Abstract
4(5)-Methylimidazole (4(5)-MEI) can be formed during the caramelization procedure and the Maillard reaction and has been classified as possibly carcinogenic to humans. Data concerning the formation of 4(5)-MEI by the reaction between amino acids and sugars are still scarce. In this study, an ultrahigh performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-Q-ToF-MS) method was developed for the determination of 4(5)-MEI in aqueous model systems containing sugars (fructose and glucose) and amino acids (proline, phenylalanine, tyrosine, and lysine), after thermal processing at 100 °C. The results showed that the 4(5)-MEI was formed in all model systems, with the highest concentrations to be determined in fructose–proline (3.5 μg mL−1) and fructose–tyrosine (3.0 μg mL−1) aqueous model systems.
1. Introduction
4(5)-Methylimidazole (4(5)-MEI) is formed in food matrices during their thermal processing as a result of the Maillard reaction and has been classified by International Agency for Research on Cancer (IARC) as potentially carcinogenic to humans [1]. This compound is also produced during the preparation of ammonia caramel colorant additives by the caramelization procedure [2].
Early research reports have led to the hypothesis that 4(5)-MEI can be produced from the reaction between ammonia and α-dicarbonyl compounds [3]. Therefore, ammonia has been mainly used as a nitrogen source for the study of 4(5)-MEI in Maillard reaction systems, in combination with sugars (L-rhamnose, D-glucose) or methylglyoxal [4]. However, studies employing amino acids in Maillard model systems for investigating 4(5)-MEI formation are still scarce. Amino acids could be a source of nitrogen-containing compounds in foods from the Strecker degradation and exist in significant amounts in honey [5]. The possibility of honey adulteration with caramel color raises concerns about the existence of 4(5)-MEI under specific conditions, apart from the addition of caramel colorants [6]. 4(5)-Methylimidazole is a highly polar molecule characterized by the absence of chromophores; hence, liquid chromatography-mass spectrometry methods (LC-MS/MS) have been mainly applied for its determination in foods [7,8,9,10]. Nevertheless, the complexity of food matrices suggests the need for the development of accurate and sensitive analytical methods for the quantification of 4(5)-MEI. Quadrupole time-of-flight (Q-ToF) mass analyzer offers rapid food analysis, providing high resolution, sensitivity, and selectivity. To the best of our knowledge, there is no literature report employing liquid chromatography in combination with a Q-ToF mass analyzer for the quantification of 4(5)-MEI. In line with the information presented above, the aim of this study is the development of an ultrahigh performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-Q-ToF-MS) method in order to determine 4(5)-MEI in sugar–amino acid model systems, after thermal processing.
2. Materials and Methods
2.1. Reagents
Glucose, fructose, proline, phenylalanine, tyrosine, lysine, 4(5)-MEI, and ammonium acetate were supplied by Sigma-Aldrich (St. Louis, MO, USA). Formic acid, hydrochloric acid, ammonium hydroxide, methanol (MeOH) (HPLC grade), and acetonitrile (ACN) (LC-MS grade) were purchased from Fisher Scientific Co. (Chicago, IL, USA). Ultrahigh-purity water was produced using a Genie Water System from RephiLe Bioscience Ltd. (Shanghai, China). Solid-phase extraction (SPE) cartridges (Bond Elut SCX, 500 mg) were supplied by Agilent Technologies (Santa Clara, CA, USA).
2.2. Standard Solutions
Stock solution (1000 mg L−1) of 4(5)-MEI was prepared using ACN and stored in a dark glass vial at −20 °C. The calibration curve of 4(5)-MEI was constructed using the standard concentrations of 10.0, 8.0, 5.0, 3.0, 1.0, 0.8, 0.5, 0.3, and 0.1 mg L−1 via dilution with ACN.
2.3. Preparation of Aqueous Model Systems
Eight model systems were prepared by mixing the appropriate amounts of sugar and amino acids (Table 1). Subsequently, water was added in order to reach the quantity of 100 g for each model system. The aqueous model systems were heated at 100 °C for 60 h in a Tv10b heating oven (Memmert GmbH, Schwabach, Germany).

Table 1.
Aqueous model systems of sugars and amino acids analyzed by UPLC-Q-ToF-MS and the concentration of 4(5)-MEI after their thermal treatment.
2.4. SPE Procedure
The SPE procedure was performed based on a previous study [11] with some modifications: 10 g of sample was weighted into a glass vial and diluted with 10 mL of water. The solution was acidified with 20 µL of 0.1 M HCl. SCX cartridges were activated with 2 mL of methanol and 2 mL of 1% (v/v) formic acid solution. Then, the sample solution was loaded into the column under a vacuum, and impurities were washed out with 4 mL of methanol and 4 mL of 1% (v/v) formic acid solution. Elution of 4(5)-MEI was achieved using 5 mL of 5% (v/v) methanolic ammonia solution. Subsequently, the solvent was removed under the N2 gas flow stream, and the residue was dissolved with 1 mL of 10% (v/v) aqueous acetonitrile.
2.5. UPLC-Q-ToF-MS Analysis
An Agilent 6530 quadrupole time-of-flight system (Q-ToF-MS) with an electron spray ionization (ESI) source was used. The Q-ToF-MS was coupled with ultra performance liquid chromatography (UPLC) system (Agilent 1290 Infinity, Agilent Technologies, Santa Clara, CA, USA). The MS experiments were performed in positive and negative ESI modes, and nitrogen was used as the collision gas. The Q-TOF conditions were as follows: fragmentor, 100 V; drying gas, 12 L/min; nebulizer gas, 50 psi; capillary voltage, 3000 V; skimmer, 65 V; gas temperature, 350 °C; acquisition rate, 1 spectra/s (threshold 200 Abs, 0.01% rel.). The MS system was calibrated before each analysis using a calibrant solution. Furthermore, a constant infusion of a solution (Agilent Technologies) with reference ions was also used during the analysis for mass calibration of the MS system. The Agilent MassHunter software (version B.06.00, Santa Clara, CA, USA) was used for the acquisition of data, while data processing was performed using the Agilent MassHunter Qualitative Analysis software (version B.07.00).
2.6. Chromatographic Study
A Nucleoshell Bluebird (RP 18 EC, 2.7 µm particle size, 100 mm length, 4.6 mm i.d.) (Macherey-Nagel, Düren, Germany) column was used for the chromatographic study with a flow rate of 1.0 mL min−1. The mobile phases were A = water/ammonium acetate 5 mM and B = ACN. A gradient elution program was used with the following conditions: 0 min 4% B, 5 min 40% B, 10 min 100% B, 17 min 4% B, and 25 min 4% B. The column temperature was 40 °C, with an injection volume of 5 μL.
3. Results and Discussion
3.1. UPLC-Q-ToF-MS Method
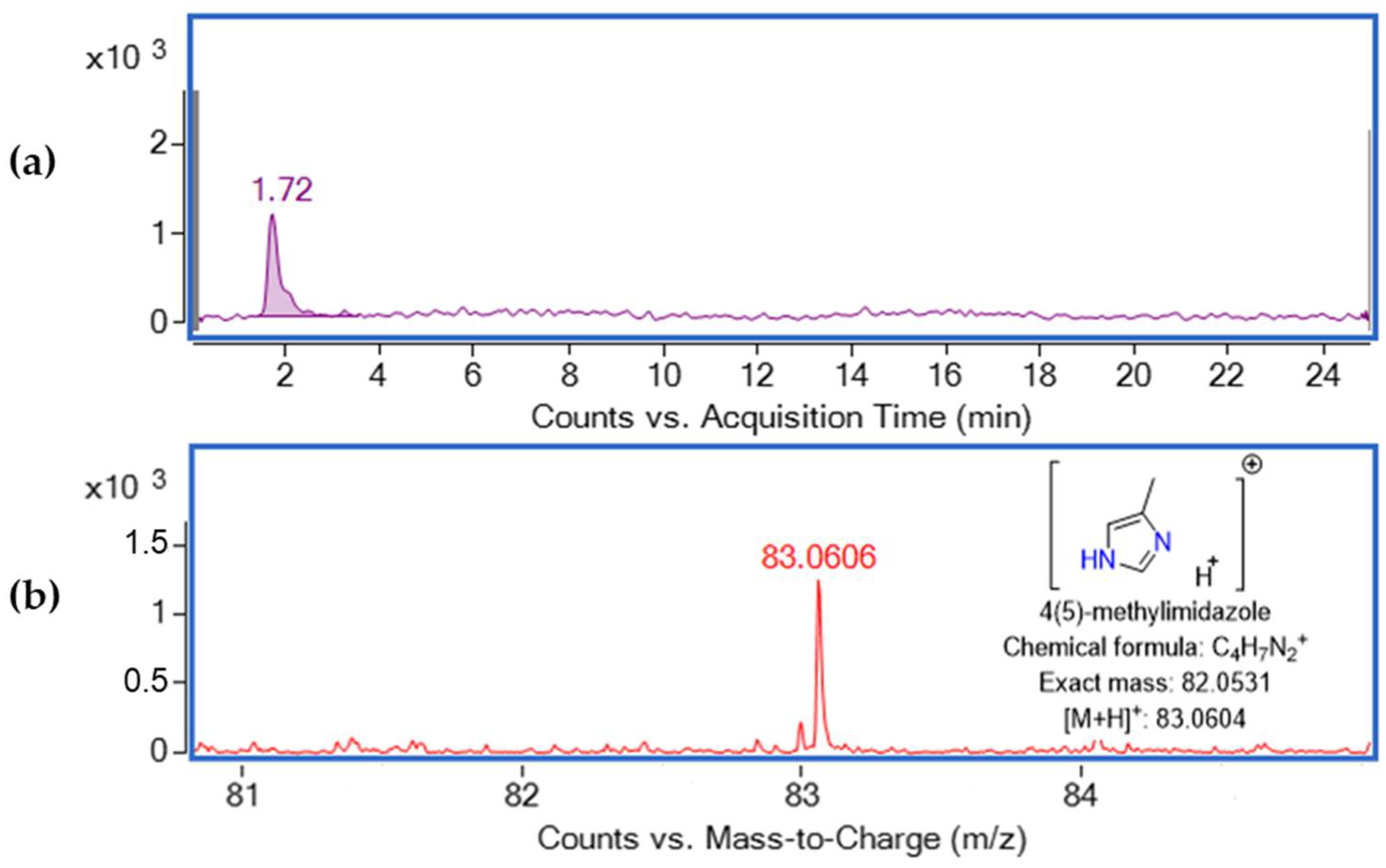
4(5)-Methylimidazole was studied in positive and negative ESI modes at different fragmentor (100 V, 120 V, 150 V) and capillary voltage conditions (3000 V, 4000 V). However, in negative ESI mode, the [M-H]− ion was not detected, which confirms previous research that determined this compound only at positive ESI [12,13]. Proceeding with the experiments in positive ESI (Figure 1), the optimum abundance of the [M+H]+ ion was observed at fragmentor 100 V and capillary voltage 3000 V. Nevertheless, the [M+Na]+ ion was not observed. 4(5)-Methylimidazole was detected at retention time 1.72 min at m/z 83.0606 (Δ 2.41 ppm).
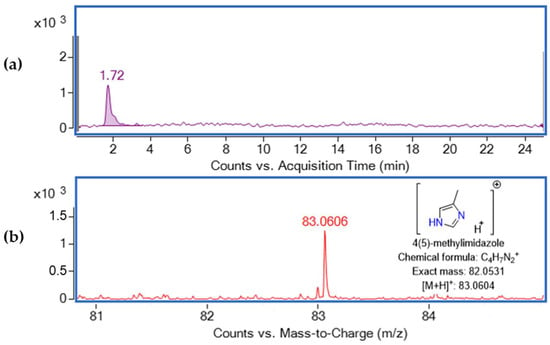
Figure 1.
(a) Extracted ion chromatogram of 1 mg L−1 standard solution of 4(5)-MEI at retention time 1.72 and (b) mass spectrum of 1 mg L−1 standard solution.
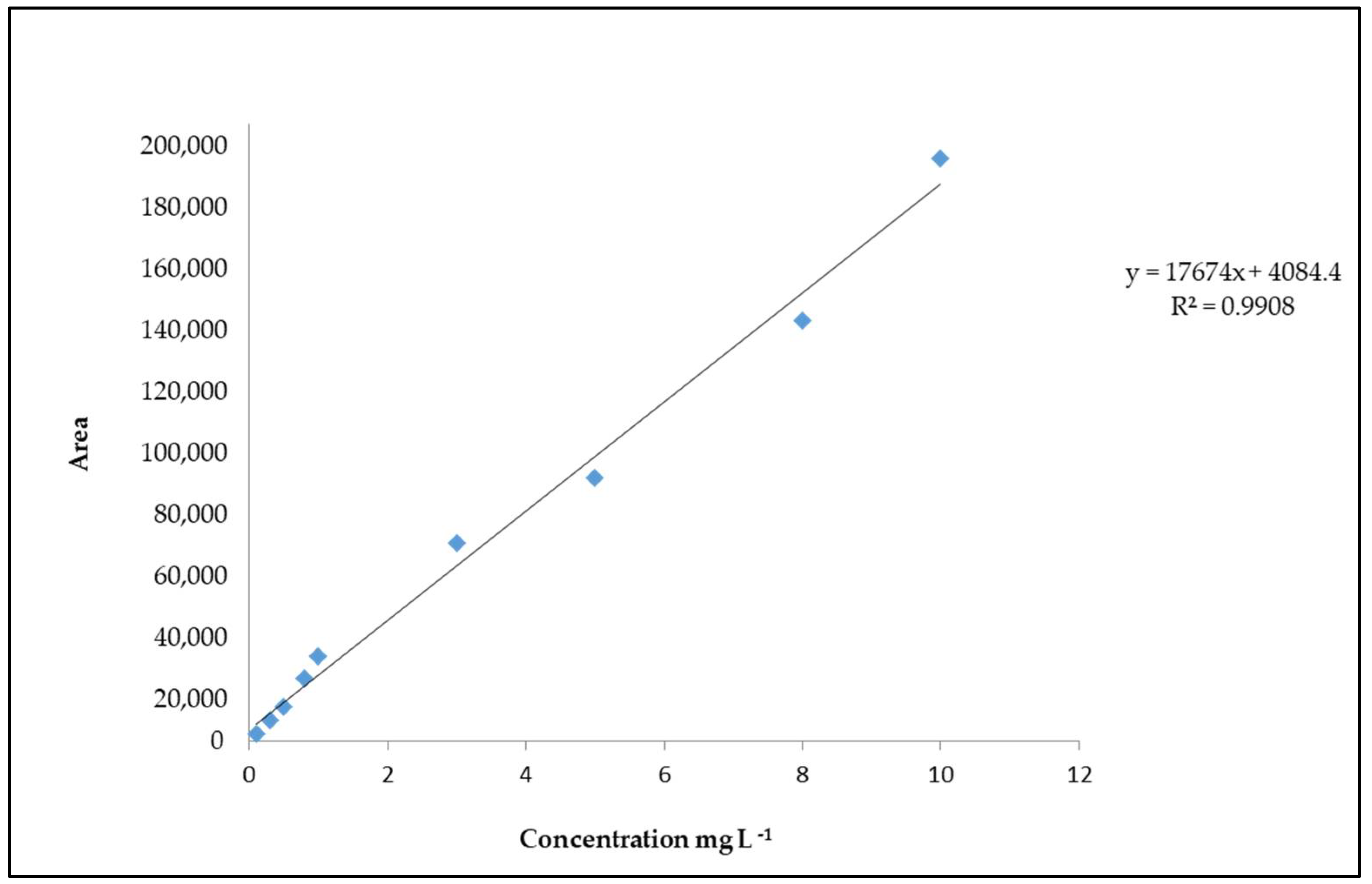
The peak area of the extracted ion chromatograms was utilized for the quantification of 4(5)-MEI. The linearity of the UPLC-Q-ToF-MS method was determined by the construction of a calibration curve at different concentrations (Figure 2). The limits of detection (LOD) and quantification (LOQ) were 1.7 mg L−1 and 5.1 mg L−1, respectively, while linearity was R2 = 0.9908.
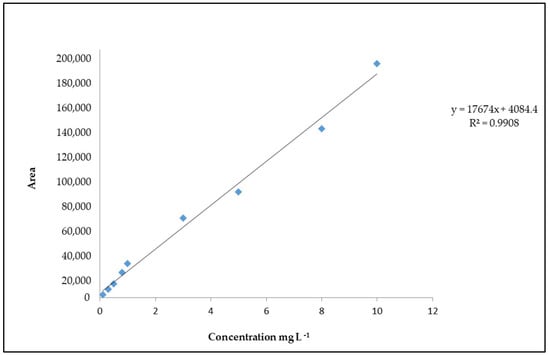
Figure 2.
Calibration curve of 4(5)-MEI.
3.2. Analysis of Aqueous Model Systems
Prolonged heating of the aqueous model systems was applied at 100 °C for 60 h in order to ascertain the formation of 4(5)-MEI. Additionally, different concentrations of the amino acids added in the model systems were used to test the effect of concentration on the formation of 4(5)-MEI). 4(5)-Methylimidazole was detected in all model systems (Table 1). The combination of fructose and proline provided the highest concentration of 4(5)-MEI (3.5 μg mL−1). The lowest concentration was detected in the glucose–tyrosine system (0.2 μg mL−1); however, in the fructose–tyrosine system, the concentration of 4(5)-MEI was significantly elevated (3.0 μg mL−1). In all model systems of amino acids with fructose, the concentration of 4(5)-MEI was increased, except for fructose–lysine, where it was lower (0.9 μg mL−1), compared with glucose–lysine (1.3 μg mL−1), which indicates that the formation of 4(5)-MEI is differently affected from each amino acid.
Furthermore, the formation of 4(5)-MEI may be affected by the amount of sugar, as in the current study, where the amount of fructose was increased by 10 g in relation to glucose. These results are in agreement with a research study on glucose–ammonia systems heated at 150 °C for 2 h, where the authors observed that the concentration of 4(5)-MEI increases with increasing glucose concentration [14].
4. Conclusions
An accurate analytical method for the determination of 4(5)-MEI was developed employing UPLC-Q-ToF-MS at positive ESI mode. Eight aqueous model systems were prepared with sugar (glucose, fructose) and amino acid (proline, phenylalanine, tyrosine, and lysine), which are the main components of honey. The model systems were heated at 100 °C for 60 h in order to study the formation of 4(5)-MEI. The results indicate that the formation of 4(5)-MEI is differently affected by each amino acid as well as the amount of sugar. The proposed UPLC-Q-ToF-MS method can be used for future applications in other food matrices.
Author Contributions
Conceptualization, E.A. and P.-K.R.; methodology, E.A. and P.-K.R.; software, P.-K.R.; validation, P.-K.R. and M.X.; formal analysis, P.-K.R.; investigation, P.-K.R.; resources, E.A. and P.A.T.; data curation, P.-K.R.; writing—original draft preparation, P.-K.R.; writing—review and editing, P.A.T., E.A., C.S.P., P.-K.R. and M.X.; visualization, P.-K.R.; supervision, P.A.T., E.A. and C.S.P.; project administration, P.A.T. and E.A.; funding acquisition, E.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was co-funded 50% by the Hellenic Ministry of Rural Development and Food and 50% by the EU, under the Commission Delegated Regulation (EU) 2015/1366 of 11 May 2015 supplementing Regulation (EU) No 1308/2013 of the European Parliament and the Council with regard to aid in the apiculture sector.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 4-Methylimidazole. IARC Monogr. Eval. Carcinog. Risks Hum. 2003, 101, 447–459. [Google Scholar]
- Revelou, P.-K.; Xagoraris, M.; Alissandrakis, E.; Pappas, C.S.; Tarantilis, P.A. A Review of the Analytical Methods for the Determination of 4(5)-Methylimidazole in Food Matrices. Chemosensors 2021, 9, 322. [Google Scholar] [CrossRef]
- Radzisewski, B. Ueber Glyoxalin Und Seine Homologe. Ber. der Dtsch. Chem. Ges. 1882, 15, 2706–2708. [Google Scholar] [CrossRef]
- Moon, J.-K.; Shibamoto, T. Formation of Carcinogenic 4(5)-Methylimidazole in Maillard Reaction Systems. J. Agric. Food Chem. 2011, 59, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Hermosín, I.; Chicón, R.M.; Dolores Cabezudo, M. Free Amino Acid Composition and Botanical Origin of Honey. Food Chem. 2003, 83, 263–268. [Google Scholar] [CrossRef]
- Zábrodská, B.; Vorlová, L. Adulteration of Honey and Available Methods for Detection—A Review. Acta Vet. Brno 2015, 83, 85–102. [Google Scholar] [CrossRef]
- Wu, C.; Wang, L.; Li, H.; Yu, S. Determination of 4(5)-Methylimidazole in Foods and Beverages by Modified QuEChERS Extraction and Liquid Chromatography-Tandem Mass Spectrometry Analysis. Food Chem. 2019, 280, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, C. Simultaneous Analysis of 2-Methylimidazole, 4-Methylimidazole, and 5-Hydroxymethylfurfural Potentially Formed in Fermented Soy Sauce by “Quick, Easy, Cheap, Effective, Rugged, and Safe” Purification and UHPLC with Tandem Mass Spectrometry. J. Sep. Sci. 2019, 42, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.-T.; Wu, J.-H.; Liang, X.; Du, M.; Qin, L.; Xu, X.-B. Isotope Dilution Determination for the Trace Level of 4(5)-Methylimidazole in Beverages Using Dispersive Liquid-Liquid Microextraction Coupled with ESI-HPLC–MS/MS. Food Chem. 2018, 245, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, G.; Voorspoels, S.; Vloemans, P.; Fierens, T.; Van Holderbeke, M.; Cornelis, C.; Sioen, I.; De Maeyer, M.; Vinkx, C.; Vanermen, G. Caramel Colour and Process By-Products in Foods and Beverages: Part I—Development of a UPLC-MS/MS Isotope Dilution Method for Determination of 2-Acetyl-4-(1,2,3,4-Tetrahydroxybutyl)Imidazole (THI), 4-Methylimidazole (4-MEI) and 2-Methylimidazol (2-MEI). Food Chem. 2018, 255, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tang, X.; Wu, C.; Yu, S. Maillard Reaction in Chinese Household-Prepared Stewed Pork Balls with Brown Sauce: Potentially Risky and Volatile Products. Food Sci. Hum. Wellness 2021, 10, 221–230. [Google Scholar] [CrossRef]
- Mottier, P.; Mujahid, C.; Tarres, A.; Bessaire, T.; Stadler, R.H. Process-Induced Formation of Imidazoles in Selected Foods. Food Chem. 2017, 228, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, L.; Li, H.; Yu, S. Combination of Solid-Phase Extraction with Microextraction Techniques Followed by HPLC for Simultaneous Determination of 2-Methylimidazole and 4-Methylimidazole in Beverages. Food Chem. 2020, 305, 125389. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.W.; Jiang, Y.; Hengel, M.; Shibamoto, T. Formation of 4(5)-Methylimidazole and Its Precursors, α-Dicarbonyl Compounds, in Maillard Model Systems. J. Agric. Food Chem. 2013, 61, 6865–6872. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).