α-Amylase Inhibitory Secondary Metabolites from Artemisia pallens Wall ex DC—Biochemical and Docking Studies †
Abstract
:1. Introduction
2. Methods
2.1. Collection of Plant Material
2.2. Preparation of Plant Extracts
2.3. Qualitative Phytochemical Analysis of Extracts
2.4. α-Amylase Inhibition Assay
2.5. 3,5-Dinitrosalicylic Acid (DNSA) Assay
2.6. Determination of Total Flavonoid Content of Acetone Extract
2.7. Determination of Total Phenol Content of Acetone Extract
2.8. Determination of Total Terpenoid Content of Acetone Extract
2.9. LC-MS/MS Analysis of Acetone Extract
2.10. Molecular Docking Studies
2.10.1. Softwares and Tools
2.10.2. Ligand Preparation
2.10.3. Protein Preparation
2.10.4. Docking Studies
2.10.5. Protein-Ligand Interactions
3. Results
3.1. Qualitative Phytochemical Analysis of A. pallens Leaf and Buds Extracts
3.2. Determination of Total Phytochemicals Content
3.3. LC-MS/MS Analysis of Leaf and Bud Acetone Extract
3.4. Qualitative Starch-Iodide Assay for PPA Inhibition
3.5. Quantitative DNSA Assay for PPA Inhibition
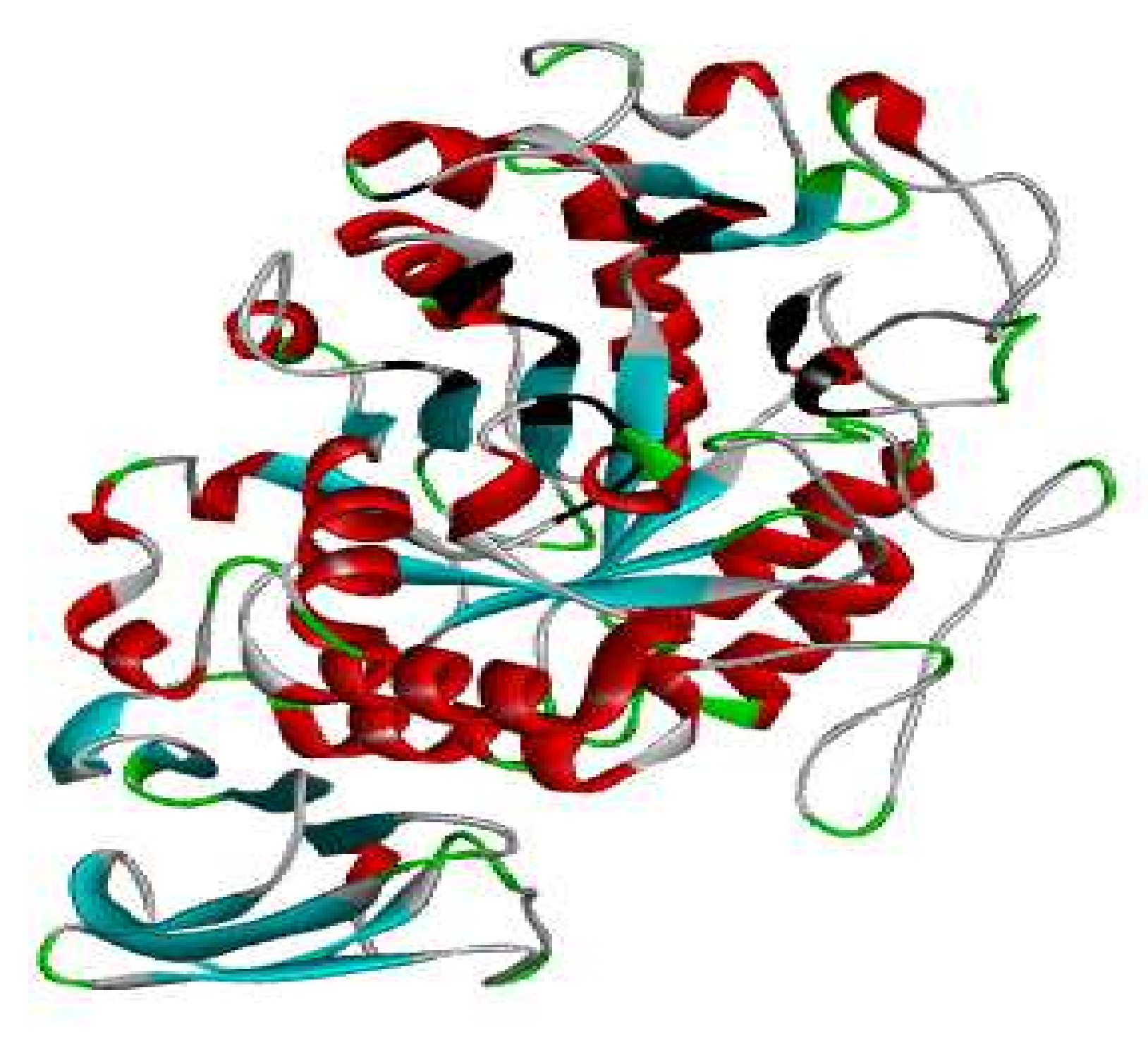
3.6. Molecular Docking Studies
Ligand Preparation
3.7. Protein Preparation
3.8. Docking Studies by Using AutoDock 4.2.6
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DM2 | Diabetes mellitus Type-II |
| LC-MS | Liquid Chromatography-Mass Spectrometry |
| PPA | Porcine Pancreatic Amylase |
| DMSO | Dimethyl Sulfoxide |
| SC | Substrate Control |
| DNSA | 3,5-Dinitrosalicylic acid |
References
- World Health Organization (WHO). Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 5 November 2020).
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Bogan, J.S. Regulation of Glucose Transporter Translocation in Health and Diabetes. Annu. Rev. Biochem. 2012, 81, 507–532. [Google Scholar] [CrossRef] [PubMed]
- Tarling, C.A.; Woods, K.; Zhang, R.; Brastianos, H.C.; Brayer, G.D.; Andersen, R.J.; Withers, S.G. The search for novel human pancreatic α-amylase inhibitors: High-throughput screening of terrestrial and marine natural product extracts. ChemBioChem 2008, 9, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Grover, J.; Yadav, S.; Vats, V. Medicinal plants of India with anti-diabetic potential. J. Ethnopharmacol. 2002, 81, 81–100. [Google Scholar] [CrossRef]
- Hayat, M.Q.; Ashraf, M.; Khan, M.A.; Yasmin, G.; Shaheen, N.; Jabeen, S. Diversity of foliar trichomes and their systematic implications in the genus Artemisia (Asteraceae). Int. J. Agric. Biol. 2009, 11, 542–546. [Google Scholar]
- Su, X.-Z.; Miller, L.H. The discovery of artemisinin and the Nobel Prize in Physiology or Medicine. Sci. China Life Sci. 2015, 58, 1175–1179. [Google Scholar] [CrossRef] [Green Version]
- Islam, N.; Jung, H.A.; Sohn, H.S.; Kim, H.M.; Choi, J.S. Potent α-glucosidase and protein tyrosine phosphatase 1B inhibitors from Artemisia capillaris. Arch. Pharmacal Res. 2013, 36, 542–552. [Google Scholar] [CrossRef]
- Nofal, S.M.; Mahmoud, S.S.; Ramadan, A.; Soliman, G.A.; Fawzy, R. Anti-diabetic effect of Artemisia judaica extracts. Res. J. Med. Med. Sci. 2009, 4, 42–48. [Google Scholar]
- Anaya-Eugenio, G.D.; Rivero-Cruz, I.; Rivera-Chávez, J.; Mata, R. Hypoglycemic properties of some preparations and compounds from Artemisia ludoviciana Nutt. J. Ethnopharmacol. 2014, 155, 416–425. [Google Scholar] [CrossRef]
- Aniya, Y.; Shimabukuro, M.; Shimoji, M.; Kohatsu, M.; Gyamfi, M.A.; Miyagi, C.; Kunii, D.; Takayama, F.; Egashira, T. Antioxidant and hepatoprotective actions of the medicinal herb Artemisia campestris from the Okinawa Islands. Biol. Pharm. Bull. 2000, 23, 309–312. [Google Scholar] [CrossRef] [Green Version]
- Herz, W.; Bhat, S.; Santhanam, P. Coumarins of Artemisia dracunculoides and 3′,6-dimethoxy-4′,5,7-trihydroxyflavone in A. arctica. Phytochemistry 1970, 9, 891–894. [Google Scholar] [CrossRef]
- Li, H.; Yao, Y.; Li, L. Coumarins as potential antidiabetic agents. J. Pharm. Pharmacol. 2017, 69, 1253–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Logendra, S.; Ribnicky, D.M.; Yang, H.; Poulev, A.; Ma, J.; Kennelly, E.J.; Raskin, I. Bioassay-guided isolation of aldose reductase inhibitors from Artemisia dracunculus. Phytochemistry 2006, 67, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Subramoniam, A.; Pushpangadan, P.; Rajasekharan, S.; Evans, D.; Latha, P.; Valsaraj, R. Effects of Artemisia pallens Wall. on blood glucose levels in normal and alloxan-induced diabetic rats. J. Ethnopharmacol. 1996, 50, 13–17. [Google Scholar] [CrossRef]
- Trease, G.E.; Evans, W.C. Pharmacognosy, 15th ed.; Saunders Publishers: London, UK, 2002; pp. 42–44. [Google Scholar]
- Sudha, P.; Zinjarde, S.S.; Bhargava, S.Y.; Kumar, A.R. Potent α-amylase inhibitory activity of Indian Ayurvedic medicinal plants. BMC Complement. Altern. Med. 2011, 11, 5. [Google Scholar] [CrossRef] [Green Version]
- Miller, G.L. Use of Dinitrosalicylic Acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Baba, S.A.; Malik, S.A. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. J. Taibah Univ. Sci. 2015, 9, 449–454. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.; Zhang, W.; Yin, L.; Yan, F.; Xu, Y.; Chen, F. Extraction optimization of total triterpenoids from Jatropha curcas leaves using response surface methodology and evaluations of their antimicrobial and antioxidant capacities. Electron. J. Biotechnol. 2015, 18, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Fakhri, S.; Moradi, S.Z.; Farzaei, M.H.; Bishayee, A. Modulation of dysregulated cancer metabolism by plant secondary metabolites: A mechanistic review. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar] [CrossRef]
- Saleem, S.; Khan, R.; Kazmi, I.; Afzal, M. Medicinal plants in the treatment of arthritis. In Plant and Human Health; Springer: Cham, Switzerland, 2019; Volume 3, pp. 101–137. [Google Scholar] [CrossRef]
- Hien, D.F.D.S.; Dabiré, K.R.; Roche, B.; Diabate, A.; Yerbanga, R.S.; Cohuet, A.; Yameogo, B.K.; Gouagna, L.-C.; Hopkins, R.; Ouedraogo, G.A.; et al. Plant-mediated effects on mosquito capacity to transmit human malaria. PLoSPathog. 2016, 12, e1005773. [Google Scholar] [CrossRef] [Green Version]
- Ota, A.; Ulrih, N.P. An overview of herbal products and secondary metabolites used for management of Type Two Diabetes. Front. Pharmacol. 2017, 8, 436. [Google Scholar] [CrossRef]
- Ruikar, A.D.; Khatiwora, E.; Ghayal, N.A.; Misar, A.V.; Mujumdar, A.M.; Puranik, V.G.; Deshpande, N.R. Studies on aerial parts of Artemisia pallens Wall for phenol, flavonoid and evaluation of antioxidant activity. J. Pharm. Bioallied Sci. 2011, 3, 302–305. [Google Scholar] [CrossRef]
- Svensson, B.; Søgaard, M. Mutational analysis of glycosylase function. J. Biotechnol. 1993, 29, 1–37. [Google Scholar] [CrossRef]
- Rydberg, E.H.; Li, C.; Maurus, R.; Overall, C.M.; Brayer, G.D.; Withers, S.G. Mechanistic analyses of catalysis in human pancreatic α-amylase: Detailed kinetic and structural studies of mutants of three conserved carboxylic acids. Biochemistry 2002, 41, 4492–4502. [Google Scholar] [CrossRef] [PubMed]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of α-glucosidase and α-amylase by flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piparo, E.L.; Scheib, H.; Frei, N.; Williamson, G.; Grigorov, M.; Chou, C.J. Flavonoids for controlling starch digestion: Structural requirements for inhibiting human α-amylase. J. Med. Chem. 2008, 51, 3555–3561. [Google Scholar] [CrossRef]
- Kidane, Y.; Bokrezion, T.; Mebrahtu, J.; Mehari, M.; Gebreab, Y.B.; Fessehaye, N.; Achila, O.O. In Vitro inhibition of α-amylase and α-glucosidase by extracts from Psiadia punctulata and Meriandra bengalensis. Evid.-Based Complement. Altern. Med. 2018, 2018, 2164345. [Google Scholar] [CrossRef] [Green Version]
- Yin, Z.; Zhang, W.; Feng, F.; Zhang, Y.; Kang, W. α-glucosidase inhibitors isolated from medicinal plants. Food Sci. Hum. Wellness 2014, 3, 136–174. [Google Scholar] [CrossRef] [Green Version]
- Magaña-Barajas, E.; Buitimea-Cantúa, G.V.; Hernández-Morales, A.; Torres-Pelayo, V.D.R.; Vázquez-Martínez, J.; Buitimea-Cantúa, N.E. In vitro α-amylase and α-glucosidase enzyme inhibition and antioxidant activity by capsaicin and piperine from Capsicum chinense and Piper nigrum fruits. J. Environ. Sci. Health Part B 2021, 56, 282–291. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, W.; Sun, W.; Chen, S.; Liu, D.; Kong, X.; Tian, J.; Ye, X. Inhibition of porcine pancreatic α-amylase activity by chlorogenic acid. J. Funct. Foods 2019, 64, 103587. [Google Scholar] [CrossRef]
- Jelenkovic, L.; Jovanovic, V.; Palic, I.; Mitic, V.; Radulovic, M. In vitro screening of α-amylase inhibition by selected terpenes from essential oils. Trop. J. Pharm. Res. 2014, 13, 1421. [Google Scholar] [CrossRef] [Green Version]


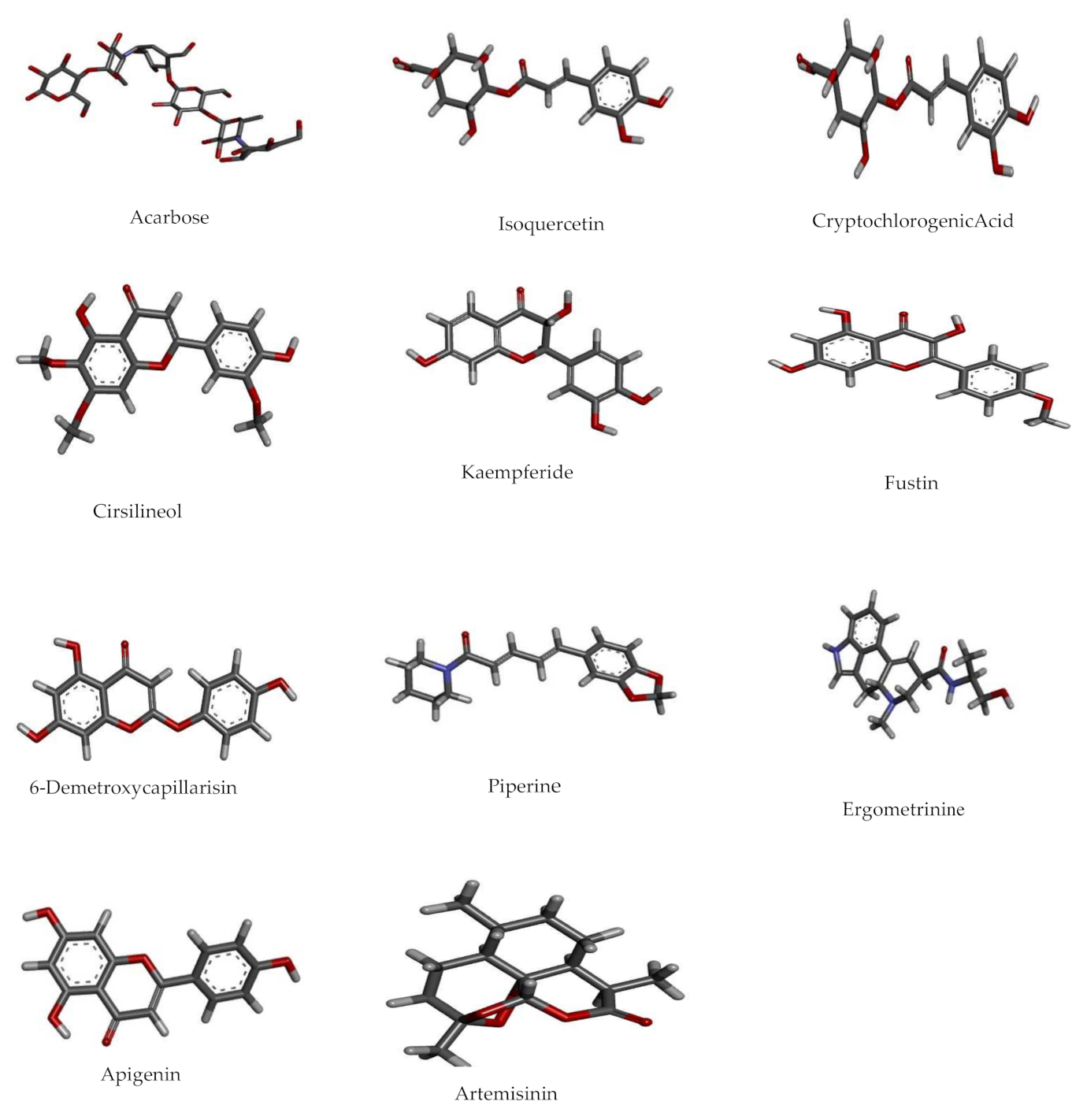


| No. | Pubchem ID | Compound Name | Binding Energy Kcal/mol | Interaction | Amino Acid Residues |
|---|---|---|---|---|---|
| 1 | 41774 | Acarbose | −17.58 | 5-H Bonds | Tyr59, Arg195, His201, Glu233, Asp300 |
| Van Der Waal | Trp58, Tyr62, Tyr151, Asp197, Lys200, Ile235, Gly306, His305, Ala307 | ||||
| 2 | 5280804 | Isoquercetin | −11.57 | 4-HBond | Trp59, Gln63, Glu233, Gly306 |
| Van Der Waal | Trp58, Arg195, His299 | ||||
| Pi-Pi | His201, Ile235, Leu162, Val163, Ala198 | ||||
| 3 | 9798666 | Cryptochlorogenic Acid | −11.17 | 3-H Bonds | Trp59, Tyr62, Tyr151 |
| Van Der Waal | Glu60, Gln63, Trp58, Val163, Leu165, His101, Arg195, Asp197, Lys200, His201, Ile235, His299, Asp300 | ||||
| 4 | 162464 | Cirsilineol | −10.24 | 1-H Bond | Tyr62 |
| Van Der Waal | Gln63, Val98, His101, Tyr151, Val163, Ser199, Val234 | ||||
| Pi-Pi | His201 | ||||
| 5 | 5281666 | Kaempferide | −9.99 | 4-H Bonds | Gln63, Glu233, His299, Asp300 |
| Van Der Waal | Trp58, Leu162, Arg195 | ||||
| Pi-Pi | Trp59, Tyr62 | ||||
| 6 | 5317435 | Fustin | −9.86 | 4-H Bonds | Trp62, Leu162, Asp197, Asp197 |
| 7 | 5316511 | 6-Demethoxy-capillarisin | −9.82 | 4-H Bond | His101, Val163, Asp197, Asp300 |
| Van Der Waal | Trp58, Trp59, Gln63, Leu162 | ||||
| Pi-Pi | Tyr62, Lue165, Ala198 | ||||
| 8 | 638024 | Piperine | −9.45 | Van Der Waal | Gln63, His101, Val163, Leu165, Asp197, Ser199, Val234 |
| Pi-Pi | His201 | ||||
| 9 | 443884 | Ergometrine | −9.43 | 3-H Bond | Asp197, Glu233, Asp300 |
| Van Der Waal | Trp58, Arg195 | ||||
| Pi-Pi | Tyr62, His201, Ala198, Lys200 | ||||
| 10 | 5280443 | Apigenin | −9.38 | 3 H Bonds | Gln63, Glu233, His299 |
| Van Der Waal | Trp58, Leu162, Val163, Leu165, Arg195, His201 | ||||
| Pi-Pi | Tyr62, Trp59 | ||||
| 11 | 68827 | Artemisinin | −9.27 | H bond | Gln63 |
| Van Der Waal | Asp300, Asp197, Alu162, Alu165 | ||||
| Pi-Pi | Trp59, Tyr62, His101, Val163, His305 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulkarni, A.A.; Kamble, R.P. α-Amylase Inhibitory Secondary Metabolites from Artemisia pallens Wall ex DC—Biochemical and Docking Studies. Biol. Life Sci. Forum 2022, 11, 73. https://doi.org/10.3390/IECPS2021-11978
Kulkarni AA, Kamble RP. α-Amylase Inhibitory Secondary Metabolites from Artemisia pallens Wall ex DC—Biochemical and Docking Studies. Biology and Life Sciences Forum. 2022; 11(1):73. https://doi.org/10.3390/IECPS2021-11978
Chicago/Turabian StyleKulkarni, Anjali A., and Rohit P. Kamble. 2022. "α-Amylase Inhibitory Secondary Metabolites from Artemisia pallens Wall ex DC—Biochemical and Docking Studies" Biology and Life Sciences Forum 11, no. 1: 73. https://doi.org/10.3390/IECPS2021-11978
APA StyleKulkarni, A. A., & Kamble, R. P. (2022). α-Amylase Inhibitory Secondary Metabolites from Artemisia pallens Wall ex DC—Biochemical and Docking Studies. Biology and Life Sciences Forum, 11(1), 73. https://doi.org/10.3390/IECPS2021-11978






