Abstract
This correlation study aimed to assess global trends of lip and oral cavity (LOC) cancer mortality due to chewing tobacco in the period 1990–2019. Among women, the highest proportion of deaths by LOC cancer attributed to chewing tobacco was 27.7% in 2019, with a positive correlation between mortality of LOC cancer and chewing tobacco in the studied period (r = +0.832, p < 0.001). Among men, the contribution of chewing tobacco to LOC cancer burden globally was 14.1% in 2019, with a negative correlation between mortality of LOC cancer in males and chewing tobacco (r = −0.564, p < 0.01). The percentage of deaths for LOC cancer attributable to chewing tobacco is concentrated in certain world regions (mainly in South East Asia and Eastern Mediterranean), with significantly increasing trends.
1. Introduction
Cancers of the lip and oral cavity (LOC) are highly frequent in South Central Asia, and Melanesia, as well as in Eastern and Western Europe, and have been linked to alcohol consumption, tobacco smoking, HPV infection, and to ultraviolet radiation [1,2].
Many studies focused on chewing tobacco use have found many associated health risks [3]. A recent meta-analysis showed a significantly positive association between smokeless tobacco use and the risk of oral cancer in South East Asia, the Eastern Mediterranean Region, and among women users [4]. Additionally, the same study reported that chewing products was associated with a higher risk of oral cancer than other types of smokeless tobacco [4]. However, the association between chewing tobacco and mortality rates from LOC cancer was found to vary in significance and magnitude across countries [5]. The aim of this study was to analyze the global and regional LOC cancer mortality in relation to chewing tobacco use, as well as to assess differences by sex.
2. Materials and Methods
A correlation study was performed to analyze global trends of mortality from LOC cancer attributable to chewing tobacco.
Lip and oral cavity cancer mortality and chewing tobacco data were obtained from the database of the Global Burden of Disease (GBD) 2019 study [6]. The age-standardized rates (ASRs, expressed per 100,000 persons) were presented, based on standardization performed by direct methods (GBD population was used as the standard population). In our study, “lip and oral cavity cancer, (LOC)” mortality includes deaths from malignant neoplasm: site codes 140–145, 210, 235 according to revision 9 and site codes C01-C08, D00, D10, D11, D37 according to revision 10 of the International Classification of Diseases—to classify death, injury and cause of death.
To estimate trends of LOC cancer mortality, joinpoint regression analysis was used: the average annual percent change (AAPC) with the corresponding 95% confidence interval (95% CI) was calculated [7]. Additionally, trends were evaluated by regions of the World Health Organization (WHO). In this manuscript, trends of LOC mortality were presented using a straight line in the whole period, even if there were changes in trends in the observed period. Additionally, disparities in mortality trends by sex were tested using a comparability test. All statistical analyses were performed using the Joinpoint regression software, Version 4.9.0.0 (National Cancer Institute, Bethesda, MA, USA—March 2021). A p value of <0.05 was considered statistically significant for all tests.
The relationship between mortality rates and chewing tobacco was examined by calculating a bivariate correlation coefficient—the Pearson’s correlation coefficient (r). All statistical analyses were conducted using the Statistical Package for Social Sciences software (v. 20.0, SPSS Inc., Chicago, IL, USA).
3. Results
In 2019, the global ASR of LOC cancer mortality was 3.4 per 100,000 in males vs. 1.6 per 100,000 in females (Figure 1). Males had higher ASRs compared with females in 2019 in all WHO regions. The highest ASR in males was found in the South East Asia Region (6.1 per 100,000), while the highest rate in females was reported in the Eastern Mediterranean Region (4.0 per 100,000).
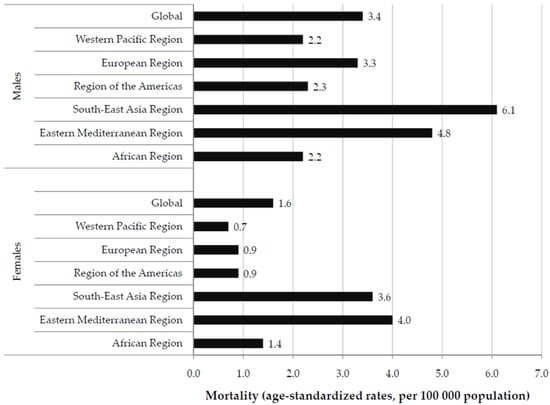
Figure 1.
Mortality of lip and oral cavity cancer (global and by regions), by sexes, in 2019.
Over the 1990–2019 period, the global ASRs of LOC cancer mortality significantly decreased in males (AAPC = −0.2%, 95%CI = −0.3 to −0.2), but significantly increased in females (AAPC = 0.2%, 95% CI = 0.1–0.3) (Figure 2). According to the comparability test, global trends in mortality of LOC cancer in males and females were not parallel (final selected model rejected parallelism, p < 0.05).
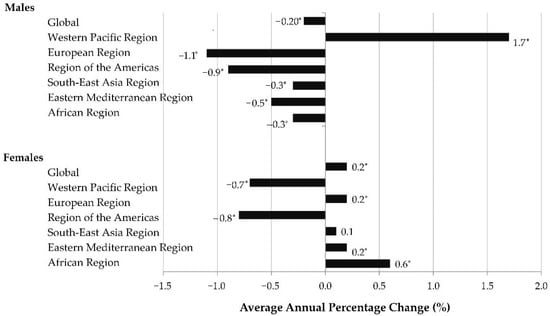
Figure 2.
Global mortality trends of lip and oral cavity cancer, by regions and by sexes, in the period 1990–2019: a joinpoint regression analysis. * Statistically significant, p < 0.05.
The most significant changes in ASRs of LOC cancer mortality over the 1990–2019 period were detected in males in the Western Pacific Region, with an increase of +1.7% per year. All other WHO regions showed significantly decreasing trends in males. In females, a significant increase in mortality of LOC cancer was registered in the European and Eastern Mediterranean Region (equally by 0.2% per year) and African Region (by 0.6% per year).
Only in females in the South East Asia Region was a non-significant increase observed (by +0.1% per year). In only one WHO region—Region of the Americas, in both sexes—a significant decrease in LOC cancer mortality rates was observed: AAPC = −0.9% (95%CI = −1.0 to −0.9) in males, and AAPC = −0.8%, (95%CI = −0.8 to −0.7) in females.
The contribution of chewing tobacco to the LOC cancer burden globally in males was 14.1% in 2019, with a significantly increasing trend (by 0.1% per year in the study period): the Pearson coefficient showed significant negative correlation between global mortality of LOC cancer in males and chewing tobacco (r = −0.564, p < 0.01) (Table 1). The highest contribution of chewing tobacco to the LOC cancer burden in males in 2019 was observed in the South East Asia Region (29.7%), while the lowest was observed in the European Region (0.6%). Except for the European Region, decreasing trends of the proportion of deaths by LOC cancer attributed to chewing tobacco were observed in males in all regions.

Table 1.
Pearson’s correlation coefficients (r) and trends (AAPC) for chewing tobacco with mortality of lip and oral cancer (age-standardized rates per 100,000 persons) by regions and sexes, 1990–2019.
Among females globally, the proportion of deaths by LOC cancer attributed to chewing tobacco was 27.7% in 2019, and increased by AAPC = +0.8% per year in the the period 1990–2019: the Pearson coefficient showed significant positive correlation between mortality of LOC cancer and chewing tobacco in the observed period (r = +0.832, p < 0.01). The highest contribution of chewing tobacco to the LOC cancer burden in females in 2019 was observed in the South East Asia Region (50.5%), while the lowest was observed in the African Region (1.0%). Significantly increased trends of the proportion of deaths by the LOC cancer attributed to chewing tobacco were observed in females in the Western Pacific, European and South East Asia Regions.
4. Discussion
One of the main findings in this study is the significantly increasing global trends of the proportion of deaths by the LOC cancer attributed to chewing tobacco both in males and females. The percentage of LOC cancer deaths attributed to chewing tobacco use in both sexes is concentrated mainly in the South East Asia Region.
Similar to that, in previous studies chewing tobacco was considered a major risk factor for oral and pharyngeal cancer in Asia, while the risk was not significant in the United States or Europe [8]. The discrepancies in risk between countries can be attributed to differences in populations (by age, socio-economic status, lifestyle, etc.), tobacco species in different regions [3,9]. This finding could probably be attributed to the chewing of product containing betel nut, which was associated with a high risk of oral and oropharyngeal cancer [10]. About half of oral cancers could be prevented if betel quid was no longer chewed: for example, in India, the population fraction attributable to betel quid chewing with added tobacco was estimated to 44.7% in males and 63.2% in females, while the fraction without added tobacco was 2.6% in males and 12.3% in females [10]. Tobacco chewing is a frequent habit in both migrants of Asian descent and the native population, making it a major risk factor for oral cancer in those populations [11].
Consistent with others [3,9], our study noted significant sex differences in LOC cancer burden due to chewing tobacco. A recent GBD 2019 study showed that the global prevalence of chewing tobacco was 6.55% among males and 2.87% among females in 2019 [12]. The majority of persons (83.29%) who used chewing tobacco in 2019 were in the South Asia Region (in India, 67.83% of global users, and in Bangladesh, 9.37% of global users). Both in males and females, the prevalence was the highest in South Asia (24.65% and 12.13%, respectively). The prevalence in males was the lowest in Latin America (0.17%), while the lowest prevalence in females was in Western Europe (0.15%). Globally, the prevalence of chewing tobacco use has increased slightly in both sexes in 1990–2019 (by 0.39% for males and 0.60% for females) [12]. Overall, these findings support the hypothesis that chewing tobacco is associated with LOC cancer risk. Besides, substantial differences according to sexes could be attributed to different exposure to lifestyle-related risk factors such as smoking habits, alcohol consumption, etc. [10]
This study has some limitations. Apart from the issue of the quality of data and the well-known shortcomings of ecological studies, epidemiological fallacy is an inherent limitation of this study. In addition, there was a lack of control of certain confounding factors, such as socio-economic status, alcohol consumption, and cigarette smoking.
5. Conclusions
Males had higher rates of LOC cancer mortality compared with females in all WHO regions in 2019. The highest rate in males was found in the South East Asia Region, while the highest rate in females was reported in the Eastern Mediterranean Region. Over the 1990–2019 period, the global rates of LOC cancer mortality significantly decreased in males, while significantly increasing in females. Our study noted sex differences in LOC cancer mortality attributable to chewing tobacco, hereby the percentage of deaths for the LOC cancer attributable to chewing tobacco is mainly concentrated in both males and females in the South East Asia Region. Additional analytic epidemiological studies addressing the impact of chewing tobacco as a risk factor for LOC cancer are needed.
Author Contributions
Conceptualization, I.I. and M.I.; methodology, I.I. and M.I.; software, I.I. and M.I.; validation, I.I. and M.I.; formal analysis, I.I. and M.I.; investigation, I.I. and M.I.; resources, I.I. and M.I.; data curation, I.I. and M.I.; writing—original draft preparation, I.I.; writing—review and editing, I.I. and M.I.; visualization, I.I. and M.I.; supervision, M.I.; project administration, M.I.; funding acquisition, M.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the Faculty of Medical Sciences, University of Kragujevac (Ref. No.: 01-14321, 13 November 2017), entitled “Epidemiology of the most common health disorders”.
Informed Consent Statement
Not applicable. No patient approvals were sought nor required for this study. Namely, as our model-based analysis used aggregated data, patients were not involved in the research.
Data Availability Statement
Data are contained within the article.
Acknowledgments
This study is supported by project No. 175042, supported by the Ministry of Education, Science and Technological development, Republic of Serbia, 2011–2020.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Han, A.Y.; Kuan, E.C.; Mallen-St Clair, J.; Alonso, J.E.; Arshi, A.; St John, M.A. Epidemiology of squamous cell carcinoma of the lip in the United States: A population-based cohort analysis. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Chewing Tobacco Collaborators. Spatial, temporal, and demographic patterns in prevalence of chewing tobacco use in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet Public Health 2021, 6, e482–e499. [Google Scholar] [CrossRef]
- Asthana, S.; Labani, S.; Kailash, U.; Sinha, D.N.; Mehrotra, R. Association of Smokeless Tobacco Use and Oral Cancer: A Systematic Global Review and Meta-Analysis. Nicotine Tob. Res. 2019, 21, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Hajat, C.; Stein, E.; Ramstrom, L.; Shantikumar, S.; Polosa, R. The health impact of smokeless tobacco products: A systematic review. Harm Reduct. J. 2021, 18, 123. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 (GBD 2019) Results; Institute for Health Metrics and Evaluation (IHME): Seattle, WA, USA, 2020; Available online: http://ghdx.healthdata.org/gbd-results-tool (accessed on 29 July 2022).
- Kim, H.J.; Fay, M.P.; Feuer, E.J.; Midthune, D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000, 19, 335–351. [Google Scholar] [CrossRef]
- Boffetta, P.; Aagnes, B.; Weiderpass, E.; Andersen, A. Smokeless tobacco use and risk of cancer of the pancreas and other organs. Int. J. Cancer 2005, 114, 992–995. [Google Scholar] [CrossRef] [PubMed]
- Rodu, B.; Jansson, C. Smokeless tobacco and oral cancer: A review of the risks and determinants. Crit. Rev. Oral Biol. Med. 2004, 15, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Guha, N.; Warnakulasuriya, S.; Vlaanderen, J.; Straif, K. Betel quid chewing and the risk of oral and oropharyngeal cancers: A meta-analysis with implications for cancer control. Int. J. Cancer 2014, 135, 1433–1443. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Hathi, M.R. A comparative and cross-sectional study on the prevalence of risk factors for mouth ulcer and oral cancers in migrants and native population of a tourist city of Rajasthan. Int. J. Community Med. Public Health 2018, 5, 3898–3902. [Google Scholar] [CrossRef]
- GBD 2019 Tobacco Collaborators. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet 2021, 397, 2337–2360. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).