. A. (2023). Assessment of Nutritional Profile of Sargassum muticum Alga from the Spanish Coastline. Biology and Life Sciences Forum, 26(1), 94. https://doi.org/10.3390/Foods2023-15028













