Diversity and Abundance Patterns of Benthic Invertebrate Assemblages on Intertidal Estuarine Seagrass Beds in Aveiro (Portugal) †
Abstract
:1. Introduction
2. Materials and Methods
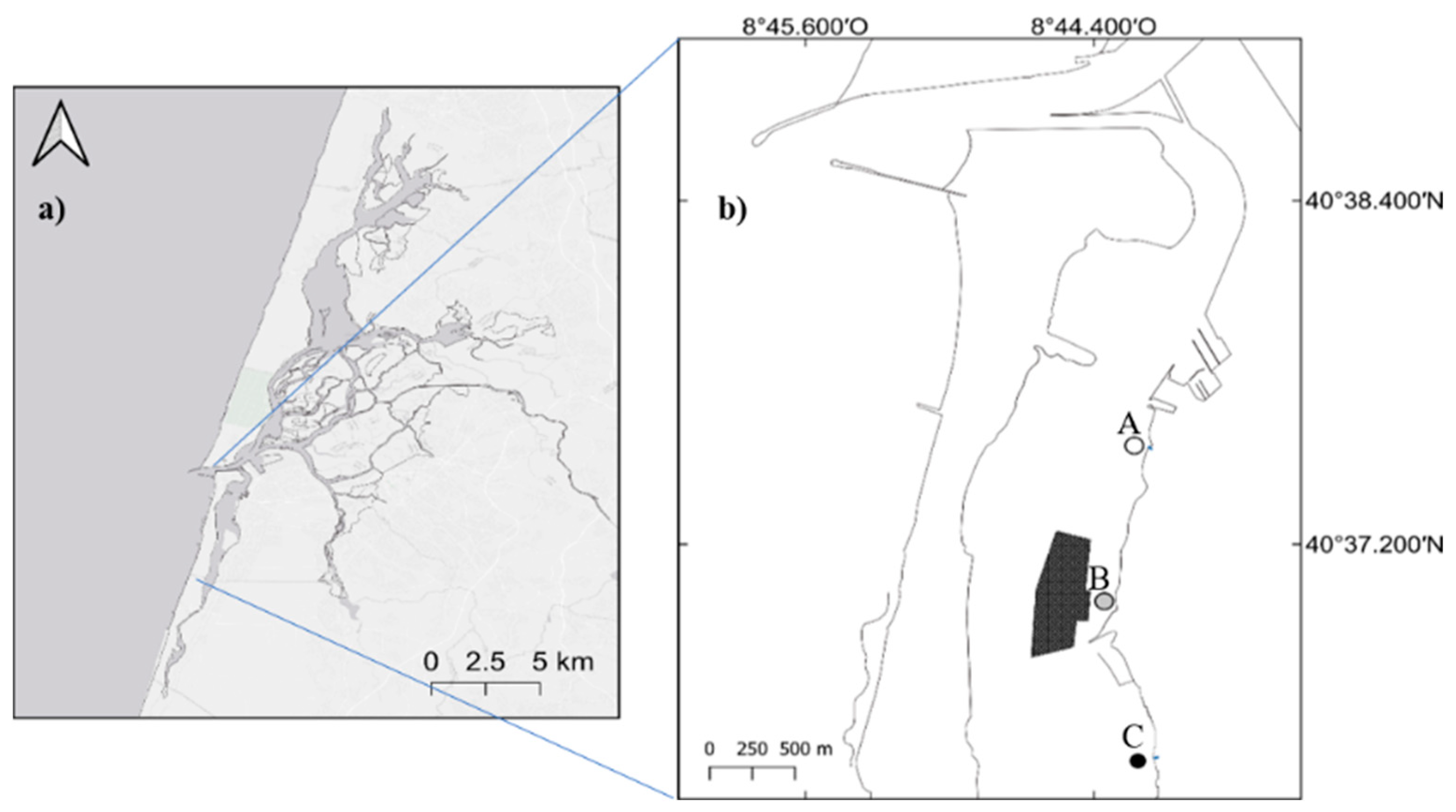
2.1. Study Area
2.2. Sampling and Sample Processing
2.3. Data Analyses
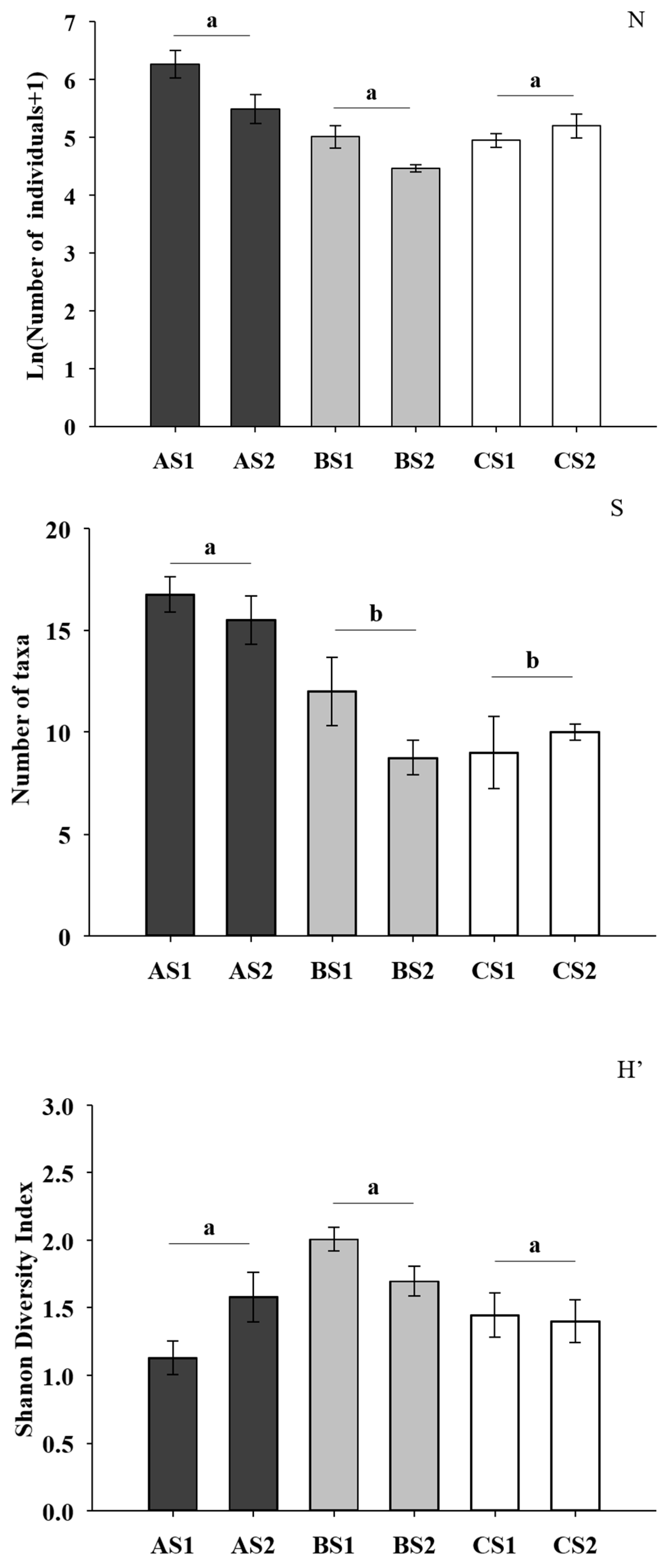
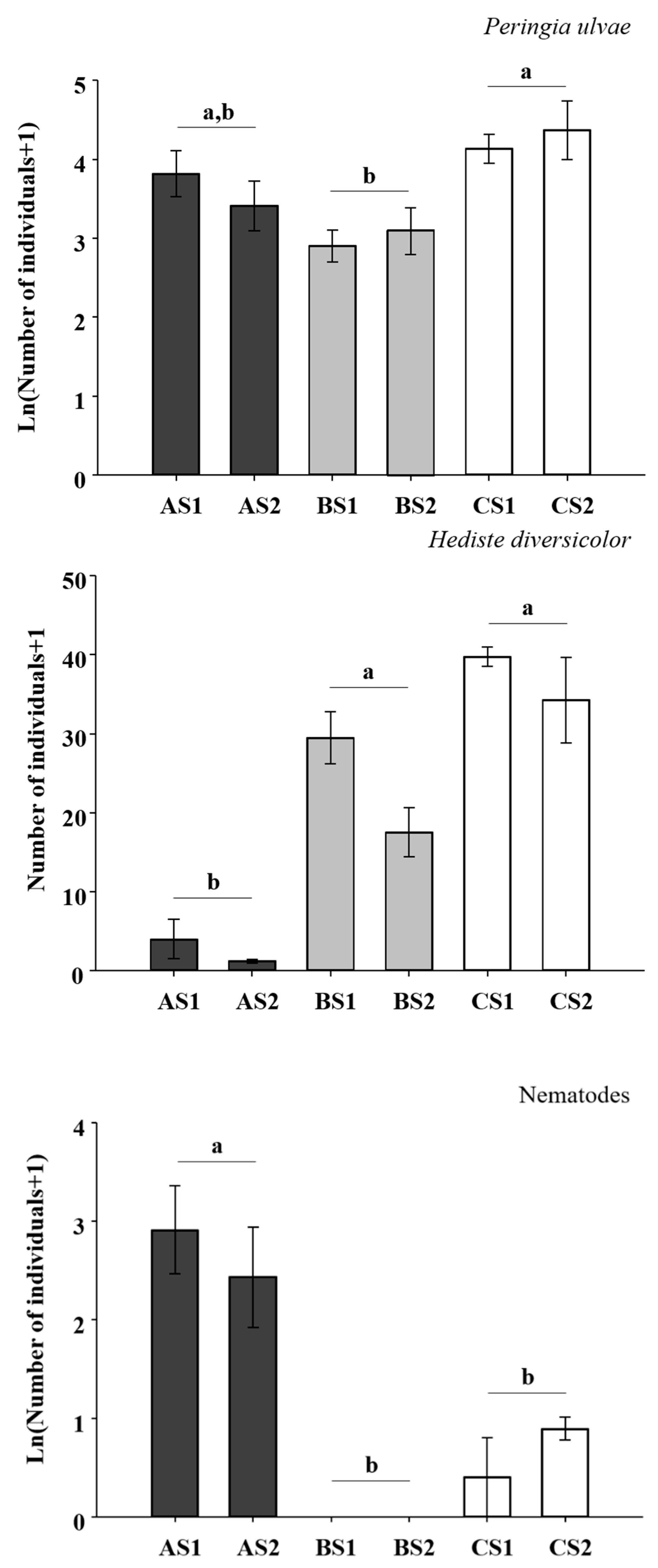
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Short, F.T.; Short, C.A.; Novak, A.B. Seagrasses. In The Wetland Book; Springer: Dordrecht, The Netherlands, 2016; pp. 1–19. [Google Scholar]
- Hemminga, M.A.; Duarte, C.M. Seagrass Ecology; Cambridge University Press: Cambridge, UK, 2000; ISBN 9780521661843. [Google Scholar]
- Blanchet, H.; de Montaudouin, X.; Lucas, A.; Chardy, P. Heterogeneity of macrozoobenthic assemblages within a Zostera noltii seagrass bed: Diversity, abundance, biomass and structuring factors. Estuar. Coast. Shelf Sci. 2004, 61, 111–123. [Google Scholar] [CrossRef]
- Bouma, T.J.; Ortells, V.; Ysebaert, T. Comparing biodiversity effects among ecosystem engineers of contrasting strength: Macrofauna diversity in Zostera noltii and Spartina anglica vegetations. Helg. Mar. Res. 2009, 63, 3–18. [Google Scholar] [CrossRef]
- Orth, R.J.W.; Carruthers, J.B.T.J.B.; Dennison, W.C.; Duarte, C.M.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; Kendrick, G.A.; Kenworthy, W.J.; Olyarnik, S.; et al. A global crisis for seagrass ecosystems. BioScience 2006, 56, 987. [Google Scholar] [CrossRef]
- Román, M.; Fernández, E.; Zamborain-Mason, J.; Martínez, L.; Méndez, G. Decadal changes in the spatial coverage of Zostera noltei in two seagrass meadows (Ría de Vigo; NW Spain). Reg. Stud. Mar. Sci. 2020, 36, 101264. [Google Scholar] [CrossRef]
- Cesbron, F.; Geslin, E.; Jorissen, F.J.; Delgard, M.L.; Charrieau, L.; Deflandre, B.; Jézéquel, D.; Anschutz, P.; Metzger, E. Vertical distribution and respiration rates of benthic foraminifera: Contribution to aerobic remineralization in intertidal mudflats covered by Zostera noltei meadows. Estuar. Coast. Shelf Sci. 2016, 179, 23–38. [Google Scholar] [CrossRef]
- Moreira, M.H.; Queiroga, H.; Machado, M.M.; Cunha, M.R. Environmental gradients in a southern Europe estuarine system: Ria de Aveiro, Portugal implications for soft bottom macrofauna colonization. Neth. J. Aquat. Ecol. 1993, 27, 465–482. [Google Scholar] [CrossRef]
- Silva, F.; Duck, R. Historical changes of bottom topography and tidal amplitude in the Ria de Aveiro, Portugal–trends for future evolution. Clim. Res. 2001, 18, 17–24. [Google Scholar] [CrossRef]
- Mosbahi, N.; Boudaya, L.; Dauvin, J.C.; Neifar, L. Spatial distribution and abundance of intertidal benthic macrofauna in the Kneiss Islands (Gulf of Gabès, Tunisia). Cah. Biol. Mar. 2015, 56, 319–328. [Google Scholar] [CrossRef]
- Tu Do, V.; de Montaudouin, X.; Lavesque, N.; Blanchet, H.; Guyard, H. Seagrass colonization: Knock-on effects on zoobenthic community, populations and individual health. Estuar. Coast. Shelf Sci. 2011, 95, 458–469. [Google Scholar] [CrossRef]
- Lavesque, N.; Blanchet, H.; de Montaundouin, X. Development of a multimetric approach to assess perturbation of benthic macrofauna in Zostera noltii beds. J. Exp. Mar. Biol. Ecol. 2009, 368, 101–112. [Google Scholar] [CrossRef]
- Tu Do, V.; Blanchet, H.; de Montaudouin, X.; Lavesque, N. Limited Consequences of Seagrass Decline on Benthic Macrofauna and Associated Biotic Indicators. Estuaries Coasts 2013, 36, 795–807. [Google Scholar] [CrossRef]
| S | N | H′ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source | df | MS | F | P | MS | F | P | MS | F | P |
| Meadow | 2 | 103.625 | 11.840 | 0.038 | 2.771 | 4.320 | 0.131 | 0.584 | 2.910 | 0.198 |
| Site | 3 | 8.750 | 1.450 | 0.261 | 0.641 | 4.460 | 0.017 | 0.201 | 2.480 | 0.094 |
| Residuals | 18 | 6.028 | 0.144 | 0.081 | ||||||
| Total | 23 | |||||||||
| P. ulvae | H. diversicolor | Nematodes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source | df | MS | F | P | MS | F | P | MS | F | P |
| Meadow | 2 | 3.157 | 18.690 | 0.0202 | 2399.542 | 19.800 | 0.0187 | 15.551 | 49.280 | 0.0051 |
| Site | 3 | 0.169 | 0.520 | 0.6728 | 121.208 | 3.170 | 0.0495 | 0.316 | 0.750 | 0.5370 |
| Residuals | 18 | 0.323 | 38.236 | 0.421 | ||||||
| Total | 23 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marín-Aragón, R.; Sampaio, L.; Guerrero-Meseguer, L.; Veiga, P.; Rubal, M. Diversity and Abundance Patterns of Benthic Invertebrate Assemblages on Intertidal Estuarine Seagrass Beds in Aveiro (Portugal). Biol. Life Sci. Forum 2022, 15, 34. https://doi.org/10.3390/IECD2022-12421
Marín-Aragón R, Sampaio L, Guerrero-Meseguer L, Veiga P, Rubal M. Diversity and Abundance Patterns of Benthic Invertebrate Assemblages on Intertidal Estuarine Seagrass Beds in Aveiro (Portugal). Biology and Life Sciences Forum. 2022; 15(1):34. https://doi.org/10.3390/IECD2022-12421
Chicago/Turabian StyleMarín-Aragón, Raúl, Leandro Sampaio, Laura Guerrero-Meseguer, Puri Veiga, and Marcos Rubal. 2022. "Diversity and Abundance Patterns of Benthic Invertebrate Assemblages on Intertidal Estuarine Seagrass Beds in Aveiro (Portugal)" Biology and Life Sciences Forum 15, no. 1: 34. https://doi.org/10.3390/IECD2022-12421
APA StyleMarín-Aragón, R., Sampaio, L., Guerrero-Meseguer, L., Veiga, P., & Rubal, M. (2022). Diversity and Abundance Patterns of Benthic Invertebrate Assemblages on Intertidal Estuarine Seagrass Beds in Aveiro (Portugal). Biology and Life Sciences Forum, 15(1), 34. https://doi.org/10.3390/IECD2022-12421









