Changes in the Differentiation Program of Phloem Derivatives of Birch Cambium after Trunk Girdling †
Abstract
:1. Introduction
2. Materials and Methods
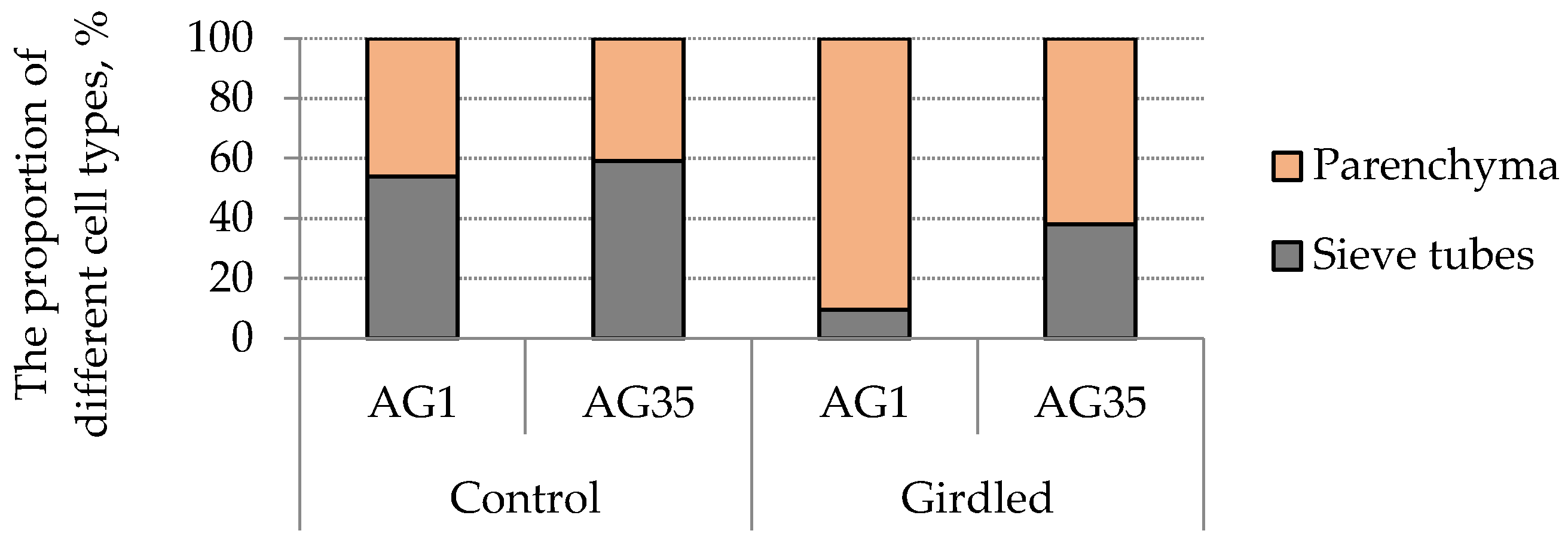
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barbier, F.; Péron, T.; Lecerf, M.; Perez-Garcia, M.-D.; Barrière, Q.; Rolčík, J.; Boutet-Mercey, S.; Citerne, S.; Lemoine, R.; Porcheron, B.; et al. Sucrose Is an Early Modulator of the Key Hormonal Mechanisms Controlling Bud Outgrowth in Rosa hybrida. J. Exp. Bot. 2015, 66, 2569–2582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, J.; Li, W.; Mi, B.; Ma, Z.; Dawuda, M.M.; Zuo, C.; Zhang, Y.; Jiang, X.; Chen, B. Transcriptome Analysis Revealed Glucose Application Affects Plant Hormone Signal Transduction Pathway in “Red Globe” Grape Plantlets. Plant Growth Regul. 2018, 84, 45–56. [Google Scholar] [CrossRef]
- Novitskaya, L.L.; Tarelkina, T.V.; Galibina, N.A.; Moshchenskaya, Y.L.; Nikolaeva, N.N.; Nikerova, K.M.; Podgornaya, M.N.; Sofronova, I.N.; Semenova, L.I. The Formation of Structural Abnormalities in Karelian Birch Wood Is Associated with Auxin Inactivation and Disrupted Basipetal Auxin Transport. J. Plant Growth Regul. 2020, 39, 378–394. [Google Scholar] [CrossRef]
- Tarelkina, T.V.; Novitskaya, L.L.; Galibina, N.A.; Moshchenskaya, Y.L.; Nikerova, K.M.; Nikolaeva, N.N.; Sofronova, I.N.; Ivanova, D.S.; Semenova, L.I. Expression Analysis of Key Auxin Biosynthesis, Transport, and Metabolism Genes of Betula Pendula with Special Emphasis on Figured Wood Formation in Karelian Birch. Plants 2020, 9, 1406. [Google Scholar] [CrossRef] [PubMed]
- De Schepper, V.; Steppe, K.; Van Labeke, M.-C.; Lemeur, R. Detailed Analysis of Double Girdling Effects on Stem Diameter Variations and Sap Flow in Young Oak Trees. Environ. Exp. Bot. 2010, 68, 149–156. [Google Scholar] [CrossRef]
- Galibina, N.A.; Tarelkina, T.V.; Chirva, O.V.; Moshchenskaya, Y.L.; Nikerova, K.M.; Ivanova, D.S.; Semenova, L.I.; Serkova, A.A.; Novitskaya, L.L. Molecular Genetic Characteristics of Different Scenarios of Xylogenesis on the Example of Two Forms of Silver Birch Differing in the Ratio of Structural Elements in the Xylem. Plants 2021, 10, 1593. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, U.; Jalkanen, R.; Eckstein, D. Cambium Dynamics of Pinus Sylvestris and Betula Spp. in the Northern Boreal Forest in Finland. Silva Fenn. 2004, 38, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Tippett, J.T.; Shigo, A.L. Barrier Zone Formation: A Mechanism of Tree Defense Against Vascular Pathogens. IAWA J. 1981, 2, 163–168. [Google Scholar] [CrossRef]
- Johnsen, K.; Maier, C.; Sanchez, F.; Anderson, P.; Butnor, J.; Waring, R.; Linder, S. Physiological Girdling of Pine Trees via Phloem Chilling: Proof of Concept. Plant Cell Environ. 2007, 30, 128–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López, R.; Brossa, R.; Gil, L.; Pita, P. Stem Girdling Evidences a Trade-off between Cambial Activity and Sprouting and Dramatically Reduces Plant Transpiration Due to Feedback Inhibition of Photosynthesis and Hormone Signaling. Front. Plant Sci. 2015, 6, 397. [Google Scholar] [CrossRef] [PubMed]
- Oberhuber, W.; Gruber, A.; Lethaus, G.; Winkler, A.; Wieser, G. Stem Girdling Indicates Prioritized Carbon Allocation to the Root System at the Expense of Radial Stem Growth in Norway Spruce under Drought Conditions. Environ. Exp. Bot. 2017, 138, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.F. Effect of Girdling on Cambial Activity in White Pine. Can. J. Bot. 1968, 46, 141–146. [Google Scholar] [CrossRef]
- Krabel, D. Influence of sucrose on cambial activity. In Cell and Molecular Biology of Wood Formation; Savidge, R.A., Barnett, J.R., Napier, R., Eds.; BIOS Scientific Publishers Limited: Oxford, UK, 2000; pp. 113–125. [Google Scholar]
- Alonso-Serra, J.; Safronov, O.; Lim, K.; Fraser-Miller, S.J.; Blokhina, O.B.; Campilho, A.; Chong, S.; Fagerstedt, K.; Haavikko, R.; Helariutta, Y.; et al. Tissue-specific Study across the Stem Reveals the Chemistry and Transcriptome Dynamics of Birch Bark. New Phytol. 2019, 222, 1816–1831. [Google Scholar] [CrossRef] [PubMed]
- Noel, A.R.A. The Girdled Tree. Bot. Rev. 1970, 36, 162–195. [Google Scholar] [CrossRef]
- Juan, D.; Hong-Li, X.; De-Qiang, Z.; Xin-Qiang, H.; Min-Jie, W.; Ying-Zhang, L.; Ke-Ming, C.; Meng-Zhu, L. Regeneration of the Secondary Vascular System in Poplar as a Novel System to Investigate Gene Expression by a Proteomic Approach. Proteomics 2006, 6, 881–895. [Google Scholar] [CrossRef] [PubMed]

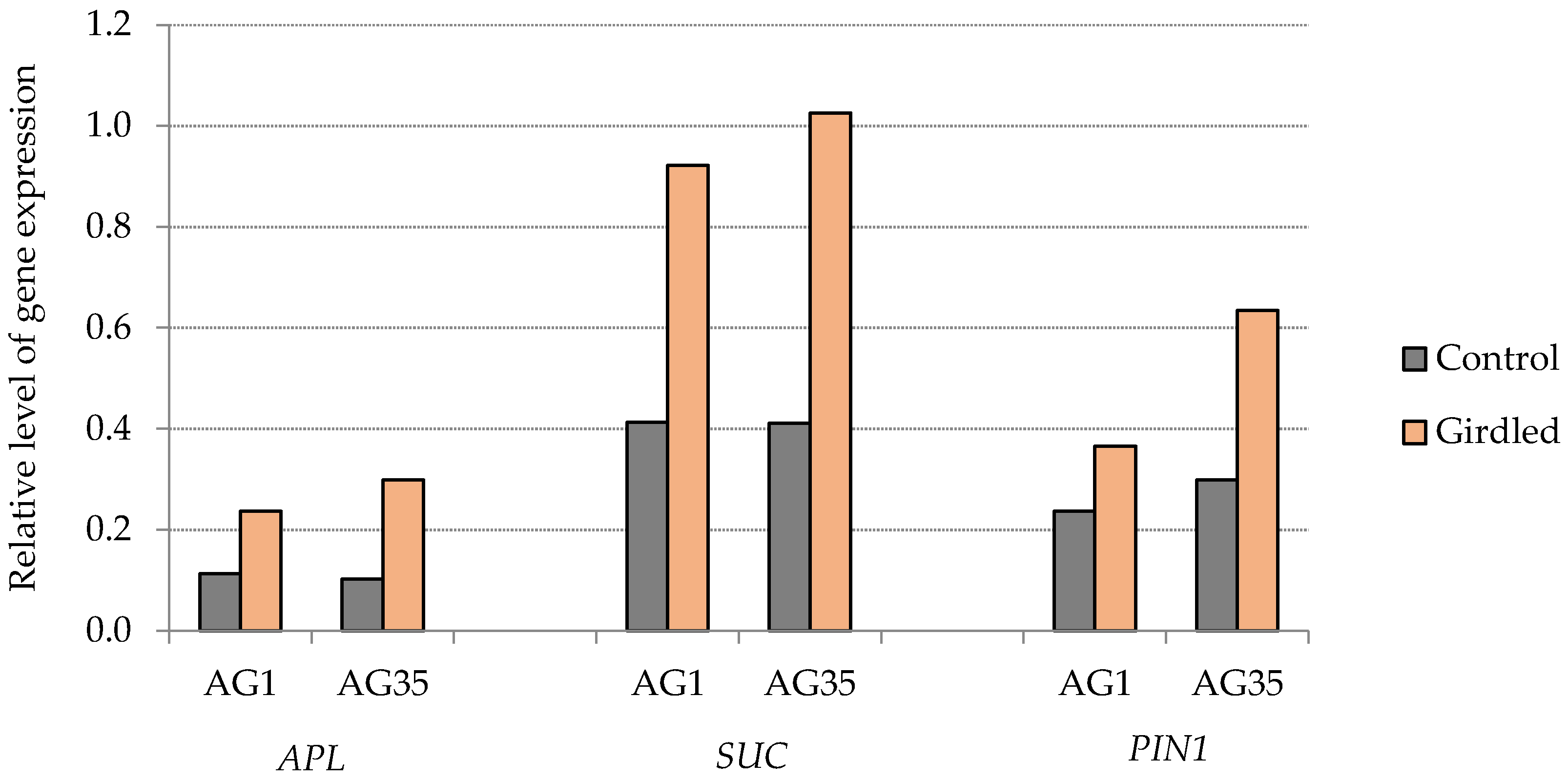
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serkova, A.A.; Tarelkina, T.V.; Galibina, N.A.; Moshchenskaya, Y.L.; Sofronova, I.N.; Ivanova, D.S.; Semenova, L.I. Changes in the Differentiation Program of Phloem Derivatives of Birch Cambium after Trunk Girdling. Biol. Life Sci. Forum 2022, 11, 56. https://doi.org/10.3390/IECPS2021-11928
Serkova AA, Tarelkina TV, Galibina NA, Moshchenskaya YL, Sofronova IN, Ivanova DS, Semenova LI. Changes in the Differentiation Program of Phloem Derivatives of Birch Cambium after Trunk Girdling. Biology and Life Sciences Forum. 2022; 11(1):56. https://doi.org/10.3390/IECPS2021-11928
Chicago/Turabian StyleSerkova, Aleksandra A., Tatiana V. Tarelkina, Natalia A. Galibina, Yulia L. Moshchenskaya, Irina N. Sofronova, Diana S. Ivanova, and Ludmila I. Semenova. 2022. "Changes in the Differentiation Program of Phloem Derivatives of Birch Cambium after Trunk Girdling" Biology and Life Sciences Forum 11, no. 1: 56. https://doi.org/10.3390/IECPS2021-11928
APA StyleSerkova, A. A., Tarelkina, T. V., Galibina, N. A., Moshchenskaya, Y. L., Sofronova, I. N., Ivanova, D. S., & Semenova, L. I. (2022). Changes in the Differentiation Program of Phloem Derivatives of Birch Cambium after Trunk Girdling. Biology and Life Sciences Forum, 11(1), 56. https://doi.org/10.3390/IECPS2021-11928





