Evaluation of the Oxidative Stability of Emulsifiers of an Acylglicerol Origin †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Study of Ultraviolet Absorption Spectra
2.3. Determination of the Specific Absorption Coefficient
2.4. Study of the Primary Products of the Oxidation of Lipids
2.5. Statistical Analysis
3. Results and Discussion
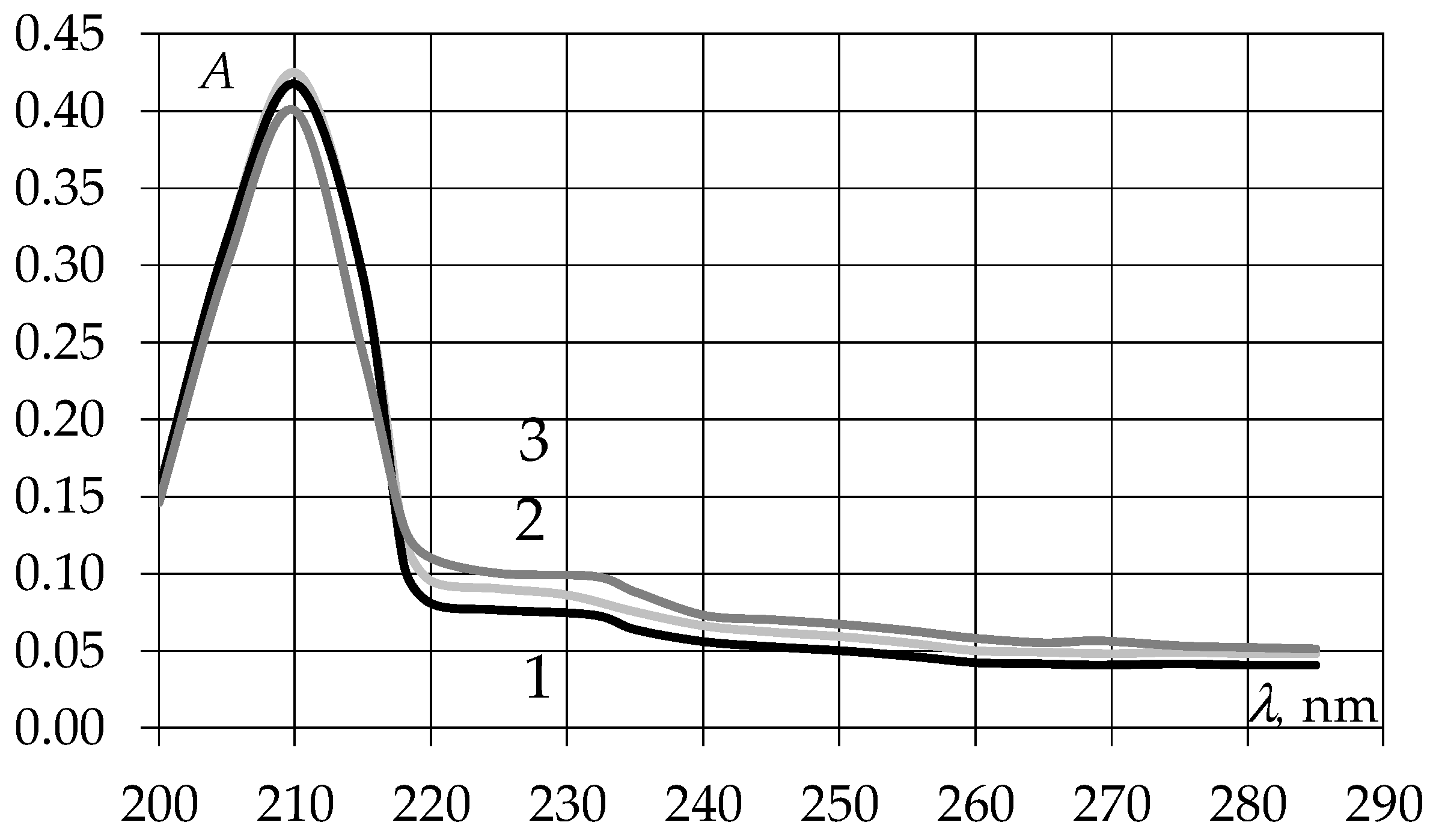
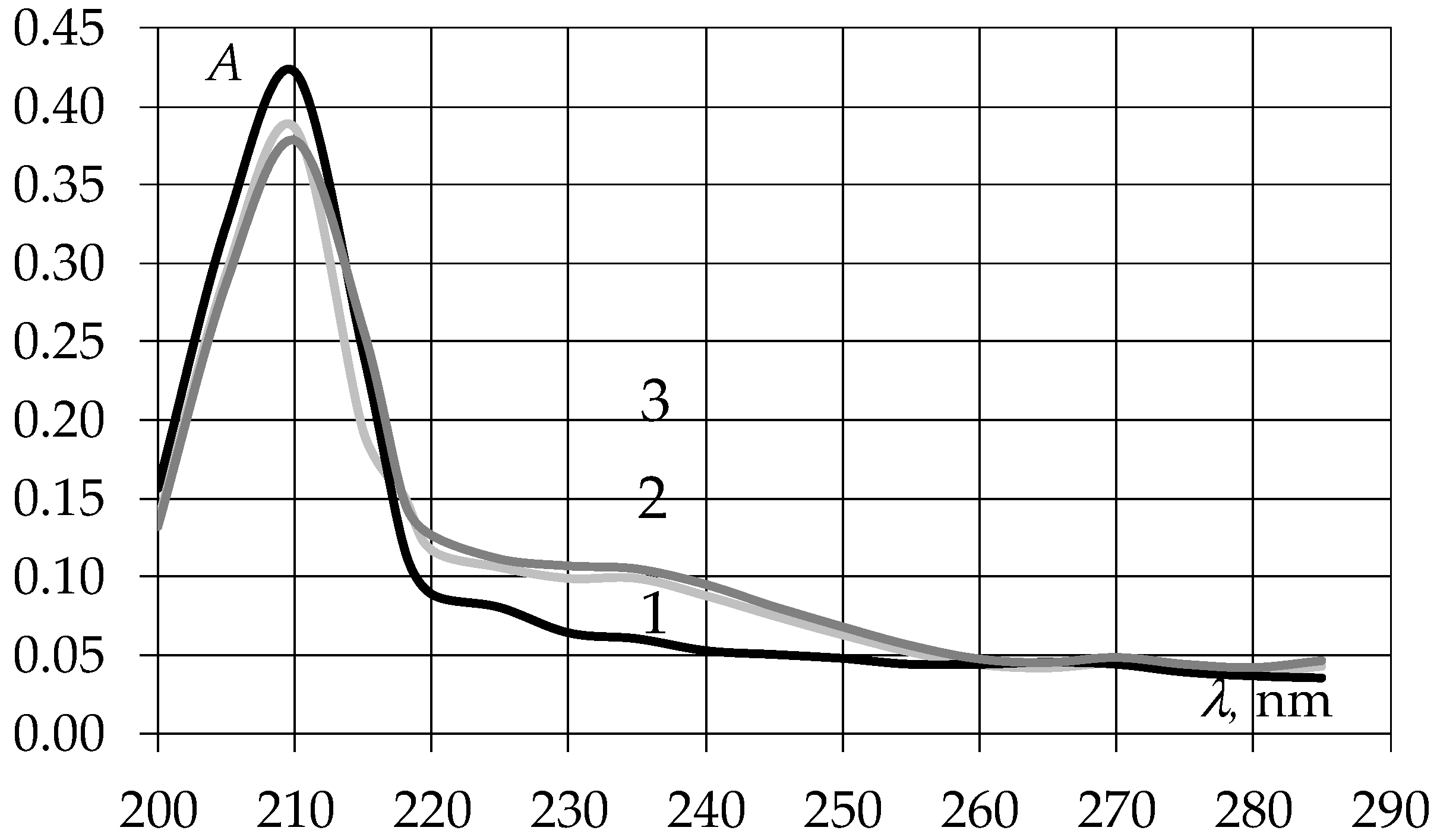
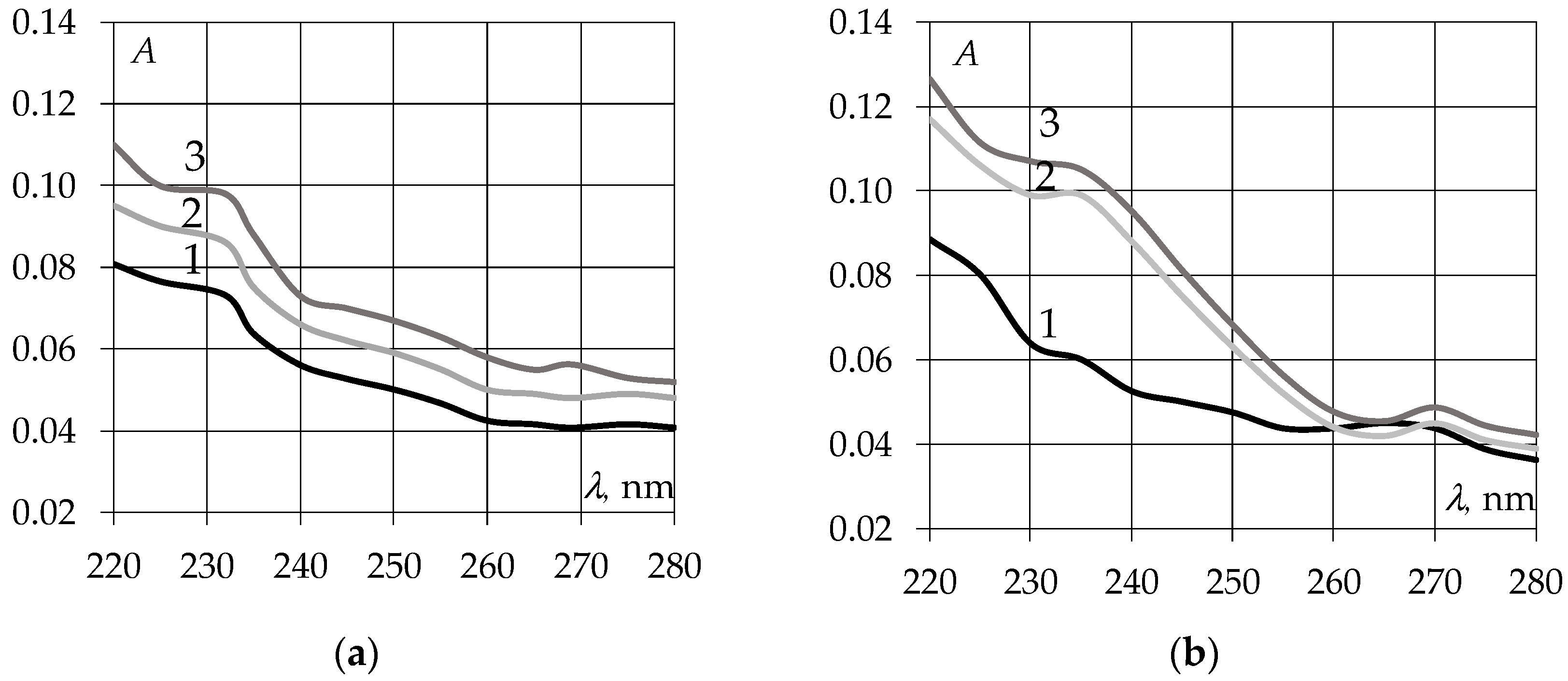
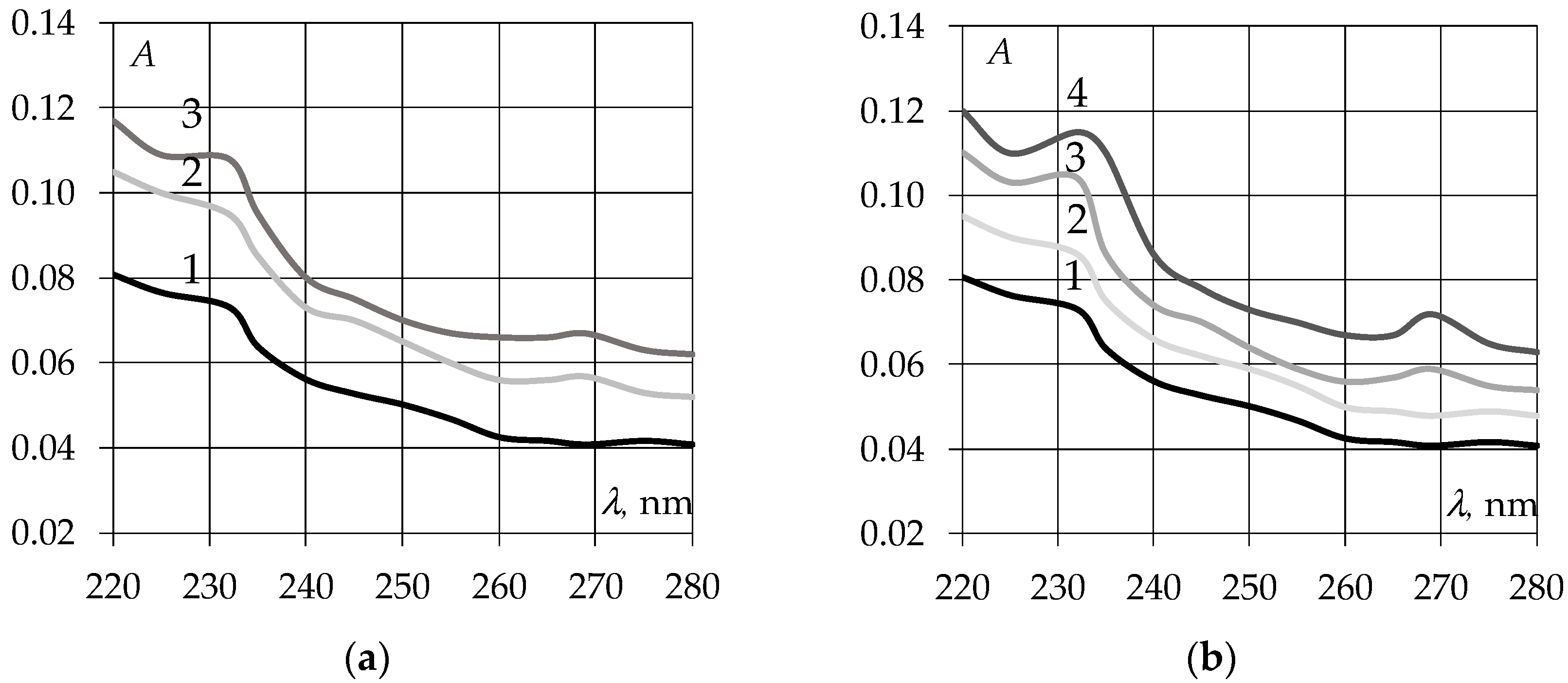
3.1. Study of Ultraviolet Absorption Spectra of EAGO and Sunflower Oil Samples
3.2. Studying the Primary Products of the Oxidation of Lipids in EAGO and Sunflower Oil Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santamaria-Echart, A.; Fernandes, I.P.; Silva, S.C.; Rezende, S.C.; Colucci, G.; Dias, M.M.; Barreiro, M.F. New Trends in Natural Emulsifiers and Emulsion Technology for the Food Industry. In Natural Food Additives; Lage, M.Á.P., Otero, P., Eds.; Intech Open: London, UK, 2021; pp. 5–17. [Google Scholar]
- Akoh, C.C.; Min, D.B. Food Lipids: Chemistry, Nutrition, and Biotechnology, 4th ed.; CRC Press, Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2017. [Google Scholar]
- Zhang, Z.; Ye, J.; Lee, W.J.; Akoh, C.C.; Li, A.; Wang, Y. Modification of palm-based oil blend via interesterification: Physicochemical properties, crystallization behaviors and oxidative stabilities. Food Chem. 2021, 347, 129070. [Google Scholar] [CrossRef] [PubMed]
- Murlykina, N.; Upatova, O.; Yancheva, M.; Murlykina, M. Application of infrared spectroscopy for quantitative analysis of new food emulsifiers. Ukr. Food J. 2015, 4, 299–309. [Google Scholar]
- Jespersen, N. Chapter 5. General principles of spectroscopy and spectroscopic analysis. In Modern Instrumental Analysis: Comprehensive Analytical Chemistry, 1st ed.; Ahuja, S., Jespersen, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 47, pp. 111–155. [Google Scholar]
- Arshad, M.S.; Hina, G.; Anjum, F.M.; Suleria, H.A.R. Effect of milk-derived bioactive peptides on the lipid stability and functional properties of beef nuggets. Sci. Rep. 2022, 12, 1242. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N. Chapter 5—Methods to determine extent of oxidation. In Lipid Oxidation, 2nd ed.; Oily Press Lipid Library Series; Woodhead Publishing: Cambridge, UK, 2012; pp. 99–127. [Google Scholar]
- Halvorsen, B.U.; Blomhoff, R. Determination of lipid oxidation products in vegetable oils and marine omega-3 supplements. Food Nutr. Res. 2011, 55, 5792. [Google Scholar] [CrossRef] [PubMed]
| Duration, Days | PV, mmol 1/2O/kg | |||
|---|---|---|---|---|
| 20 ± 1 °C | 50 ± 1 °C | |||
| EAGO | Sunflower Oil | EAGO | Sunflower Oil | |
| 0 | 3.34 ± 0.01 | 3.30 ± 0.01 | 3.34 ± 0.01 | 3.30 ± 0.01 |
| 10 | 3.35 ± 0.01 | 3.39 ± 0.01 | 3.40 ± 0.02 | 3.67 ± 0.02 |
| 20 | 3.36 ± 0.02 | 3.57 ± 0.02 | 3.65 ± 0.02 | 4.28 ± 0.03 |
| 30 | 3.39 ± 0.02 | 3.73 ± 0.03 | 3.86 ± 0.03 | 4.94 ± 0.03 |
| 40 | 3.61 ± 0.02 | 4.28 ± 0.03 | 4.67 ± 0.02 | 5.65 ± 0.02 |
| 60 | 4.06 ± 0.03 | 5.00 ± 0.04 | 5.12 ± 0.03 | 6.98 ± 0.04 |
| 80 | 4.56 ± 0.04 | 5.26 ± 0.03 | – | – |
| 100 | 5.01 ± 0.03 | 5.67 ± 0.03 | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murlykina, N.; Upatova, O. Evaluation of the Oxidative Stability of Emulsifiers of an Acylglicerol Origin. Biol. Life Sci. Forum 2022, 18, 32. https://doi.org/10.3390/Foods2022-13026
Murlykina N, Upatova O. Evaluation of the Oxidative Stability of Emulsifiers of an Acylglicerol Origin. Biology and Life Sciences Forum. 2022; 18(1):32. https://doi.org/10.3390/Foods2022-13026
Chicago/Turabian StyleMurlykina, Natalia, and Olena Upatova. 2022. "Evaluation of the Oxidative Stability of Emulsifiers of an Acylglicerol Origin" Biology and Life Sciences Forum 18, no. 1: 32. https://doi.org/10.3390/Foods2022-13026
APA StyleMurlykina, N., & Upatova, O. (2022). Evaluation of the Oxidative Stability of Emulsifiers of an Acylglicerol Origin. Biology and Life Sciences Forum, 18(1), 32. https://doi.org/10.3390/Foods2022-13026






