Exploring the Inter- and Intra-Specific Variability of Androctonus Scorpion Venoms †
Abstract
1. Introduction
2. Materials and Methods
2.1. Venoms
2.2. Venom Analysis Using Mass Spectrometry Coupled with Reverse-Phase High-Performance Liquid Chromatography (RP-HPLC)
3. Results
3.1. Fractionation of Venoms Using Reverse-Phase High-Performance Liquid Chromatography (RP-HPLC)
3.2. Analysis by Mass Spectrometry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El Oufir, R. Piqures et Envenimations Scorpioniques (PES); Rapports General et Specifiques; Revue Toxicologie Maroc; Centre Anti Poison du Maroc (CAPM): Rabat, Morocco, 2019; Volume 43, p. 11. [Google Scholar]
- Touloun, O. Liste Actualisee et commentee de la Faune scorpionique du Maroc (Arachnida: Scorpiones). Rev. Ibérica Aracnol. 2019, 34, 126–132. [Google Scholar]
- Hilal, I.; Khourcha, S.; Safi, A.; Hmyene, A.; Asnawi, S.; Othman, I.; Stöcklin, R.; Oukkache, N. Comparative Proteomic Analysis of the Venoms from the Most Dangerous Scorpions in Morocco: Androctonus mauritanicus and Buthus occitanus. Life 2023, 13, 1133. [Google Scholar] [CrossRef] [PubMed]
- Cid-Uribe, J.I.; Veytia-Bucheli, J.I.; Romero-Gutierrez, T.; Ortiz, E.; Possani, L.D. Scorpion venomics: A 2019 overview. Expert Rev. Proteom. 2020, 17, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Escoubas, P.; Quinton, L.; Nicholson, G.M. Venomics: Unravelling the complexity of animal venoms with mass spectrometry. J. Mass. Spectrom. 2008, 43, 279–295. [Google Scholar] [CrossRef]
- Oukkache, N.; Chgoury, F.; Lalaoui, M.; Cano, A.A.; Ghalim, N. Comparison between two methods of scorpion venom milking in Morocco. J. Venom. Anim. Toxins Trop. Dis. 2013, 19, 5. [Google Scholar] [CrossRef]
- Vestal, M.L. High-Performance Liquid Chromatography-Mass Spectrometry. Science 1984, 226, 275–281. [Google Scholar] [CrossRef]
- Jenkins, T.P.; Ahmadi, S.; Bittenbinder, M.A.; Stewart, T.K.; Akgun, D.E.; Hale, M.; Nasrabadi, N.N.; Wolff, D.S.; Vonk, F.J.; Kool, J.; et al. Terrestrial venomous animals, the envenomings they cause, and treatment perspectives in the Middle East and North Africa. PLoS Negl. Trop. Dis. 2021, 15, e0009880. [Google Scholar] [CrossRef]
- Pimenta, A.M.C.; Stöcklin, R.; Favreau, P.; Bougis, P.E.; Martin-Eauclaire, M.F. Moving pieces in a proteomic puzzle: Mass fingerprinting of toxic fractions from the venom of Tityus serrulatus (Scorpiones, Buthidae): Mass fingerprinting of Tityus serrulatus toxic fractions. Rapid Commun. Mass Spectrom. 2001, 15, 1562–1572. [Google Scholar] [CrossRef]
- Diego-García, E.; Batista, C.V.F.; García-Gómez, B.I.; Lucas, S.; Candido, D.M.; Gómez-Lagunas, F.; Possani, L.D. The Brazilian scorpion Tityus costatus Karsch: Genes, peptides and function. Toxicon 2005, 45, 273–283. [Google Scholar] [CrossRef]
- Barona, J.; Batista, C.V.F.; Zamudio, F.Z.; Gomez-Lagunas, F.; Wanke, E.; Otero, R.; Possani, L.D. Proteomic analysis of the venom and characterization of toxins specific for Na+- and K+-channels from the Colombian scorpion Tityus pachyurus. Biochim. Biophys. Acta BBA-Proteins Proteom. 2006, 1764, 76–84. [Google Scholar] [CrossRef]
- Batista, C.V.F.; D’Suze, G.; Gómez-Lagunas, F.; Zamudio, F.Z.; Encarnación, S.; Sevcik, C.; Possani, L.D. Proteomic analysis of Tityus discrepans scorpion venom and amino acid sequence of noveltoxins. Proteomics 2006, 6, 3718–3727. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, D.G.; Rates, B.; Santos, D.M.; Verano-Braga, T.; Barbosa-Silva, A.; Dutra, A.A.A.; Biondi, I.; Martin-Eauclaire, M.F.; De Lima, M.E.; Pimenta, A.M.C. Moving pieces in a taxonomic puzzle: Venom 2D-LC/MS and data clustering analyses to infer phylogenetic relationships in some scorpions from the Buthidae family (Scorpiones). Toxicon 2006, 47, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Diego-García, E.; Peigneur, S.; Clynen, E.; Marien, T.; Czech, L.; Schoofs, L.; Tytgat, J. Molecular diversity of the telson and venom components from Pandinus cavimanus (Scorpionidae Latreille 1802): Transcriptome, venomics and function. Proteomics 2012, 12, 313–328. [Google Scholar] [CrossRef]
- Romero-Gutiérrez, M.; Santibáñez-López, C.; Jiménez-Vargas, J.; Batista, C.; Ortiz, E.; Possani, L. Transcriptomic and Proteomic Analyses Reveal the Diversity of Venom Components from the Vaejovid Scorpion Serradigitus gertschi. Toxins 2018, 10, 359. [Google Scholar] [CrossRef]
- Caliskan, F.; García, B.I.; Coronas, F.I.V.; Batista, C.V.F.; Zamudio, F.Z.; Possani, L.D. Characterization of venom components from the scorpion Androctonus crassicauda of Turkey: Peptides and genes. Toxicon 2006, 48, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Batista, C.V.F.; Román-González, S.A.; Salas-Castillo, S.P.; Zamudio, F.Z.; Gómez-Lagunas, F.; Possani, L.D. Proteomic analysis of the venom from the scorpion Tityus stigmurus: Biochemical and physiological comparison with other Tityus species. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 146, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Daoudi, K.; Malosse, C.; Lafnoune, A.; Darkaoui, B.; Chakir, S.; Sabatier, J.M.; Chamot-Rooke, J.; Cadi, R.; Oukkache, N. Massspectrometry-based top-down and bottom-up approaches for proteomic analysis of the Moroccan Buthus occitanus scorpion venom. FEBS Open Bio 2021, 11, 1867–1892. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Quintero-Hernandez, V.; Possani, L.D. Venom proteomic and venomous glands transcriptomic analysis of the Egyptian scorpion Scorpio maurus palmatus (Arachnida: Scorpionidae). Toxicon 2013, 74, 193–207. [Google Scholar] [CrossRef]
- Erdeş, E.; Doğan, T.S.; Coşar, İ.; Danışman, T.; Kunt, K.B.; Şeker, T.; Yücel, M.; Ozen, C. Characterization of Leiurus abdullahbayrami (Scorpiones: Buthidae) venom: Peptide profile, cytotoxicity and antimicrobial activity. J. Venom. Anim. Toxins Trop. Dis. 2014, 20, 48. [Google Scholar] [CrossRef]
- Estrada-Gómez, S.; Vargas Muñoz, L.J.; Saldarriaga-Córdoba, M.; Quintana Castillo, J.C. Venom from Opisthacanthus elatus scorpion of Colombia, could be more hemolytic and less neurotoxic than thought. Acta Trop. 2016, 153, 70–78. [Google Scholar] [CrossRef]
- Martin-Eauclaire, M.F.; Bosmans, F.; Céard, B.; Diochot, S.; Bougis, P.E. A first exploration of the venom of the Buthus occitanus scorpion found in southern France. Toxicon 2014, 79, 55–63. [Google Scholar] [CrossRef] [PubMed][Green Version]
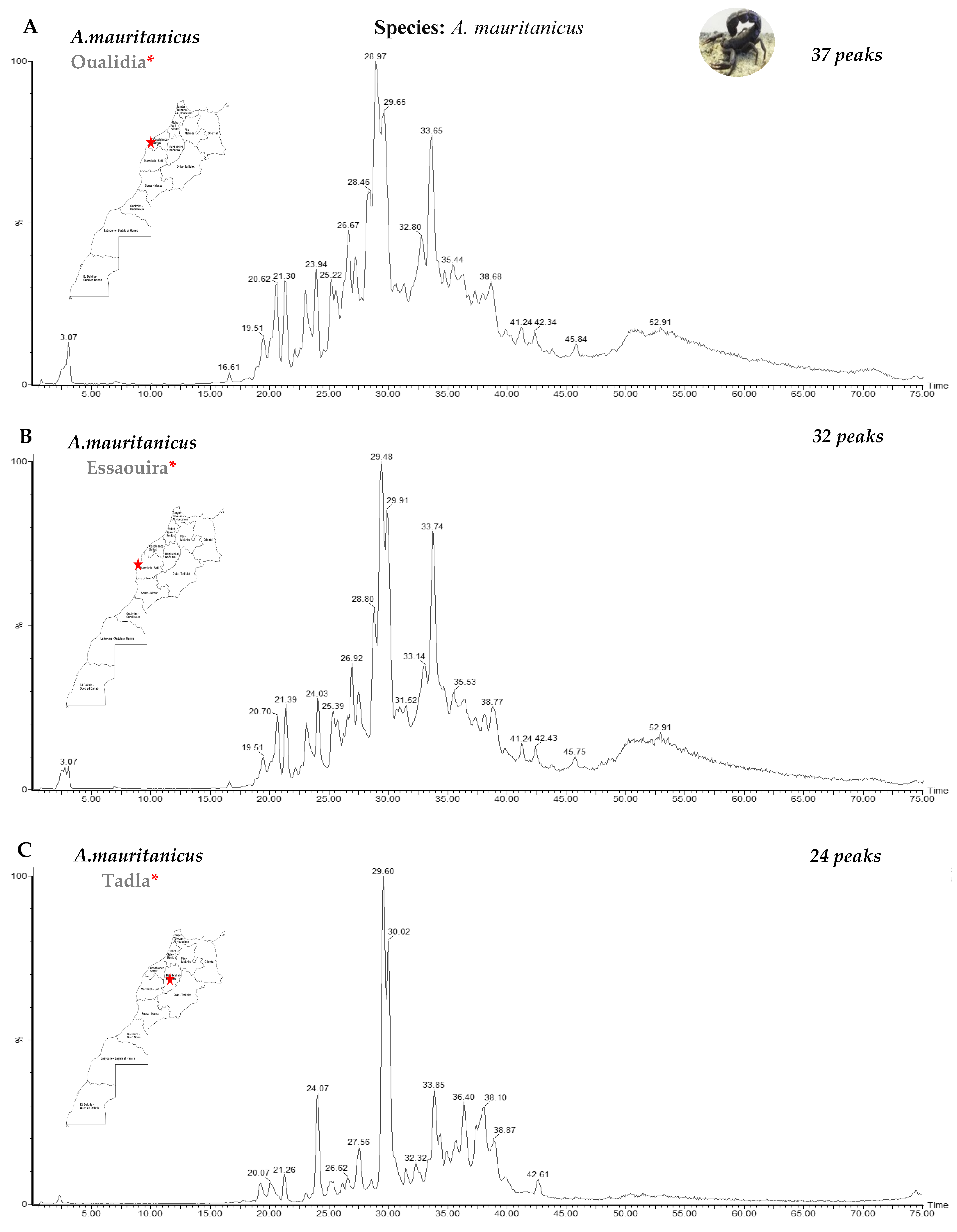
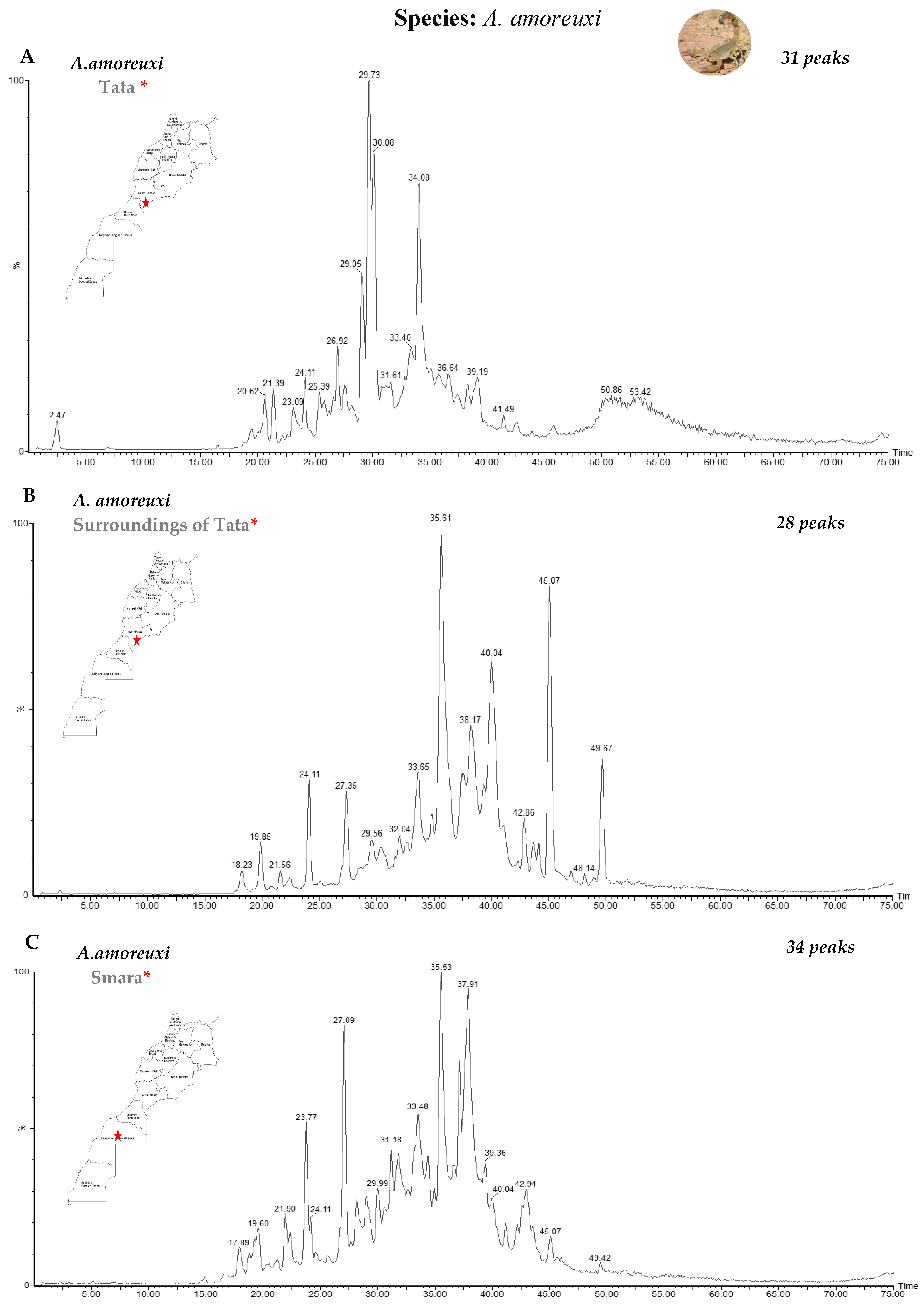

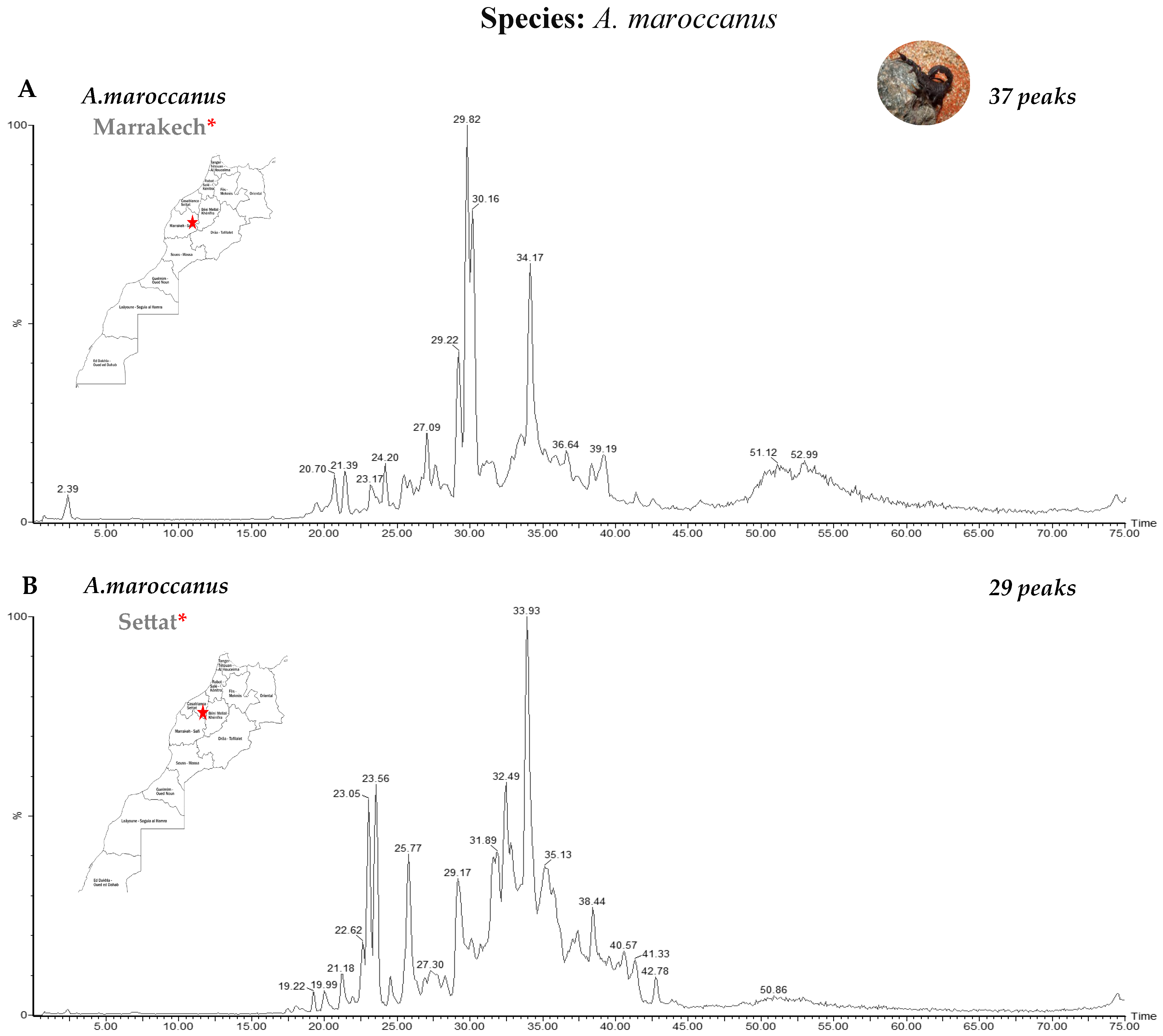

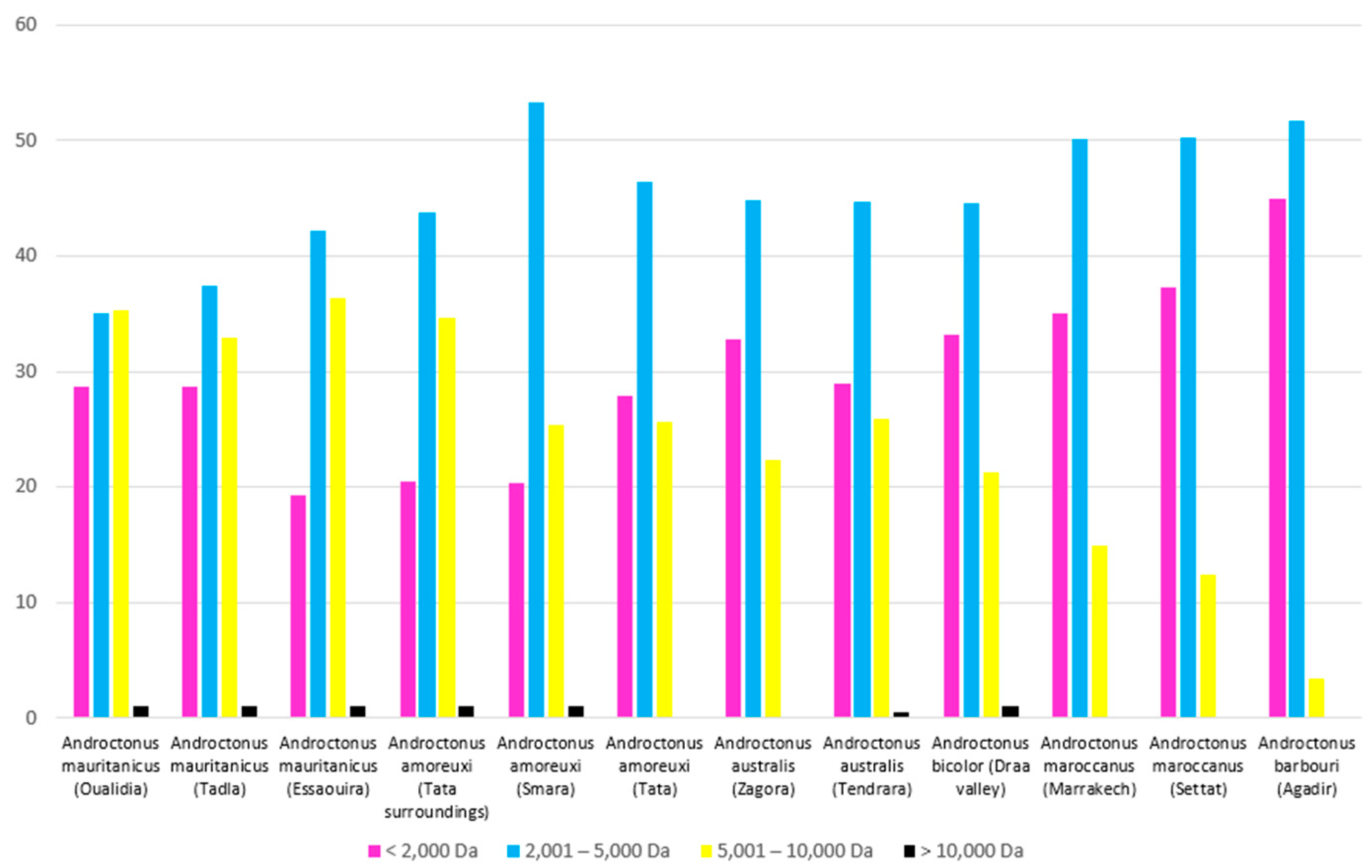
| Genus | Species | Number | Region | Molecular Masses |
|---|---|---|---|---|
| Androctonus | Androctonus mauritanicus | 3 | Oualidia | 469 |
| Essaouira | 410 | |||
| Tadla | 328 | |||
| Androctonus amoreuxi | 3 | Tata | 374 | |
| Tata surroundings | 452 | |||
| Smara | 309 | |||
| Androctonus australis | 2 | Zagora | 336 | |
| Tendrara | 359 | |||
| Androctonus bicolor | 1 | Draa Valley | 578 | |
| Androctonus maroccanus | 2 | Marrakech | 312 | |
| Settat | 338 | |||
| Androctonus barbouri | 1 | Agadir | 236 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hilal, I.; Khourcha, S.; Safi, A.; Hmyene, A.; Stöcklin, R.; Oukkache, N. Exploring the Inter- and Intra-Specific Variability of Androctonus Scorpion Venoms. Biol. Life Sci. Forum 2023, 24, 12. https://doi.org/10.3390/IECT2023-14797
Hilal I, Khourcha S, Safi A, Hmyene A, Stöcklin R, Oukkache N. Exploring the Inter- and Intra-Specific Variability of Androctonus Scorpion Venoms. Biology and Life Sciences Forum. 2023; 24(1):12. https://doi.org/10.3390/IECT2023-14797
Chicago/Turabian StyleHilal, Ines, Soukaina Khourcha, Amal Safi, Abdelaziz Hmyene, Reto Stöcklin, and Naoual Oukkache. 2023. "Exploring the Inter- and Intra-Specific Variability of Androctonus Scorpion Venoms" Biology and Life Sciences Forum 24, no. 1: 12. https://doi.org/10.3390/IECT2023-14797
APA StyleHilal, I., Khourcha, S., Safi, A., Hmyene, A., Stöcklin, R., & Oukkache, N. (2023). Exploring the Inter- and Intra-Specific Variability of Androctonus Scorpion Venoms. Biology and Life Sciences Forum, 24(1), 12. https://doi.org/10.3390/IECT2023-14797







