Characterization of Functional Proteins from Edible Bird’s Nest Using Proteomic Techniques in Combination with Bioinformatics Analyses †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Protein Extraction from EBN
2.3. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.4. Characterization of Functional Proteins from EBN Using Liquid Chromatography–Mass Spectrometry
2.5. Antioxidant and Anti-Inflammatory Activity
2.6. Statistical Analysis
3. Results and Discussion
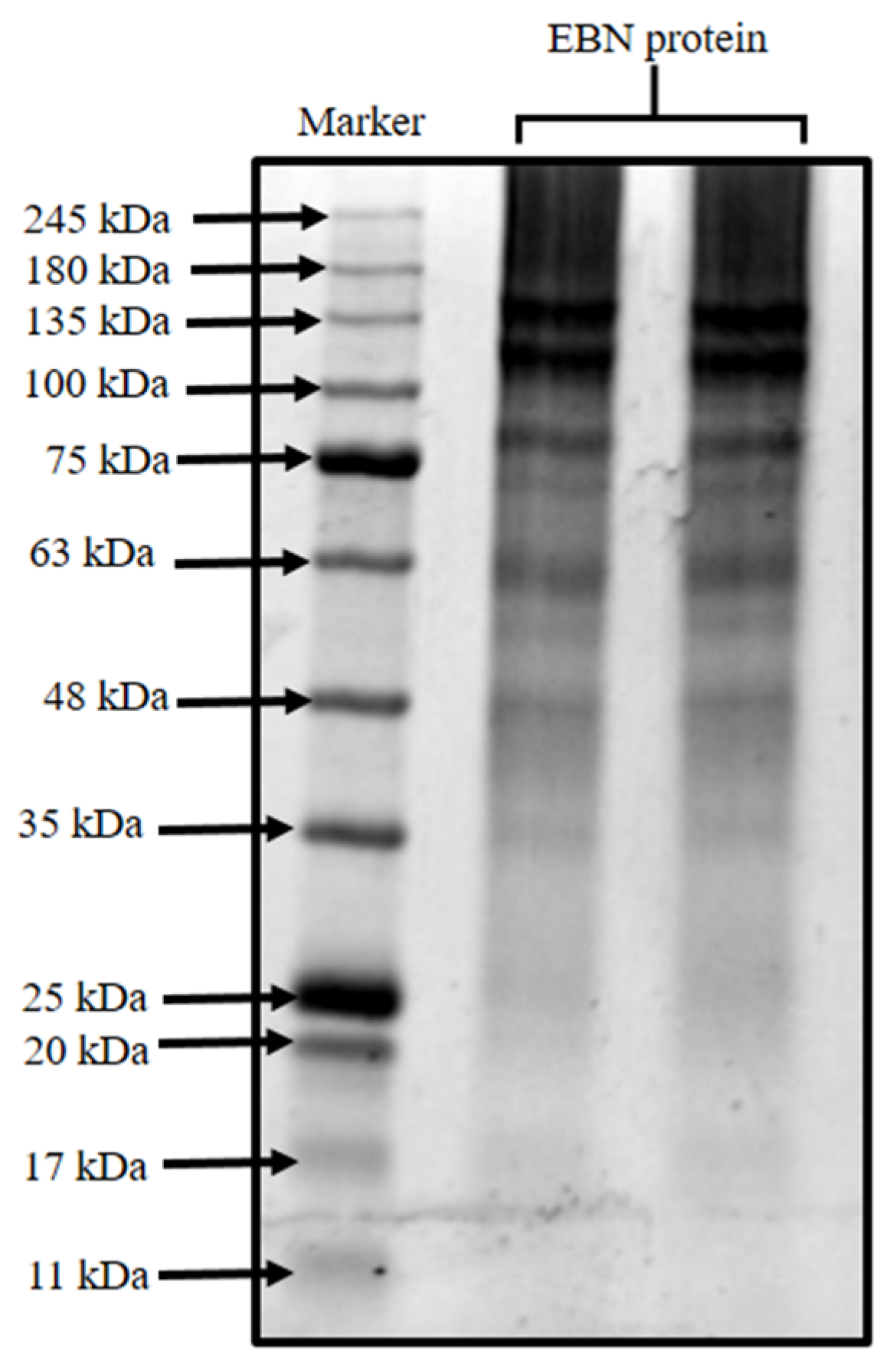
3.1. Identification of EBN Proteins Using SDS-PAGE
3.2. Characterization of EBN Proteins and Functional Proteins Prediction
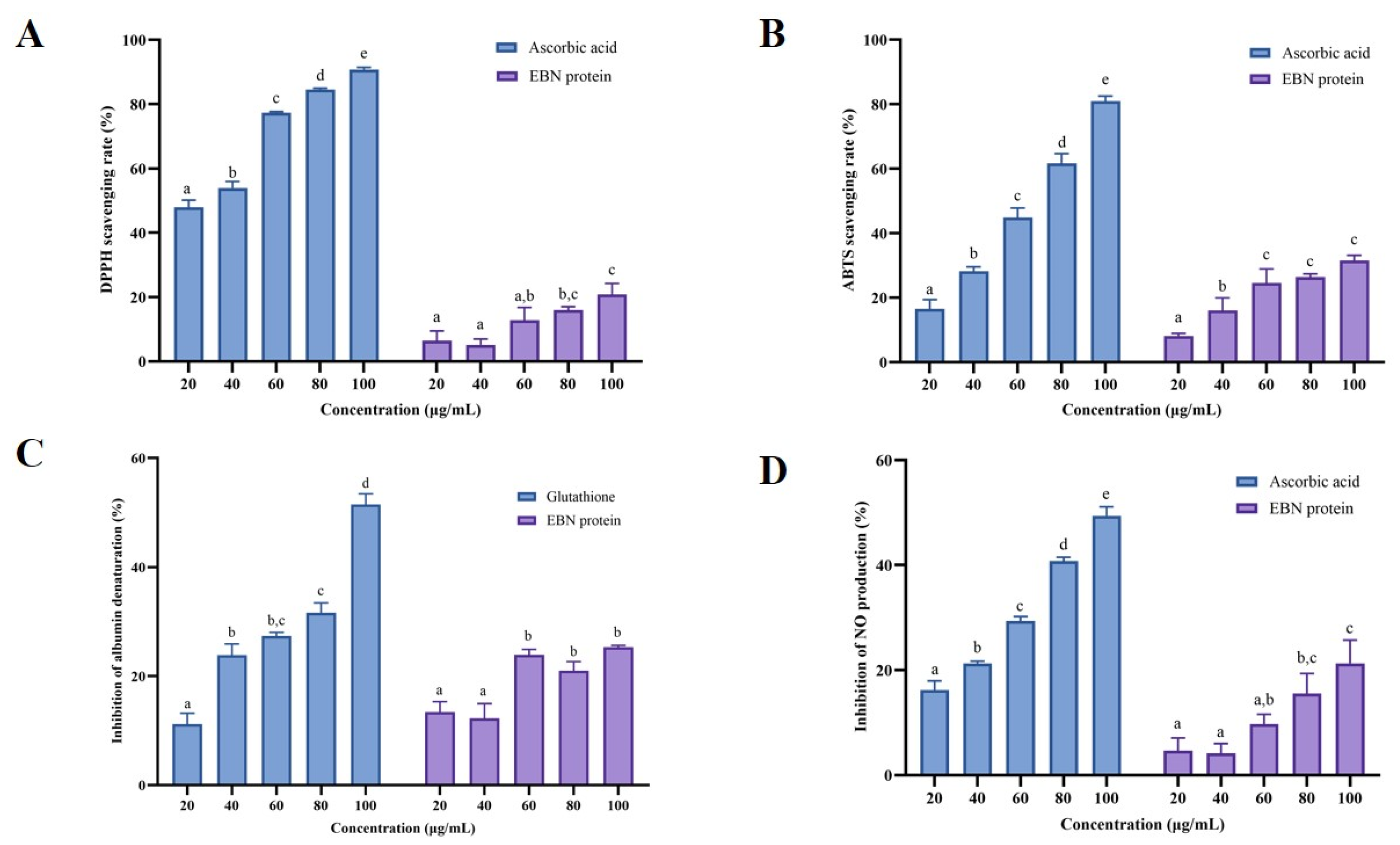
3.3. Evaluation of Antioxidant and Anti-Inflammatory Activity of EBN Proteins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, T.H.; Babji, A.S.; Lim, S.J.; Sarbini, S.R. A Systematic Review of Edible Swiftlet’s Nest (ESN): Nutritional bioactive compounds, health benefits as functional food, and recent development as bioactive ESN glycopeptide hydrolysate. Trends Food Sci. Technol. 2021, 115, 117–132. [Google Scholar] [CrossRef]
- Lee, C.H.; Lee, T.H.; Wong, S.L.; Nyakuma, B.B.; Hamdan, N.; Khoo, S.C.; Ramachandran, H.; Jamaluddin, H. Characteristics and trends in global Edible Bird’s Nest (EBN) research (2002–2021): A review and bibliometric study. J. Food Meas. Charact. 2023, 17, 4905–4926. [Google Scholar] [CrossRef]
- Puspa Liza, G.; Mustafa, M.; Roslida, A.R. Location area and premise size of successful edible bird nest (EBN) swiftlet houses in Terengganu, Malaysia. Int. J. Adv. Sci. Technol. 2020, 29, 9356–9362. [Google Scholar]
- Ghassem, M.; Arihara, K.; Mohammadi, S.; Sani, N.A.; Babji, A.S. Identification of two novel antioxidant peptides from edible bird’s nest (Aerodramus fuciphagus) protein hydrolysates. Food Funct. 2017, 8, 2046–2052. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.; Park, S.W.; Yang, J.E. Anti-aging, anti-inflammatory, and wound-healing activities of edible bird’s nest in human skin keratinocytes and fibroblasts. Pharmacogn. Mag. 2020, 16, 336–342. [Google Scholar] [CrossRef]
- Dobutr, T.; Kantamala, W.; Phimwapi, S.; Jangpromma, N.; Tippayawat, P.; Boonlue, S.; Daduang, J.; Klaynongsruang, S.; Poopornchai, S.; Daduang, S. The effects of edible bird’s nest on T-lymphocyte proliferation, secondary lymphoid organs, and interleukin-2 production. J. Funct. Foods 2022, 90, 104977. [Google Scholar] [CrossRef]
- Hun, L.T.; Lee, C.H.; Azmi, N.A.; Liew, R.K.; Hamdan, N.; Wong, S.L.; Ong, P.Y. Amino acid determination by HPLC combined with multivariate approach for geographical classification of Malaysian edible bird’s nest. J. Food Compos. Anal. 2022, 107, 104399. [Google Scholar] [CrossRef]
- Lee, T.H.; Wong, S.; Lee, C.H.; Azmi, N.A.; Darshini, M.; Kavita, S.; Cheng, K.K. Identification of Malaysia’s edible bird’s nest geographical origin using gel electrophoresis analysis. Chiang Mai Univ. J. Nat. Sci. 2018, 19, 379. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Liang, R.; Yang, Y.; Sun, N.; Lin, S. Optimization of pea protein hydrolysate preparation and purification of antioxidant peptides based on an in silico analytical approach. LWT 2020, 123, 109126. [Google Scholar] [CrossRef]
- Hu, S.; Yuan, J.; Gao, J.; Wu, Y.; Meng, X.; Tong, P.; Chen, H. Antioxidant and anti-inflammatory potential of peptides derived from in vitro gastrointestinal digestion of germinated and heat-treated foxtail millet (Setaria italica) proteins. J. Agric. Food Chem. 2020, 68, 9415–9426. [Google Scholar] [CrossRef]
- Liu, X.Q.; Lai, X.T.; Zhang, S.W.; Huang, X.L.; Lan, Q.X.; Li, Y.; Li, B.F.; Chen, W.; Zhang, Q.L.; Hong, D.Z.; et al. Proteomic profile of edible bird’s nest proteins. J. Agric. Food. Chem. 2012, 60, 12477–12481. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.L.; Wu, Y.J.; Liu, M.C.; Wang, B.; Ge, Y.Q.; Chen, Y. Determination of edible bird’s nests by FTIR and SDS-PAGE coupled with multivariate analysis. Food Control 2017, 80, 259–266. [Google Scholar] [CrossRef]
- Hun, L.T.; Hau Lee, C.; Alia Azmi, N.; Kavita, S.; Wong, S.; Znati, M.; Ben Jannet, H. Characterization of polar and non-polar compounds of house edible bird’s nest (EBN) from Johor, Malaysia. Chem. Biodivers. 2020, 17, e1900419. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.T.; Zhang, J.K.; Liang, J.Z.; Ma, X.L.; Xing, R.R.; Han, J.X.; Guo, L.H.; Chen, Y. Authentication of edible bird’s nest (EBN) and its adulterants by integration of shotgun proteomics and scheduled multiple reaction monitoring (MRM) based on tandem mass spectrometry. Food Res. Int. 2019, 125, 108639. [Google Scholar] [CrossRef] [PubMed]
- Wong, Z.C.F.; Chan, G.K.L.; Wu, L.; Lam, H.H.N.; Yao, P.; Dong, T.T.X.; Tsim, K.W.K. A comprehensive proteomics study on edible bird’s nest using new monoclonal antibody approach and application in quality control. J. Food Compos. Anal. 2018, 66, 145–151. [Google Scholar] [CrossRef]
- Zhao, Y.; Tao, Q.; Wu, J.; Liu, H. DMBT1 has a protective effect on allergic rhinitis. Biomed. Pharmacother. 2020, 121, 109675. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wan, S.J.; Metruccio, M.M.; Ma, S.; Nazmi, K.; Bikker, F.J.; Evans, D.J.; Fleiszig, S.M. DMBT1 inhibition of Pseudomonas aeruginosa twitching motility involves its N-glycosylation and cannot be conferred by the Scavenger Receptor Cysteine-Rich bacteria-binding peptide domain. Sci. Rep. 2019, 9, 13146. [Google Scholar] [CrossRef] [PubMed]
- Lechner, A.; Bohnacker, S.; Esser-von Bieren, J. Macrophage regulation & function in helminth infection. In Seminars in Immunology; Academic Press: Cambridge, MA, USA, 2021; p. 101526. [Google Scholar]
- Yuan, M.; Lin, X.; Wang, D.; Dai, J. Proteins: Neglected active ingredients in edible bird’s nest. Chin. Herb. Med. 2023, 15, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Morón, E.; Abad-Jiménez, Z.; Martinez de Maranon, A.; Iannantuoni, F.; Escribano-López, I.; López-Domènech, S.; Salom, C.; Jover, A.; Mora, V.; Roldan, I. Relationship between oxidative stress, ER stress, and inflammation in type 2 diabetes: The battle continues. J. Clin. Med. 2019, 8, 1385. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.H.; Hamdan, N.; Nyakuma, B.B.; Jamaluddin, H.; Wong, S.L.; Wong, K.Y.; Lee, T.H. Characterization of Functional Proteins from Edible Bird’s Nest Using Proteomic Techniques in Combination with Bioinformatics Analyses. Biol. Life Sci. Forum 2023, 26, 49. https://doi.org/10.3390/Foods2023-15008
Lee CH, Hamdan N, Nyakuma BB, Jamaluddin H, Wong SL, Wong KY, Lee TH. Characterization of Functional Proteins from Edible Bird’s Nest Using Proteomic Techniques in Combination with Bioinformatics Analyses. Biology and Life Sciences Forum. 2023; 26(1):49. https://doi.org/10.3390/Foods2023-15008
Chicago/Turabian StyleLee, Chia Hau, Norfadilah Hamdan, Bemgba Bevan Nyakuma, Haryati Jamaluddin, Syie Luing Wong, Keng Yinn Wong, and Ting Hun Lee. 2023. "Characterization of Functional Proteins from Edible Bird’s Nest Using Proteomic Techniques in Combination with Bioinformatics Analyses" Biology and Life Sciences Forum 26, no. 1: 49. https://doi.org/10.3390/Foods2023-15008
APA StyleLee, C. H., Hamdan, N., Nyakuma, B. B., Jamaluddin, H., Wong, S. L., Wong, K. Y., & Lee, T. H. (2023). Characterization of Functional Proteins from Edible Bird’s Nest Using Proteomic Techniques in Combination with Bioinformatics Analyses. Biology and Life Sciences Forum, 26(1), 49. https://doi.org/10.3390/Foods2023-15008








