Abstract
Biological amendments, namely Pseudomonas florescence, Azotobacter chroococcum, K mobilizers, and AM fungi, were expedited during an air-layering operation on litchi (Litchi chinensis Sonn.). Twenty-five-year healthy progeny of mother plants were maintained for the air-layering operation. The treatments comprised the following combinations: T1, litchi orchard soil + sand (1:1); T2, sand + AM fungi + Azotobacter chroococcum (1:2:1); T3, sand + Pseudomonas florescence + K mobilizers (1:1:1); T4, AM fungi + K mobilizers (1:1); T5, P. florescence + A. chroococcum + K mobilizers (1:1:1); T6, sand + P. florescence (1:2); and T7, uninoculated control. Treatment T2 significantly improved the survival rate, plant height, stem diameter, leaf number, leaf area, and total leaf chlorophyll of the saplings. The microbial biomass of A. chroococcum Pseudomonas, K mobilizers, and AM fungi tremendously increased. The soil–enzyme activity in the rhizosphere increased, which indicated better P nutrition. The study indicated that biological amendments inoculation can be a promising technology to improve the survival rate to produce elite litchi planting material.
1. Introduction
Biological amendments have the well-known property of symbiotic effects in the rhizosphere. Litchi trees are reported to have a high dependence on AM fungi and associated beneficial PGPR because of farmers’ inoculation of new plantations with the soil of old litchi orchards. Air-layered saplings are devoid of a microbial consortium at the time of layering on shoots [1]. Further, these air-layered litchi plants in the absence of natural inoculants usually take 3–4 years for establishment after transplanting in fields, even when provided with well-fertilized soils and irrigated conditions. The bottlenecks are high mortality of layers once detached from the mother plant of their own root system [2]. Biological amendments, including PGPR, are rhizosphere-inhabiting bacteria colonizing the root system of plants that could stimulate plant growth and development processes [3]. The genus Metarrhizium (also known as green muscardine) comprises entomo-pathogenic fungi with a worldwide distribution. The fungi have long been recognized as biological pesticides (myco-insecticides) with the advent of genetic profiling known to colonize the roots of different species [4]. The fungal species are most frequently found as soil saprophytes in agricultural fields [5] compared to forest ecosystems. The earlier literature has suggested that the fungi form associations with plant roots in the rhizosphere zone [6] for better survival over extended periods [7], which are alleviated in drought-stress conditions [8]. In rhizosphere soil, the inhabiting microbial communities have direct interactions with the host plants in relation to nutrient acquisition and organic matter recycling. Several experimental attempts have been made for the establishment of air layers in field conditions [1].
It has been shown that plant-growth-promoting endophytic bacteria can help their host plants to cope with various biotic and abiotic stresses [9]. PGPR are rhizosphere-inhabiting bacteria, colonizing the root system of plants, that could stimulate plant growth and development processes. The saplings planted during the initial phase of air layering have low survival due to a poorly developed root system. Several experimental attempts on rooting media have been made toward the success of the establishment of air layers in field conditions. These investigations, therefore, were planned and focused with the objective to study the effectiveness of PGPR probiotics on air layer root development in mother litchi plants and a further evaluation of their interactive effects with Metarrhizium inoculation on the growth, survival, physiological profiling, and rhizosphere stoichiometry of transplanted air-layered litchi saplings at the nursery stage, which could be an innovative scientific approach with a consistent outcome for the production of elite planting material.
2. Materials and Methods
The experiment was conducted in the RHRTS of Dr. YS Parmar University of Horticulture and Forestry at Dhaulakuan, Sirmour (HP). The trial site is located at an elevation of 468 m above the mean sea level, with geographical coordinates of 28°25′ North (latitude) and 75°48′ East (longitude). The experiment was carried out on Litchi chinensis Sonn. cv. Early Large Red between late September and October until June, commencing with the monsoon for two consecutive years of 2015 and 2016. The climate of the experimental area is typically sub-tropical. Winters are cold, and the summers are hot. The maximum mean temperature was 39.5 °C, while the minimum mean temperature was 17.3 °C during the growth periods. The normal annual rainfall is 1100 mm, which is almost unevenly distributed. The southwest monsoon contributes 90%, which sets in the last week of June and withdraws in the middle of September. July and August are rainy months. The maximum soil temperature was 28.4 °C. Soil solarization increased the maximum daily temperature to 35.3 °C and the average minimum daily soil temperature to 22.7 °C.
Biological amendments in growing media were used with the purpose of producing quality planting material with a better root system in air layers and an enhanced final survival of litchi air layers within the nursery. PGPR bioinoculants, namely Pseudomonas florescence, Azotobacter chroococcum, AM fungi consortia (Glomus fasciculatum, G. clarum, and G. mosseae), and potash (K) mobilizers, were included. The treatments comprised the following combinations: T1, litchi orchard soil + sand + Metarrhizium (1:1:1); T2, sand + AM fungi + A. chroococcum + Metarrhizium (1:2:1:1); T3, sand + P. florescence + K mobilizers (1:1:1); T4, AM fungi + K mobilizers + Metarrhizium (1:1:2); T5, P. florescence + A. chroococcum + K mobilizers + Metarrhizium (1:1:1:1); T6, sand + P. florescence + Metarrhizium (1:2); and T7, uninoculated control, supplemented with farmyard manure along with a recommended dose of N:P:K fertilizer in the ratio of 60:30:30 for field performance. Additionally, the air layer saplings were root-dipped with Metarrhizium for 10 min, following a dual application of PGPR probiotics in each treatment combination in bulk as well as rhizosphere soil. The detached rooted air layers from the mother plants were further transplanted in different rooting media. The uniform air layers were transplanted at 30 × 60 cm using a double-row planting method replicated thrice during the month of October. The probiotic application was performed using a dipping method in which plant roots were inoculated with the respective microbial suspensions for about 20 min prior to transplantation. Control plants were dipped in sterile water. The nursery air-layered plantlets were also covered for 7–8 months by a shade net for improved viability of the propagation period and success percentage to regulate the micro-climate with temperature and humidity for improved survival of detached layers.
3. Results and Discussion
3.1. Survival and Growth Traits of Saplings
Results showed that the maximum number of roots was obtained in layers formed in the third week of June, which actually appeared in early September (Table 1, Figure 1). The variation in the intensity of rooting emergence in layers could be due to the fluctuations in temperature, especially high temperature coupled with high relative humidity in the month of June, and thus increased the respiration of the plants with low net photosynthates for rooting. In addition, air temperatures dropped, which resulted in lesser utilization of carbohydrates for respiration; thus, extra energy was diverted to root development. Treatment T2 contributed more nutrients, especially P and N, which provided an ideal condition for the growth of roots in air layers. Moreover, the potential of AM fungi and their ability to colonize roots appeared to depend on the relationship between the fungi and the host. Effectiveness of this colonization might be due to better root colonization, which has a direct relationship with growth [10]. Application of bio-organics, especially PGPR, enhanced the absorption of nutrients by plants, especially the availability of N, which led to higher levels of proteins [11], thereby increasing photosynthetic pigments, which could accordingly strengthen photosynthetic activity and ultimately provide balanced nutrition compared to traditional fertilizers for the conversion process and sink–source relationships. The stimulative effects of biological amendments improved the acquisition and uptake of nutrients, the release of growth-promoting substances in the rhizosphere, and the suppression of deleterious soil-borne microbial communities due to the inoculation of Metarrhizium, following the dual application of biological amendments in each treatment combination. Moreover, the saplings’ growth after transplant showed that the litchi-rooted layers took 95.4 days for plant establishment to achieve better survival and vegetative growth traits.

Table 1.
Effects of microbial inoculants on growth traits of Litchi chinensis Sonn. cv. Early Large Red saplings.

Figure 1.
Effects of PGPR transplant treatments on root behavior during the layering process in Litchi chinensis Sonn. cv. Early Large Red saplings: (A) sand + AM fungi + Azotobacter chroococcum (1:2:1), (B) litchi orchard soil + sand (1:1), (C) uninoculated control, and (D) hardening.
3.2. Rooting Characteristics
The highest fresh and dry weight of roots (11.2 g and 6.2 g, respectively) was achieved with PGPR transplant amendment media (T2), followed by T1 and T5, whereas the lowest was recorded with the uninoculated control. Moreover, the promotional effects on root characteristics were ascribed to the variation in the intensity of colonization due to the capacity to form an extensive and effective network of external hyphae around the root zone for nutrient acquisition [12,13]. In addition, the production of plant-growth-regulating substances, such as auxins, cytokinins, and gibberellins by PGPR probiotics, that interfere with resident soil microbial communities, especially AM fungi, by colonizing the roots causes an increased root growth and exudation rate. AM fungi promoted plant growth and improved plant establishment by increasing nutrient and water relationships, especially ascribed to increased uptake of immobile P and plant tolerance to biotic and abiotic stresses [8].
3.3. Microbial Population and Soil Enzymes
In general, the resident microbial population in rhizosphere and non-rhizosphere soils with the dual and/or triple inoculation of different PGPR in air-layered transplants increased compared to the uninoculated control. PGPR probiotics had a significant effect on the total cultural microbial population of both rhizosphere and non-rhizosphere zones. The microbial biomass estimated less than 5% of propagules of the total culturable bacterial population in soil in terms of AM fungi, A. chroococcum, and Pseudomonas sp., and K mobilizers which were significantly higher in the rhizosphere zone than in the non-rhizosphere zone. Among different treatment combinations, the respective plate count of A. chroococcum, Pseudomonas sp., and K mobilizers also varied between the corresponding values of 13.0 × 106–25.7 × 106 cfu g−1, 9.3 × 105–25.8 × 105 cfu g−1, and 9.7 × 104–19.6 × 104 cfu g−1, respectively, in the rhizosphere zone and 9.9 × 106–10.8 × 106 cfu g−1, 7.6 × 105–16.1 × 105 cfu g−1, and 8.1 × 104–16.2 × 104 cfu g−1, respectively, in the non-rhizospheric zone. Similarly, the propagules of AM fungi (per 50 g) ranged between 143.0, 282.8 and 102.0, 195.8 per 50 g of rhizosphere and non-rhizosphere moist soil (Figure 2).
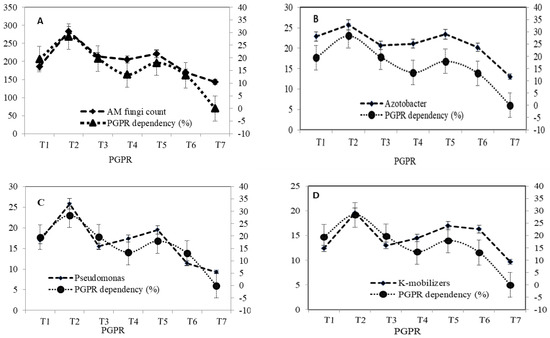
Figure 2.
PGPR dependency and indigenous microflora affected by biological amendments in Litchi chinensis Sonn. cv. Early Large Red Saplings (A) AM Fungi, (B) Azotobacter population, (C) Pseudomonas count, and (D) K–mobilizers population.
In the rhizosphere zone, acid phosphatase (AcP) activity was significantly higher after treatment T2 (163.2 µg PNP g−1 h−1), followed by treatments T1 (149.3 µg PNP g−1 h−1), T3 (139.3 µg PNP g−1 h−1), and T4 (138.5 µg PNP g−1 h−1) than in the uninoculated control. When compared to the uninoculated control, the AcP activity after treatment T2 was 1.65-fold within and 2.53 times more between the rhizosphere and non-rhizosphere zones. The order of alkaline phosphatase (AlP) activity varied significantly among the PGPR treatments as T2 > T3 > T1 > T6 > T5 in the rhizosphere zone and as T2 > T3 > T1 > T5 > T6 > T4 in the non-rhizosphere zone. In addition, dehydrogenases activity (DHA) showed the same trends with respect to AcP and AlP in both rhizosphere and non-rhizosphere zones. In the rhizosphere zone, DHA activity expressed in terms of µg TPF g−1 h−1 was significantly higher after treatment T2 (11.5), followed by treatments T1 (10.6), T3 (10.0), and T4 (9.6), with no significant differences between treatments T1, T3, T4, and T5, whereas it was the least after treatment T7 (9.6). Similar trends in DHA activity were observed in the non-rhizosphere zone in the order T2 > T1 > T3 > T4 > T5; however, the differences among these were not significant. After treatment T2 in the rhizosphere zone, superior AcP, AlP, and DHA activities in layered transplants were recorded to be 2.53, 2.08, and 30.2 times higher than in the non-rhizosphere uninoculated control, respectively (Figure 3). Considering physico-chemical and biological properties in the rhizosphere zone, the flow of organic substrates markedly influenced higher microbial population densities and also the microbial community structure [12]. Soil enzymatic bioassay is critically important for soil productivity, which has provided indications of changes in metabolic capacity and nutrient cycling [14].
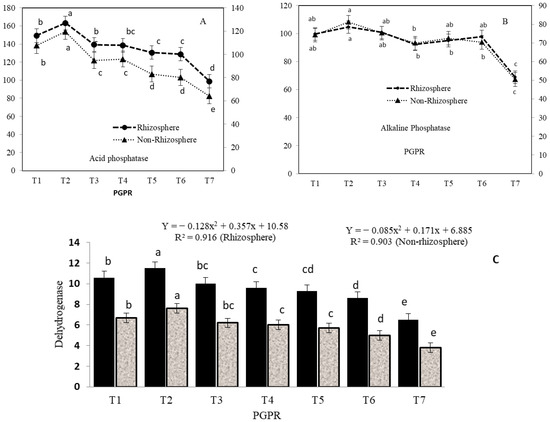
Figure 3.
Soil enzymes affected by biological amendments in Litchi chinensis Sonn. cv. Early Large Red saplings in rhizosphere and non-rhizosphere, (A) Acid Phosphatase, (B) Alkaline Phosphate, and (C) Dehydrogenases. The values followed by the same letters within same graphical line (A,B) and bars (C) are not sig-nificantly different from each other according to Duncan’s Multiple Range Test (DMRT, p ≤ 0.05).
4. Conclusions
The application of AM fungi + A. chroococcum + Metarrhizium (2:1:1) at the hardening stage in the nursery recorded the maximum percentage survival (34.7%) of air-layered litchi plantlets compared to the control and better rooting and survival and the establishment of the layered guttee in soil. The study indicated PGPR transplant amendments coupled with soil solarization as a promising technology to maintain a healthy rhizosphere in litchi at the nursery stage, which could be an innovative scientific approach with a consistent outcome for the production of elite planting material in the Shiwalik foothills of the north-west Himalayas.
Author Contributions
Conceptualization, P.K. and A.K.J.; methodology, P.K., S.L. and N.S.; validation, P.K., A.K.J. and B.K.G.; formal analysis, S.L. and N.S.; resources, A.K.J.; data curation, P.K., S.L. and N.S.; writing—original draft preparation, P.K.; writing—review and editing, A.K.J., B.K.G. and N.S.; supervision, A.K.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sharma, S.D.; Kumar, P.; Gautam, H.R.; Bhardwaj, S.K. Isolation of arbuscular mycorrhizal fungi and Azotobacter chroococcum from local litchi orchards and evaluation of their activity in air-layers system. Sci. Hortic. 2009, 123, 117–123. [Google Scholar] [CrossRef]
- Sarita, B.P.; Kour, K.; Mehla, U.; Bhawana, S.S.; Jasrotia, A.; Bushan, B. Effect of different potting media on survival and growth of air layered litchi cv. Dehradun. Int. J. Curr. Microbio. App. Sci. 2019, 8, 1196–1204. [Google Scholar]
- Dinesh, R.; Anandaraj, M.; Kumar, A.; Srinivasan, V.; Bini, Y.K.; Subila, K.P.; Aravind, R.; Hamza, S. Effects of plant growth-promoting rhizobacteria and NPK fertilizers on biochemical and microbial properties of soils under ginger (Zingiber officinale) cultivation. Agric. Res. 2013, 2, 346–353. [Google Scholar] [CrossRef]
- Sasan, R.K.; Bidochka, M.J. The insect-pathogenic fungus Metarhizium robertsii (Clavicipitaceae) is also an endophyte that stimulates plant root development. Am. J. Bot. 2012, 99, 101–107. [Google Scholar] [CrossRef]
- Meyling, N.; Eilenberg, J. Ecology of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in temperate agro-ecosystems: Potential for conservation biological control. Biol. Cont. 2007, 43, 145–155. [Google Scholar] [CrossRef]
- Hu, G.; St. Leger, R.J. Field studies using a recombinant mycoinsecticide (Metarhizium anisopliae) reveal that it is rhizosphere competent. Appl. Environ. Microbiol. 2002, 68, 6383–6387. [Google Scholar] [CrossRef]
- Bruck, D.J. Ecology of Metarhizium anisopliae in soilless potting media and the rhizosphere: Implications for pest management. Biol. Cont. 2005, 32, 155–163. [Google Scholar] [CrossRef]
- Behrooz, A.; Vahdati, K.; Rejali, F.; Lotfi, M.; Sarikhani, S.; Leslie, C.A. Arbuscular mycorrhiza and plant growth-promoting bacteria alleviate drought stress in walnut. HortScience 2019, 54, 1087–1092. [Google Scholar] [CrossRef]
- Forouzi, A.; Ghasemnezhad, A.; Ghorbani Nasrabad, R. Effects of growth stimulator microbes on growth and ions concentration of Stevia under salinity stress conditions. Int. J. Hortic. Sci. Technol. 2019, 6, 217–236. [Google Scholar]
- Abbott, L.K.; Robson, A.D. Factors influencing the occurrence of VA-mycorrhizae. Agric. Ecosyst. Environ. 1985, 35, 121–150. [Google Scholar] [CrossRef]
- Zaferanchi, S.; Salmasi, S.; Salehi Lisar, S.; Sarikhani, M. Influence of organics and biofertilizers on biochemical properties of Calendula officinalis L. Int. J. Hortic. Sci. Technol. 2019, 6, 125–136. [Google Scholar]
- Sharma, S.D.; Devi, M.; Kumar, P.; Bhardwaj, S.K.; Raj, H. Potential use of bio-organic and inorganic nutrient source dynamics for improving cropping behavior, soil biological properties, nutrient content and quality attributes of apricot. Commun. Soil Sci. Plant Anal. 2011, 42, 1659–1674. [Google Scholar] [CrossRef]
- Sharma, S.D.; Kumar, P.; Bhardwaj, S.K.; Chandel, A. Symbiotic effectiveness of arbuscular mycorrhizal technology and Azotobacterization for citrus nursery management under soil disinfestations and moisture conservation mulch practices. Sci. Hortic. 2011, 132, 27–36. [Google Scholar] [CrossRef]
- Zhou, X.G.; Yu, G.B.; Wu, F.Z. Effects of intercropping cucumber with onion or garlic on soil enzyme activities, microbial communities and cucumber yield. Eur. J. Soil Bio. 2011, 47, 279–287. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).