Abstract
In various parts of India, tomatoes are grown using eco-friendly indigenous organic manures prepared from cow products for sustainable food production by small and marginal farmers. The main objective of the study was to compare the postharvest quality characteristics and storability between organically grown tomatoes using indigenous organic manures and those grown conventionally. The organic (OT) and conventional (CT) tomatoes procured from selected farms were observed for 28 days under ambient and refrigerated storage conditions. The postharvest quality characteristics and storability of tomatoes were assessed at intervals of 0, 7, 14, 21, and 28 days and observed till senescence. Physiological loss of weight (PLW), total soluble solids (TSS), titratable acidity (TA), pH, colour, lycopene, ascorbic acid content, respiration rate, and microbial stability were assessed to determine the postharvest quality and storability of OT and CT respectively. The study revealed that organic tomatoes stored in refrigerated conditions had a lower physiological loss of weight (2.78%), respiration rate (27.61 µL CO2 g−1 h−1), and loss in firmness (27.14%) compared to conventional tomatoes indicating a higher storability and delayed senescence. The titratable acidity showed a decreasing trend while pH increased significantly for both samples stored under ambient and refrigerated conditions. A slower rate of increment in redness and chroma values was observed for OT at refrigerated storage conditions compared to ambient temperature. Ascorbic acid content was also found to be significantly higher in OT (23.53 mg/100 g) compared to CT (13.85 mg/100 g). Additionally, the result showed increased lycopene content in CT during storage compared to OT. The microbial study revealed that total aerobic mesophilic count and yeasts–molds were highest in CT on the 28th day of storage. Therefore, the study revealed that OT under refrigerated storage conditions had superior postharvest quality, storability and longevity compared to CT, which may be due to the sustained release of nutrients and useful elements from liquid organic manures and their uptake by plants.
Keywords:
organic; conventional; tomato; storability; postharvest quality; lycopene; organic manures 1. Introduction
India experienced a ‘Green revolution’ to greater abundance with increased use of synthetic agrochemicals and the adoption of high-yielding varieties of genetically modified crops, which led to the deterioration of soil quality, environment, and also human health [1]. In the current scenario, recognizing the impact of excessive use of chemical fertilizers, organic farming has gained a central focus amongst the consumers. There is a huge difference between organic farming and conventional farming practices, which has an enormous impact on postharvest quality and physicochemical composition of produce [2]. Numerous studies have also highlighted that the difference detected between organic and conventional fresh produce are linked to differences in crop management practices [3]. The concept of organic agriculture is native to the Indian farming community and has its roots in an intimate understanding of nature [4]. Several organic farming methods, such as rishi krishi, panchgavya, natueco, zero budget organic farming, biodynamic agriculture, etc., are followed in different parts of the country, which are now gathered under one umbrella termed ‘Jaivik Krishi’ [5,6]. In India, cows are an integral part of organic agriculture, and cow dung is considered an important and sole fertilizer by Indian farmers [7].
Additionally, indigenous organic manures prepared from cow products and on-farm wastes such as Beejamrutha, Jivamrutha, and Panchyagavya are being widely used by small and marginal farmers due to their advantages in soil, crop health, and cost-effectiveness over synthetic farm inputs. These eco-friendly fermented concoctions are rich in microflora and plant growth promoters [7].
Jivamrutha is prepared in two forms: wet fermented slurry known as dhrava jivamrutha, while the dried form is termed as ghana jivamrutha. During the preparation of dhrava jivamrutha, 200 L of water, 10 kg of fresh cow dung, 5–10 L of cow urine, 2 kg of jaggery, 1–2 kg of pulse flour, and a handful of soil are mixed thoroughly and fermented for 48–72 h before application. The ghana jivamrutha uses 100 kg of cow dung, 5 L of cow urine, 2 kg of pulse flour, and a handful of soil. The components are then mixed properly, formed into balls, shade dried for storage, and ploughed into the soil before any crop plantation. Beejamrutha is used as a seed/seedling treatment that provides protection to young roots from fungus and soil or seed-borne diseases [8].
A significant amount of research is being carried out that deals with the study of these indigenous organic manures on yield and economics of organic fruits and vegetables in comparison to non-organic inputs [9]. For example, the application of beejamrutha, jivamrutha, and panchagavya showed higher plant growth and root length in tomatoes compared to individual manure applications [10]. However, studies with respect to the influence of the indigenous organic manure on postharvest quality and safety of the produce and storability are negligible. Therefore, in the present study, we sought to compare the postharvest quality characteristics and storability between organically grown tomatoes using indigenous organic manures with those grown conventionally to gain a holistic view of the effect of crop management practices on produce quality which is of paramount importance to health-conscious consumers.
2. Materials and Methods
2.1. Experimental Design
Tomato (Lycopersicon esculentum) ‘448 variety’ plants were planted in June 2021 under both organic and conventional management systems. Farms were located in Anantapur district, Andhra Pradesh, India (14°33′13.9284″ N and 77°39′7.884″ E) approximately 500 m apart.
Organic tomatoes were grown using indigenous on-farm organic inputs. Ghana jivamrutha (400 kg/acre) was applied to the soil prior to bedding. Once the bedding was ready, the tomato seedling roots were dipped in beejamrutha and sowed in the soil. Dhrava jivamrutha was applied through drip irrigation in 5, 10, 15 L/100 L of water/acre on 15th, 30th, and 45th day of transplantation, respectively. In the case of conventional counterpart, 20 kg/acre of NPK fertilizer (13:00:45) was applied through drip irrigation once every 3 days. Boron (2 kg/acre) was applied to the soil before transplantation. Insecticide spraying was performed twice after the fruiting started. Irrigation was provided to prevent drought-related stress.
After 60 days of transplantation, approximately 15–20 kg of fruits were harvested at breaker stage from both the farms and brought to the research laboratory, Department of Food and Nutritional Sciences, Sri Sathya Sai Institute of Higher Learning, Anantapur. Fruits were harvested in early morning hours and brought to the laboratory within an hour. Samples were washed with deionized water and allowed to air dry. After drying, tomatoes were sorted and graded, and fruits without blemishes, external injuries, and uniform sizes were selected and stored under ambient (25 ± 2 °C) and refrigerated conditions (10 ± 2 °C). Organic (OT) and conventional tomatoes (CT) stored at ambient conditions were marked as OTRo and CTRo, while those in refrigerated conditions were marked as OTRef and CTRef, respectively. Further, postharvest quality and storability at 0, 7th, 14th, 21st, and 28th days were investigated for the stored samples. All the analyses were performed in triplicates.
2.2. Physical Parameters
Postharvest physical quality indicators selected were physiological loss of weight (PLW), firmness, instrumental color, and respiration rate (RR). Physiological loss of weight (PLW) was determined by weighing fruits at beginning and subsequent storage interval and expressed as a percentage of weight loss relative to the fresh weight of the fruits [11]. Fruit firmness was determined by a digital hand-held penetrometer using an 8 mm diameter probe (Turoni-53205, T.R. Turoni SRL, Forli, Italy). Three fruits were randomly selected from each category, and measurements were taken in triplicates on the equatorial plane of the fruit and expressed in maximum force (N) to penetrate the fruit pulp [12]. Instrumental color was measured using Konica Minolta color reader CR-10 (Minolta Co. Ltd., Osaka, Japan) for the estimation of L* (lightness), a* (redness to greenness), b* (yellowness to blueness), Chroma value, and hue angle [13]. Respiration rates of tomatoes were determined in a closed system at the interval of 7 days during storage period. Tomatoes were placed in a rigid container of known volume and kept for incubation for 2 h. Gas composition (O2 and CO2%) was measured using a CO2/O2 gas analyzer (PBI Dansensor, Checkmate II, Ringsted Denmark). The concentration of CO2 evolved during the incubation period was used in the calculation of respiration rate [14].
2.3. Chemical Parameters
The chemical parameters studied were total soluble solids (TSS), titratable acidity (TA), pH, ascorbic acid, and lycopene. Total soluble solids (TSS) of the fruit were determined by using a digital pocket refractometer (model PAL-3, ATAGO, Tokyo, Japan) and expressed as ° Brix [15]. Titratable acidity of the fruit was determined by taking 5 mL of filtered juice and homogenizing it with 100 mL of distilled water. The aliquot was titrated with 0.1 N NaOH using phenolphthalein as an indicator and expressed as% citric acid equivalent [16]. Digital pH meter (model ELICO-120) was used to determine pH of the tomato juices. Ascorbic acid was estimated by homogenizing the 10 g of the sample in 3% freshly prepared metaphosphoric acid. The aliquot was mixed with 2,6-dichlorophenol indophenol after filtration, measured at 515 nm using UV-Vis spectrophotometer (Cary-60, Agilent Technology, Santa Clara, CA, USA), and expressed as mg of ascorbic acid/100 gm of the sample [17]. Lycopene content was assessed by spectrophotometric method using UV-Vis spectrophotometer (Cary-60, Agilent Technology, Santa Clara, CA, USA). Briefly, 0.3 g of tomato pulp was taken in triplicates and extracted with a solvent mixture of hexane: 0.5% of butylated hydroxy acetone: 95% ethanol (2:1:1) under dark conditions using a magnetic stirrer. After 20 min, the sample was mixed with 5 mL of water, and a hexane layer was taken for analysis of lycopene at 503 nm [18].
2.4. Microbial Analysis
Total aerobic mesophilic bacterial count (AMC) and yeasts and molds count (YMC) were determined to assess microbial stability [19]. A total of 0.1 mL of appropriate dilution of aseptically homogenized samples was plated on plate count agar containing 1% triphenyl tetrazolium chloride using spread plate method and incubated at 35 ± 1 °C for 48 h. Yeasts and mold count was determined using potato dextrose agar using spread plate method and incubated at 25 ± 1 °C for 3 to 5 days. Colonies were counted using digital colony counter (Scan100 Interscience, St Nom, France) and reported as log CFU/g of sample [14].
2.5. Statistical Analysis
The data obtained from the experiments were analyzed statistically using SPSS software (IBM SPSS Statistics 21, New York, NY, USA) and MS Excel 2019. The data obtained from chemical and physical parameters across the storage period were subjected to one-way analysis of variance (ANOVA) followed by post hoc Duncan’s Multiple Range Test (DMRT) at p ≤ 0.05 level to compare the means of different treatments.
3. Result and Discussion
3.1. Physical Parameters
Physiological loss of weight is a key factor in the shelf life of fresh fruits and vegetables. The weight loss was found to be predominant on CTRo compared to OTRo on the 28th day of ambient storage condition, as depicted in Figure 1a. In the case of refrigerated storage conditions, CTRef showed a higher range of water loss on the 28th day of storage. The physiological loss of weight was observed in both samples regardless of cultivation method and storage conditions. Similar results were obtained by Eboibi et al. [20], where the loss of fruit mass was evident regardless of production method and calcium chloride treatment. The loss of weight affects the quality of fruits and vegetables, which is mainly due to excessive loss of moisture through metabolic activity such as respiration and transpiration processes [21].
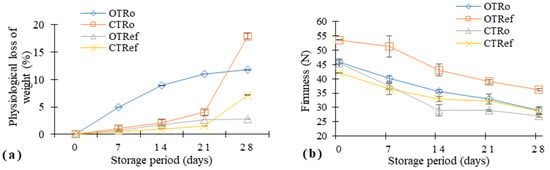
Figure 1.
Changes in physical quality attributes of organic and conventional tomatoes: (a) Physiological loss of weight (%); (b) Firmness (N); OTRo—Organic tomatoes at ambient storage; OTRef—Organic tomatoes at refrigerated storage; CTRo—Conventional tomatoes at ambient storage; CTRef—Conventional tomatoes at refrigerated storage; data are mean ± SD (n = 3).
Firmness is also one of the important quality parameters for assessing the quality of the produce. Figure 1b depicts the changes in firmness in OT and CT stored under ambient and refrigerated conditions. Organic tomatoes were observed to be firmer compared to conventional tomatoes. At day zero, the OTRef (53.55 N) showed the highest value of firmness compared to other samples; however, the decrement was seen gradually over the period of storage at both ambient and refrigerated conditions. Additionally, loss of firmness was observed more in the case of Morra et al. [22] reported implementation of organic soil amendments such as buffalo slurry resulted in increased firmness of the tomato fruit.
The evolution of color parameters during the postharvest storage of OT and CT is represented in Figure 2. The major changes were observed on L, a*, and hue angle. The decrease in lightness was observed in greater intensity in CTRo, where the lightness values decreased from 55.63 to 36.07. Apart from this, results revealed that the decrease in lightness was lesser in refrigerated samples compared to samples stored at ambient temperature. More importantly, the decrease in lightness was significantly (p < 0.05) lesser in tomatoes grown in indigenous organic manures compared to conventional. Similar results were observed by Bilalis et al. [23], who investigated higher lightness values of tomatoes treated with organic fertilizers. The a* values showed an increasing trend in all the samples over the storage period of 28 days. The green color was more dominant in tomatoes at the time of harvest, which turned into red color during the postharvest storage period. The increment in a* was higher in the case of CTRo (40.60), followed by OTRo (23.63), CTRef (23.60), and OTRef (6.87).
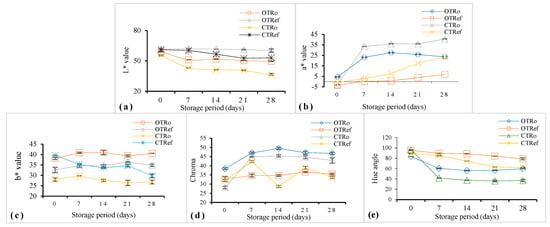
Figure 2.
Changes in color attributes of organic and conventional tomatoes during storage: (a) L* value (lightness: black—0, white—100); (b) a* value (+ redness; − greenness); (c) b* value (+ yellowness; − blueness); (d) Chroma; (e) Hue angle; OTRo—Organic tomatoes at ambient storage; OTRef—Organic tomatoes at refrigerated storage; CTRo—Conventional tomatoes at ambient storage; CTRef—Conventional tomatoes at refrigerated storage; data are mean ± SD (n = 3).
Chroma values of both organic and conventional tomatoes increased during the period of storage (Figure 2). The hue angle of the organic and conventional tomatoes ranged from 83.20 to 96.10 at the time of harvest, which decreased consecutively during the storage period. The rate of decrease in the hue angle in tomatoes was observed to be higher in the case of conventional tomatoes. Organic tomatoes stored in refrigerated storage conditions showed the smallest decrease in hue angle value representing a lower rate of postharvest ripening compared to conventional tomatoes under the same storage condition. The controlled temperature storage in the refrigerator showed a significant impact on the postharvest ripening of the organic and conventional tomatoes. The rate of postharvest ripening, indicative degradation of chlorophyll, and synthesis of lycopene were higher in the case of conventional tomatoes compared to organic tomatoes [23,24].
Respiration rate was observed to increase on the seventh day of storage which subsequently followed a decreasing trend in both OT and CT stored at ambient and refrigerated conditions during the storage. The respiration rate was significantly (p < 0.05) higher in conventional tomatoes compared to organic tomatoes, as depicted in Figure 3.
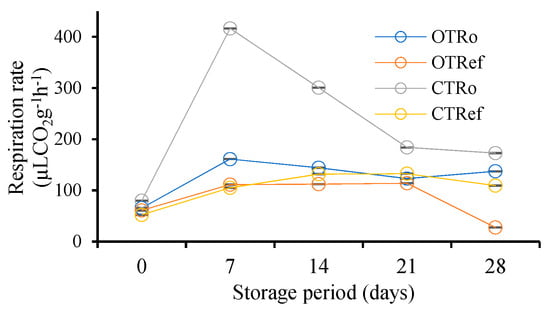
Figure 3.
Changes in respiration rate of organic and conventional tomatoes during storage; OTRo—Organic tomatoes at ambient storage; OTRef—Organic tomatoes at refrigerated storage; CTRo—Conventional tomatoes at ambient storage; CTRef—Conventional tomatoes at refrigerated storage; data are mean ± SD (n = 3).
On the seventh day of storage, the highest respiratory peak was observed in OTRo and CTRo, while in refrigerated samples, the respiratory peak was observed on the 21st day of storage, owing to delayed senescence. Ayomide et al. [25] reviewed that the increase in respiration rate occurs as tomato fruit ripens from mature green to red. Further, the depletion of organic food reserves during respiration hastens the process of senescence.
3.2. Postharvest Chemical Parameters
The changes in postharvest chemical quality parameters of OT and CT are presented in Table 1. Total soluble solids content determines the sugar content of a product in terms of °Brix. TSS ranged from 3.20 to 5.20 across the samples. It was observed that TSS was higher in conventional tomatoes at the time of harvest, which showed an increasing trend during the storage period and consecutively declined on the 28th day. A similar trend was followed by organic tomatoes; however, the rate of increment in TSS was slower compared to conventional tomatoes. This indicates a slower rate of sugar accumulation in organic tomatoes. Current findings affirm the previous study of Navarro and Munne-bosh [26] and Kim et al. [27] that those fruits grown under low nitrogen content result in low soluble sugar content during maturity than that with higher nitrogen content. According to Shehata et al. [15], the increase in TSS during storage is indicative of solubilization of cell wall components or moisture loss through transpiration. However, the decrement in TSS at the end of the storage could be due to the utilization of sugar in the postharvest metabolic process.

Table 1.
Changes in postharvest chemical quality parameters of organic and conventional tomatoes during storage.
Titratable Acidity (TA) was absorbed to be significantly higher in conventional tomatoes compared to organic tomatoes (Table 1). During storage, TA gradually increased and showed a steep decline from the 21st day for all samples. On the 28th day of storage, a decrease in acidity was observed for both samples regardless of storage condition. However, the decrement was significantly lesser in the case of organic tomatoes compared to conventional counterparts. The results are in line with the previous data provided by Shehata et al. [15].
pH ranged from 4.30 to 4.78 among the samples throughout the storage period. The detailed values are given in Table 1. The pH values were higher in the case of conventional tomatoes. There was an increase in pH in all the samples during storage. The increment at the maximum was observed in CTRef (4.78), followed by CTRo (4.72), OTRo (4.54), and OTRef (4.49). Iqbal et al. [28] and Tilahun et al. [29] reported similar results and explained that changes in pH during storage are due to numerous pectin degrading enzymatic reactions, which lead to altered physiological processes ultimately spoiling the fresh produce.
Ascorbic acid content was significantly (p < 0.05) observed to be higher in organic tomatoes (23.53 mg/100 gm) compared to conventionally grown tomatoes (13.86 mg/100 gm) at the time of harvest (Table 1). Toor et al. [30] reported similar results as in the current study, wherein the application of chicken manure and mulching influenced and improved the accumulation of ascorbic acids and phenolics in tomatoes. The increased antioxidant activity could be attributed to the self-defense mechanism triggered by slow nutrient uptake from organic inputs during the growth and development phase [2]. In the current study, a significant (p < 0.05) reduction in ascorbic acid content was observed in all the samples across the storage duration. Refrigerated storage helped in retaining the ascorbic acid in both organic and conventional samples [31].
Lycopene content of the freshly harvested organic tomato was 8.43 mg/kg at the breaker stage, while 7.30 mg/kg was observed in conventional tomatoes (Table 1). The results revealed that refrigeration significantly decreased the rate of formation of lycopene, thereby reducing the postharvest ripening. It was also observed that lycopene content drastically increased in CTRo (117.38 mg/kg) on the 28th day of storage at ambient conditions, compared to OTRo (44.84 mg/kg). Additionally, the result also revealed that even under refrigerated conditions, the rate of color development was significantly higher in the case of conventional tomatoes. Fagundes et al. [32] reported similar results with respect to lycopene content in cherry tomatoes, wherein lycopene content increased during the storage period. Further, low-temperature storage at 5 °C inhibited the enhancement of lycopene [33].
3.3. Microbial Stability
The postharvest microbial quality of OT and CT during ambient and refrigerated storage is represented in Figure 4. Yeast and mold growth was visible on the 14th day of storage for both OT and CT, which consecutively increased during storage. Significantly lower (p < 0.05) increment in YMC was observed in OTRef (3.93 log CFU/g) compared to CTRef (4.42 log CFU/g).
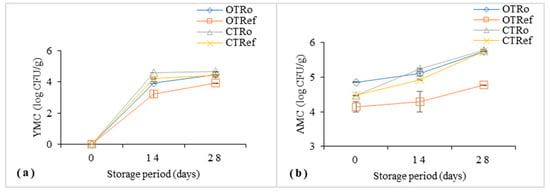
Figure 4.
Microbial stability of organic and conventional tomatoes: (a) Yeast and molds count (YMC), (b) Aerobic mesophilic count (AMC); OTRo—Organic tomatoes at ambient storage; OTRef—Organic tomatoes at refrigerated storage; CTRo—Conventional tomatoes at ambient storage; CTRef—Conventional tomatoes at refrigerated storage; data are mean ± SD (n = 3).
Similarly, AMC was significantly higher in conventional tomatoes at the initial phase of storage and showed an increasing trend during the storage. On the 28th day of ambient storage condition, AMC was significantly (p < 0.05) higher for CTRo (5.78 log CFU/g) compared to OTRo (5.74 log CFU/g). The growth of YMC and AMC in both organic and conventional tomatoes was observed to be inhibited by low-temperature storage conditions. Similar results were reported by Merlini et al. [19], wherein organically grown leafy vegetables had lower microbial counts compared to the ones grown conventionally [34]. As the fruit ripens and enters into senescence, susceptibility towards microbial attack also increases, which ultimately leads to microbial spoilage. In the current study, CT was observed to have a higher ripening rate and early senescence, which can be correlated to the increased microbial load.
4. Conclusions
In the current study, a comparative approach was implemented to assess the postharvest quality parameters of tomatoes grown with indigenous organic manures and those grown using synthetic fertilizers. Organic tomatoes were found to have higher firmness, ascorbic acid, and lycopene content at the time of harvest. Interestingly, conventional tomatoes exhibited an increased rate of lycopene synthesis during storage at ambient conditions. It was observed that the refrigerated storage condition slowed down the metabolic changes that led to senescence and low shelf stability. Tomatoes grown with indigenous liquid organic manures and stored in refrigerated conditions showed lesser PLW, loss of firmness and respiration rate, and lower microbial load compared to conventional counterparts. Additionally, the rate of ripening was slower in organic tomatoes compared to conventional tomatoes contributing to their longevity. Further, an in-depth investigation will be carried out to understand the impact of indigenous liquid organic manures on the phytochemical and antioxidant profile of tomatoes.
Author Contributions
Conceptualization, M.S.; methodology, M.S.; software, A.P.; validation, M.S.; formal analysis, A.P.; investigation, A.P.; writing—original draft preparation, A.P.; writing—review and editing, M.S.; visualization, A.P.; supervision, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the Founder Chancellor and the institute management of Sri Sathya Sai Institute of Higher Learning (SSSIHL), Andhra Pradesh, India, for providing the research facility in the Department of Food and Nutritional Sciences, and Central Research Laboratory (CRL), Anantapur.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mitra, S.; Devi, H. Organic Horticulture in India. Horticulturae 2016, 2, 17. [Google Scholar] [CrossRef]
- Mditshwa, A.; Magwaza, L.S.; Tesfay, S.Z.; Mbili, N. Postharvest Quality and Composition of Organically and Conventionally Produced Fruits: A Review. Sci. Hortic. 2017, 216, 148–159. [Google Scholar] [CrossRef]
- Rahman, S.M.E.; Mele, M.A.; Lee, Y.-T.; Islam, M.Z. Consumer Preference, Quality, and Safety of Organic and Conventional Fresh Fruits, Vegetables, and Cereals. Foods 2021, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Tutika, S.; Dara, S.; Saikia, N.; Cherukuri, S.R. Screening and Isolation of Beneficial Microorganisms from Natural and Organic Concoctions Collected from Various Parts of Andhra Pradesh and Telangana. Biopestic. Int. 2018, 14, 101–108. [Google Scholar]
- Gopinath, K.A.; Mitnala, J. Organic Farming Research in India: Present Status and Way Forward. Int. J. Econ. Plants 2018, 3, 98–101. [Google Scholar] [CrossRef]
- Panwar, A.S.; Dutta, D.; Kumar, A.; Meena, L.K.; Meena, A.L. Modern Concepts and Practices of Organic Farming for Safe Secured and Sustainable Food Production; Indian Institute of Farming Systems Research: Meerut, India, 2019; pp. 1–72. [Google Scholar]
- Hiremath, R.; Ke, U. Analysis of Diversified Rice Based Cropping Systems under Organic Management. J. Pharmacogn. Phytochem. 2019, 8, 1118–1120. [Google Scholar]
- Smith, J.; Yeluripati, J.; Smith, P.; Nayak, D.R. Potential Yield Challenges to Scale-up of Zero Budget Natural Farming. Nat. Sustain. 2020, 3, 247–252. [Google Scholar] [CrossRef]
- Boraiah, B.; Devakumar, N.; Shubha, S.; Palanna, K.B. Effect of Panchagavya, Jeevamrutha and Cow Urine on Beneficial Microorganisms and Yield of Capsicum (Capsicum annuum L. Var. Grossum). Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 3226–3234. [Google Scholar] [CrossRef]
- Gore, N.S.; Sreenivasa, M.N. Influence of Liquid Organic Manures on Growth, Nutrient Content and Yield of Tomato (Lycopersicon Esculentum Mill.) in the Sterilized Soil. Karnataka J. Agric. Sci. 2011, 24, 153–156. [Google Scholar]
- Koraddi, V.V.; Devendrappa, S. Analysis of Physiological Loss of Weight of Vegetables under Refrigerated Conditions. J. Farm Sci. 2011, 1, 61–68. [Google Scholar]
- Massantini, R.; Radicetti, E.; Frangipane, M.T.; Campiglia, E. Quality of Tomato (Solanum Lycopersicum L.) Changes under Different cover Crops, Soil Tillage and Nitrogen Fertilization Management. Agricultural 2021, 11, 106. [Google Scholar] [CrossRef]
- Pandurangaiah, S.; Sadashiva, A.T.; Shivashankar, K.S.; Sudhakar Rao, D.V.; Ravishankar, K.V. Carotenoid Content in Cherry Tomatoes Correlated to the Color Space Values L∗, A∗, B∗: A Non-Destructive Method of Estimation. J. Hortic. Sci. 2020, 15, 27–34. [Google Scholar] [CrossRef]
- Ghoora, M.D.; Srividya, N. Effect of Packaging and Coating Technique on Postharvest Quality and Shelf Life of Raphanus Sativus L. and Hibiscus Sabdariffa L. Microgreens. Foods 2020, 9, 653. [Google Scholar] [CrossRef] [PubMed]
- Shehata, S.A.; Abdelrahman, S.Z.; Megahed, M.M.A.; Abdeldaym, E.A.; El-Mogy, M.M.; Abdelgawad, K.F. Extending Shelf Life and Maintaining Quality of Tomato Fruit by Calcium Chloride, Hydrogen Peroxide, Chitosan, and Ozonated Water. Horticulturae 2021, 7, 309. [Google Scholar] [CrossRef]
- Teka, T.A. Analysis of the Effect of Maturity Stage on the Postharvest Biochemical Quality Characteristics of Tomato (Lycopersicon Esculentum Mill.) Fruit. Int. Res. J. Pharm. Appl. Sci. 2013, 3, 180–186. [Google Scholar]
- Mehta, N.; Patani, P.; Singhvi, I. Colorimetric Estimation of Ascorbic Acid from Different Varities of Tomatoes Cultivated in Gujarat. World J. Pharm. Res. 2018, 7, 1376–1384. [Google Scholar]
- Hussein, J.B.; Sanusi, M.S.; Filli, K.B. Evaluation of Drying Methods on the Content of Some Bio-Actives (Lycopene, -Carotene and Ascorbic Acid) of Tomato Slices. Afr. J. Food Sci. 2016, 10, 359–367. [Google Scholar] [CrossRef]
- Merlini, V.V.; Pena, F.D.L.; Da Cunha, D.T.; De Oliveira, J.M.; Rostagno, M.A.; Antunes, A.E.C. Microbiological Quality of Organic and Conventional Leafy Vegetables. J. Food Qual. 2018, 2018, 4908316. [Google Scholar] [CrossRef]
- Eboibi, O.; Isaac, O.; Nyorere, O.; Oghenerukevwe, P.; Uguru, H. Annals of Agricultural Sciences Effect of Pre-Harvest Applications of Organic Manure and Calcium Chloride on the Storability of Tomato Fruits. Ann. Agric. Sci. 2021, 66, 142–151. [Google Scholar] [CrossRef]
- Memon, N.; Gat, Y.; Arya, S.; Waghmare, R. Combined Effect of Chemical Preservative and Different Doses of Irradiation on Green Onions to Enhance Shelf Life. J. Saudi Soc. Agric. Sci. 2020, 19, 207–215. [Google Scholar] [CrossRef]
- Morra, L.; Cozzolino, E.; Salluzzo, A.; Modestia, F.; Bilotto, M.; Baiano, S.; Del Piano, L. Plant Growth, Yields and Fruit Quality of Processing Tomato (Solanum Lycopersicon L.) as Affected by the Combination of Biodegradable Mulching and Digestate. Agronomy 2021, 11, 100. [Google Scholar] [CrossRef]
- Bilalis, D.; Roussis, I.; Papastylianou, P.; Cheimona, N. Effects of Organic and Inorganic Fertilization on Yield and Quality of Processing Tomato (Lycopersicon Esculentum Mill.). Folia Hortic. 2018, 30, 321–332. [Google Scholar] [CrossRef]
- Ünlü, H.; Ünlü, H.Ö.; Karakurt, Y.; Padem, H. Influence of Organic and Conventional Production Systems on the Quality of Tomatoes during Storage. Afr. J. Agric. Res. 2011, 6, 538–544. [Google Scholar]
- Ayomide, O.B.; Ajayi, O.O.; Ajayi, A.A. Advances in the Development of a Tomato Postharvest Storage System: Towards Eradicating Postharvest Losses. J. Phys. Conf. Ser. 2019, 1378, 022064. [Google Scholar] [CrossRef]
- Navarro, M.; Bosch, S.M. Reduced Phosphate Availability Improves Tomato Quality Through Hormonal Modulation in Developing Fruits. J. Plant Growth Regul. 2022, 41, 153–162. [Google Scholar] [CrossRef]
- Kim, Y.X.; Son, S.; Lee, S.; Jung, E.; Lee, Y.; Sung, J.; Lee, C. Combined Effects of Nutrients × Water × Light on Metabolite Composition in Tomato Fruits (Solanum Lycopersicum L.). Plants 2021, 10, 1437. [Google Scholar] [CrossRef]
- Iqbal, H.M.; Akbar, Q.; Arif, S.; Khurshid, S. Maturity Dependent Changes in Post-Harvest Physiological, Antioxidant and Anti-Microbial Attributes of Tomato. Pak. J. Agric. Sci. 2022, 35, 144–153. [Google Scholar] [CrossRef]
- Tilahun, S.; Park, D.S.; Solomon, T.; Choi, H.R.; Jeong, C.S. Maturity Stages Affect Nutritional Quality and Storability of Tomato Cultivars. CYTA-J. Food 2019, 17, 87–95. [Google Scholar] [CrossRef]
- Toor, R.K.; Savage, G.P.; Heeb, A. Influence of Different Types of Fertilisers on the Major Antioxidant Components of Tomatoes. J. Food Compos. Anal. 2006, 19, 20–27. [Google Scholar] [CrossRef]
- Okolie, N.P. Effect of Post Harvest Treatments on Quality of Whole Tomatoes. Afr. J. Food Sci. 2012, 6, 70–76. [Google Scholar] [CrossRef]
- Fagundes, C.; Moraes, K.; Pérez-Gago, M.B.; Palou, L.; Maraschin, M.; Monteiro, A.R. Effect of Active Modified Atmosphere and Cold Storage on the Postharvest Quality of Cherry Tomatoes. Postharvest Biol. Technol. 2015, 109, 73–81. [Google Scholar] [CrossRef]
- Javanmardi, J.; Kubota, C. Variation of Lycopene, Antioxidant Activity, Total Soluble Solids and Weight Loss of Tomato during Postharvest Storage. Postharvest Biol. Technol. 2006, 41, 151–155. [Google Scholar] [CrossRef]
- Alkan, N.; Fortes, A.M. Insights into Molecular and Metabolic Events Associated with Fruit Response to Post-Harvest Fungal Pathogens. Front. Plant. Sci. 2015, 6, 889. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).