Abstract
We investigated the effects of three rootstocks (Beaumont, Daddow, and WJMAS-29) and two crown positions (north and south) on abaxial leaf stomatal density (SD) of a common macadamia scion ‘HAES741’. Manual counting and artificial intelligence (AI) image processing methods were used to measure SD from the leaves of the scion grafted onto each rootstock. In both methods, rootstock and crown position showed significant effects on scion SD (352 stomata mm−2 in WJMAS-29,310 stomata mm−2 in Beaumont, and 332 stomata mm−2 in Daddow; 358 stomata mm−2 in north and 305 stomata mm−2 in south). We discussed that variability in SD due to genotype (rootstock) and environment (differential solar irradiance due to crown position) can be used to improve productivity of future orchard systems.
Keywords:
macadamia; rootstock; stomata; crown position; image processing; AI; disease susceptibility; climate change 1. Introduction
Stomata, the pores on the leaf epidermis, facilitate gas exchange in plants. Plants balance their CO2 demands and water losses through the adjustment of stomatal size, density, and modulation (opening and closing of stomata); however, adjustments to stomatal size and density are only possible during stomatal ontogeny. Consequently, stomatal size and density is static in fully matured leaves [1,2]. Conversely, stomatal modulation is a dynamic process that occurs continuously throughout the functional life of a leaf. Plants are vexed with the competing priorities of CO2 acquisition and water conservation. Opening stomata increases CO2 concentration within the plant for photosynthesis, but this results in water losses through transpiration [3]. The adjustment of stomatal characteristics may, therefore, have significant impacts on photosynthetic performance, disease susceptibility, water-use efficiency, and climate change adaptability [4,5]. A wide array of genetic and environmental factors exert control over the stomatal phenotype [6,7]. To the authors knowledge, there has not been any research undertaken to evaluate crown heterogeneity of stomatal characteristics in macadamia, nor the potential rootstock effects on the scion. Given the large differences in stomatal response among species, it is important to measure stomatal density under different light intensities in macadamia.
Macadamia is a vegetatively propagated plant; as such, the rootstock effects on plant productivity should be an important consideration in the breeding program. In addition to this, the effects of shading on stomatal density are of interest. There is ample evidence to suggest that stomatal density is affected by crown position; however, so far as we know, no investigation has yet been undertaken in macadamia [8,9]. Optimal parameters for stomatal characteristics for sustainable and productive macadamia orchards remain unknown. The aim of this project was to determine the effects of crown position and rootstock genotype on abaxial leaf stomatal density. We also discussed the usefulness of our findings for future orchard management decisions, with respect to sustainability and climate change adaptation.
2. Materials and Methods
2.1. Plant Material and Experimental Design
This experiment utilised an existing macadamia rootstock trial, which was planted in 2017 at the Queensland Department of Agriculture and Fisheries Maroochy Research Facility [10]. In this study, three cutting-grown rootstocks (three replications each) grafted to the common scion ‘HAES741’ were chosen to investigate the effects of rootstock and crown position on leaf stomatal density: Daddow, Beaumont, and WJMAS-29.
2.2. Phenotyping for Stomatal Density
The protocol outlined below was adapted from the lab guide issued by the Gene Technology Access Centre [11]. This method uses nail polish to create a negative imprint of the leaf surface, which is later mounted to a microscope slide for imaging. Sampling was conducted within a two-hour time period; each tree was located, and branches were selected from north and south positions in the tree crown. An approximately 1cm × 3 cm strip of nail polish was spread onto the abaxial leaf surface, avoiding the midrib, of the first three fully expanded mature leaves on the branch and was left to dry. All trees were treated before returning to the first tree to commence the bagging of samples. Each painted leaf was cut from the branch and placed in a labelled bag. Samples were then taken to the lab to commence slide mounting.
In the lab, the dried nail polish was removed from the lower surface of the leaf using a 10 cm length of sticky tape placed over the entire nail polish negative. Once peeled from the leaf, this negative and sticky tape composite was placed sticky side down onto the glass microscope slide, smoothed to reduce air bubbles, trimmed with a scalpel blade, and labelled. Each slide was then photographed five times at 400× magnification using a Nikon Optiphot (Nikon, Tokyo, Japan) compound microscope equipped with a camera adapter. The locations of the five photographs taken for each sample were randomly selected by moving the microscope stage to a unique position and capturing a photograph. The field of view was calculated by using a calibration slide to measure the length and width of the area in question. At 400× magnification, the photograph represents 0.053 mm2 (0.28 mm × 0.19 mm). Photograph file names were recorded with the respective sample and sub sample numbers in an Excel spreadsheet.
2.3. Stomatal Quantification
Two methods were used to count the stomata: manual counting (M) and an artificial intelligence (AI)-based image processing tool. With the manual method, the protocol was to count only stomata that had both guard cells fully within the boundaries of the picture. The AI method utilised a beta web application [12,13]. Once all counts were recorded, stomatal density was calculated using the stomatal count and the known area to obtain a stomatal density presented as the number of stomata mm−2.
2.4. Statistical Analysis
Analysis of the data was completed in Genstat [14]. Restricted maximum likelihood (REML) mixed model analysis was conducted to identify the significant effects of the factors and to determine the variability in leaf stomatal density of the common scion ‘HAES741’.
3. Results and Discussion
3.1. Rootstock Affects Stomatal Density
REML mixed model analysis showed that stomatal density differed significantly with respect to rootstock choice (p ≤ 0.001 (manual count), p = 0.003 (AI count)) (Table 1).

Table 1.
Restricted maximum likelihood (REML) mixed model analysis output.
In this study, rootstock effect on leaf stomatal density of ‘HAES741” varied from 310 mm−2 to 352 mm−2, with the highest SD due to the effect of ‘WJMAS-29’, and the lowest SD due to the effect of Beaumont (Figure 1). These results indicate that rootstock choice does in fact play a role in the expression of the stomatal phenotype. This knowledge has important implications for the rootstock breeding program, as stomatal characteristics are likely to affect the water-use efficiency, photosynthetic performance, and disease susceptibility of macadamia [4,15,16,17]. A 2018 study on the stomatal characteristics of Arabidopsis thaliana found that stomatal size and density are negatively correlated (r = −0.5, p < 0.001) [4]. This is of interest as it was also found that stomatal size correlates with water-use efficiency, with smaller stomata responsible for higher water-use efficiency [4,18]. So far, no investigation into macadamia has been undertaken to explore the relationship of stomatal characteristics with respect to water-use efficiency. It has been proposed that smaller stomata are able to react more quickly to environmental stimulus due to their inherently larger surface area to volume ratios, allowing faster ion flow through the guard membrane cells, which are the dynamic cells of the stomatal complex responsible for the opening and closing of the stomatal aperture [2]. This would allow the plant to fine tune stomatal apertures to optimise stomatal conductance in accordance with current environmental conditions, thereby minimising evaporative losses and maximising CO2 acquisition. For the first time in macadamia, we identified that rootstocks affect stomatal characteristics, which can be used to select rootstocks to maximise water-use efficiency and CO2 acquisition. As this study used only three rootstocks, and was conducted in a single year, further investigation is necessary to confirm the repeatability of this result. We also suggest exploring the relationships of stomatal characteristics on water-use efficiency and gas exchange in macadamia.
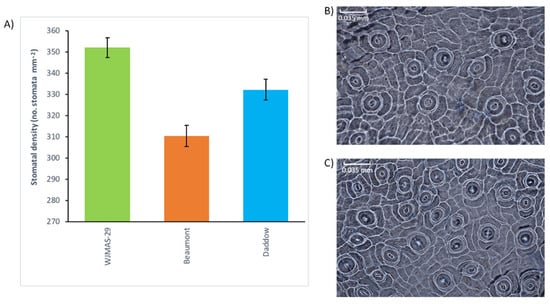
Figure 1.
Effect of rootstocks on leaf stomatal density of HAES741. Error bars show the standard error (SE) of each mean. (A) Variability in stomata density due to three rootstocks. (B) Low stomatal density due to the effect of Beaumont. (C) High stomatal density due to the effect of WJMAS-29.
One potential downfall of increased stomatal density is the possibility of increased incidence of husk spot disease caused by Pseudocercospora macadamiae. P. macadamiae is an endemic fungal pathogen in Australian macadamia production systems [16]. It germinates on the surface of the fruit husk, where it elongates and enters through open stomata, subsequently proliferating through the pericarp tissue. It is unknown whether leaf stomatal density is correlated to husk stomatal density; however, if true, more open pores on the husk surface could result in increased susceptibility to husk spot disease.
3.2. Crown Position Affects Stomatal Density
Our investigation on the north and south sides of plants identified that stomatal density differed significantly with respect to crown position (p ≤ 0.001 (manual count), p ≤ 0.001 (AI count)) (Table 1). Leaf stomatal density in the northern crown position had a mean of 358 stomata mm−2, which was significantly higher than that of leaves in the southern crown position (305 stomata mm−2) (Figure 2). Leaf stomatal density was significantly higher in the northern crown position for all rootstocks; WJMAS-29—north 381 stomata mm−2, WJMAS-29—south 315 stomata mm−2, Beaumont—north 327 stomata mm−2, Beaumont—south 281 stomata mm−2, Daddow—north 348 stomata mm−2, Daddow—south 300 stomata mm−2. This data demonstrates there was no interaction between crown position and rootstock (p = 0.455 (M), p = 0.788 (AI)) (Table 1). A possible explanation for this effect is that due to reduced light interception received on the southern crown position (in southern hemisphere production systems), there is a reduced need for gas exchange due to reduced photosynthetic rate [5]. Jumrani et al. [6] demonstrated a similar relationship in soybean, where shade cloth of increasing impermeability resulted in decreased stomatal density, as well as decreased yields. In addition to this, lower temperatures would also reduce the localised vapour pressure deficit at the leaf surface, so transpirational losses would be less severe. This highlights the importance of designing efficient orchards, and utilising small trees to minimise interrow shading and maximise solar gain [19]. This information also has important implications for future studies. Knowledge of within-tree heterogeneity, with respect to stomatal density, will reduce the chances of confounding genetic effects with those of environmental origin. Our results showing differences between sunny and shaded crown positions match those of similar studies on other tree species [8,9].
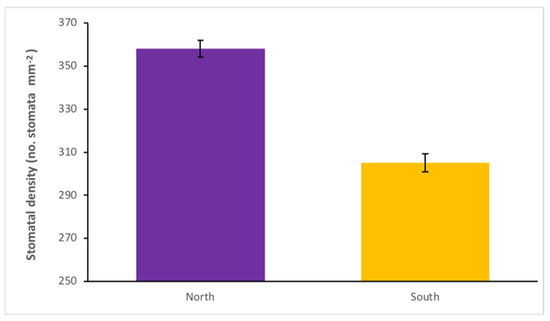
Figure 2.
Mean leaf stomatal density of three macadamia rootstocks on northern crown position (purple) and southern crown position (yellow), using HAES741 as a common scion. Error bars show the standard error (SE) of each mean.
3.3. Comparison of Manual and Artificial Intelligence Quantification Methods
Stomatal counts were carried out using manual and AI methods, with the aim of establishing whether the AI method was of comparable accuracy to manual counting. We ran a correlation on the manual and AI data sets to assess the suitability of the technology for further studies. The results indicate there is a strong correlation between the two methods (R2 = 0.7018) (Figure 3).
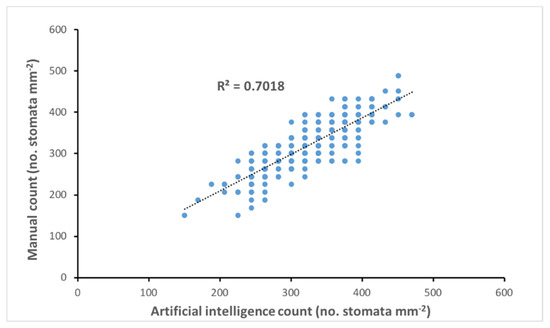
Figure 3.
Correlation of artificial intelligence (AI) vs. manual counting methods for determining stomatal density.
The counting protocol differed slightly between the AI and manual methods. The manual method did not count stomata that were half in/half out of the microscope field of vision, whereas the AI method was able to identify the outline of some of these stomata on the borders of the frame and counted them. Therefore, the correlation may in fact be higher than reported if the protocols were more closely aligned. Additionally, some stomata were not picked up by the AI software, which may be due to photo resolution or visual artifacts from the slide mounting process.
It was initially anticipated that the AI method would offer time savings over manual counting, which would help with the efficiency of phenotyping. However, due to the process requiring the individual upload of each image, and allowances for AI processing, time savings were not deemed to be substantial enough to warrant the use of this tool in its current form. The utility of this tool could be greatly increased by: (a) allowing batch processing of images; and (b) the production of a data file with automated stomatal density calculations. This would drastically reduce the amount of time required to phenotype samples, which may be useful for more extensive studies.
4. Conclusions
This preliminary research investigated the effects of rootstock and crown position on abaxial leaf stomatal density in macadamia. We found that rootstock choice affects stomatal density, which has potential implications on water-use efficiency, photosynthetic performance, and disease susceptibility of macadamia. Additionally, we found that crown position also affects stomatal density, with leaves on the northern side of the canopy (higher solar irradience in the southern hemisphere) having a higher stomatal density than those on the southern side. This result highlights the importance of canopy management for orchard productivity. Limitations of the study include small rootstock and scion genotype sample sizes, and slight differences in stomatal counting protocol between the manual and AI quantification methods. Further investigation of stomatal characteristics in macadamia is required, especially the relationship between stomatal size and density.
Author Contributions
S.W. conducted experimentation; S.W. and M.A. designed the experimentation, analysed data; B.T. and M.A. supervised the project. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been funded by the University of Queensland (UQ) and Hort Innovation (HI) Australia. UQ provided a summer research scholarship for SW. HI provided funding through MC14000, MC19000, AI13004, AS18000, using the macadamia research and development levy and contributions from the Australian Government.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be available upon request, please contact m.alam@uq.edu.au.
Acknowledgments
We acknowledge the administrative and research funding supports of UQ and HI. Hort Innovation is the grower owned, not-for-profit research and development corporation for Australian horticulture. Thanks to Duc Nguyen for his assistance during data collection and Hiranya Jayakody for his advice on AI technology.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Casson, S.A.; Hetherington, A.M. Environmental regulation of stomatal development. Curr. Opin. Plant Biol. 2010, 13, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A. Speedy small stomata. J. Exp. Bot. 2014, 65, 1415–1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewers, B.E. Understanding stomatal conductance responses to long-term environmental changes: A Bayesian framework that combines patterns and processes. Tree Physiol. 2013, 33, 119–122. [Google Scholar] [CrossRef] [Green Version]
- Dittberner, H.; Korte, A.; Mettler-Altmann, T.; Weber, A.P.; Monroe, G.; de Meaux, J. Natural variation in stomata size contributes to the local adaptation of water-use efficiency in Arabidopsis thaliana. Mol. Ecol. 2018, 27, 4052–4065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, Y.; Sugano, S.S.; Shimada, T.; Hara-Nishimura, I. Enhancement of leaf photosynthetic capacity through increased stomatal density in Arabidopsis. New Phytol. 2013, 198, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Jumrani, K.; Bhatia, V.S. Influence of different light intensities on specific leaf weight, stomatal density photosynthesis and seed yield in soybean. Plant Physiol. Rep. 2020, 25, 277–283. [Google Scholar] [CrossRef]
- MacAlister, C.A.; Ohashi-Ito, K.; Bergmann, D.C. Transcription factor control of asymmetric cell divisions that establish the stomatal lineage. Nature 2006, 445, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Poole, I.; Weyers, J.D.B.; Lawson, T.; Raven, J.A. Variations in stomatal density and index: Implications for palaeoclimatic reconstructions. Plant Cell Environ. 1996, 19, 705–712. [Google Scholar] [CrossRef]
- Herrick, J.D.; Maherali, H.; Thomas, R.B. Reduced Stomatal Conductance in Sweetgum (Liquidambar styraciflua) Sustained over Long-Term CO2 Enrichment. New Phytol. 2004, 162, 387–396. [Google Scholar] [CrossRef]
- Alam, M.; Wilkie, J.; Kelly, A.; Hardner, C.; Topp, B. Genetic diversity and variability in graft success in Australian macadamia rootstocks. In Proceedings of the International Macadamia Research Symposium, Honolulu, HI, USA, 13–14 September 2017. [Google Scholar]
- Centre, G.T.A. Measuring Stomatal Density. 2021. Available online: https://www.gtac.edu.au/wp-content/uploads/2016/01/StomatalDensity_LabPreparation.pdf (accessed on 8 February 2021).
- Jayakody, H. Computer Vision. 2020. Available online: https://www.ainz11.com/computer-vision (accessed on 8 February 2021).
- Jayakody, H.; Petrie, P.; de Boer, H.J.; Whitty, M. A Generalised Approach for High-throughput Instance Segmentation of Stomata in Microscope Images. Plant Methods 2020, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- VSN International. Genstat for Windows 21st Edition. 2021. Available online: Genstat.co.uk (accessed on 17 February 2021).
- Bertolino, L.T.; Caine, R.S.; Gray, J.E. Impact of Stomatal Density and Morphology on Water-Use Efficiency in a Changing World. Front. Plant Sci. 2019, 10, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miles, A.K.; Akinsanmi, O.A.; Sutherland, P.W.; Aitken, E.A.B.; Drenth, A. Infection, colonisation and sporulation by Pseudocercospora macadamiae on macadamia fruit. Aust. Plant Pathol. 2009, 38, 36–43. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot. 2008, 59, 3317–3325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rundel, P.W.; Ehleringer, J.R.; Nagy, K.A. Stable Isotopes in Ecological Research; Springer: New York, NY, USA, 1989; pp. 21–40. [Google Scholar]
- Helen, H.; John, W.; Paula, I. The Small Trees High Productivity Initiative: Principles and Practice in High Density Orchard Design. Multidiscip. Digit. Publ. Inst. Proc. 2020, 36, 118. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).