Nicotiana benthamiana γ-Thionin Synthesis Is Induced in Response to Foreign Nucleus-Targeted Proteins †
Abstract
:1. Introduction
2. Methods
2.1. Agroinfiltration
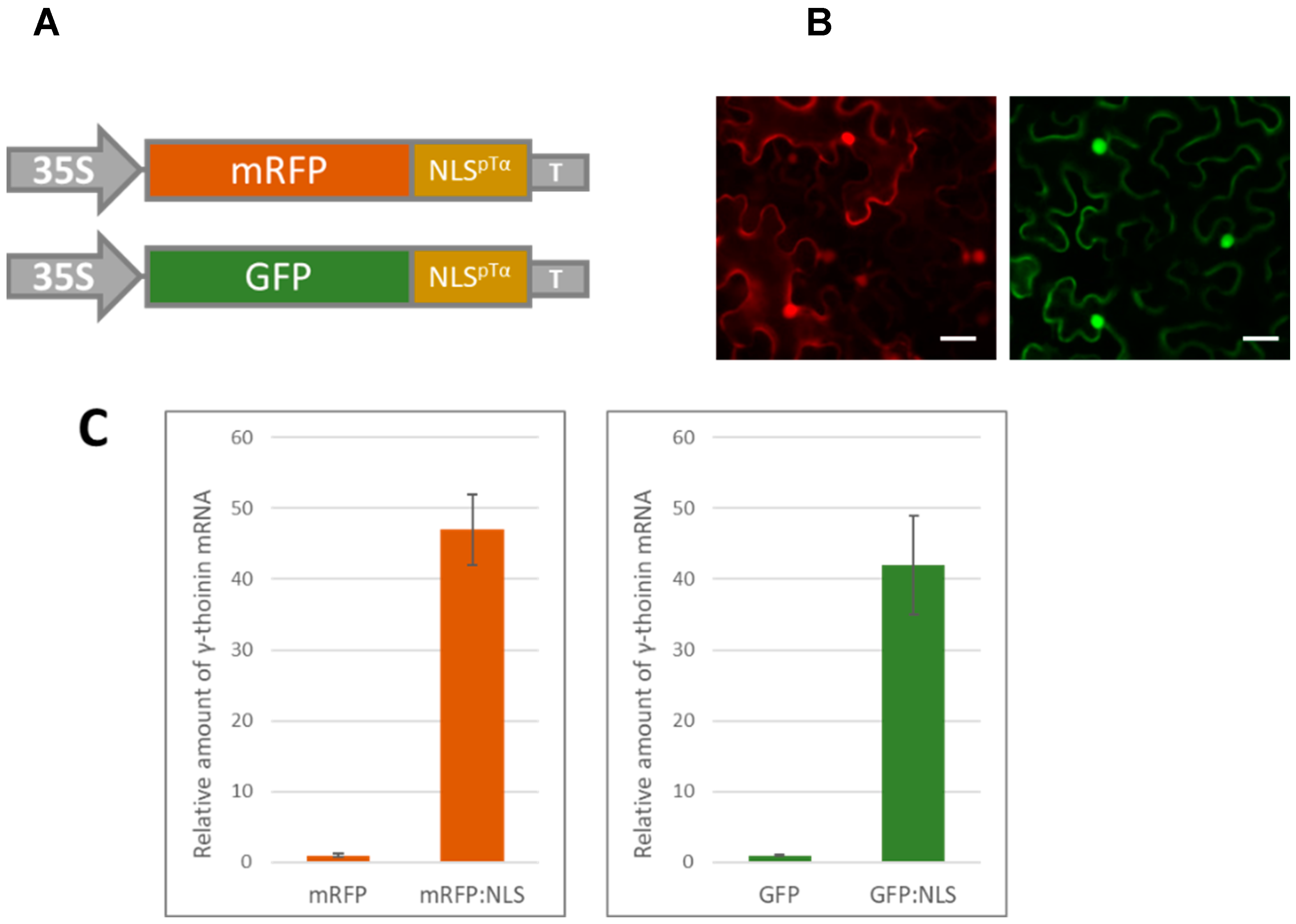
2.2. GFP and mRFP Imaging
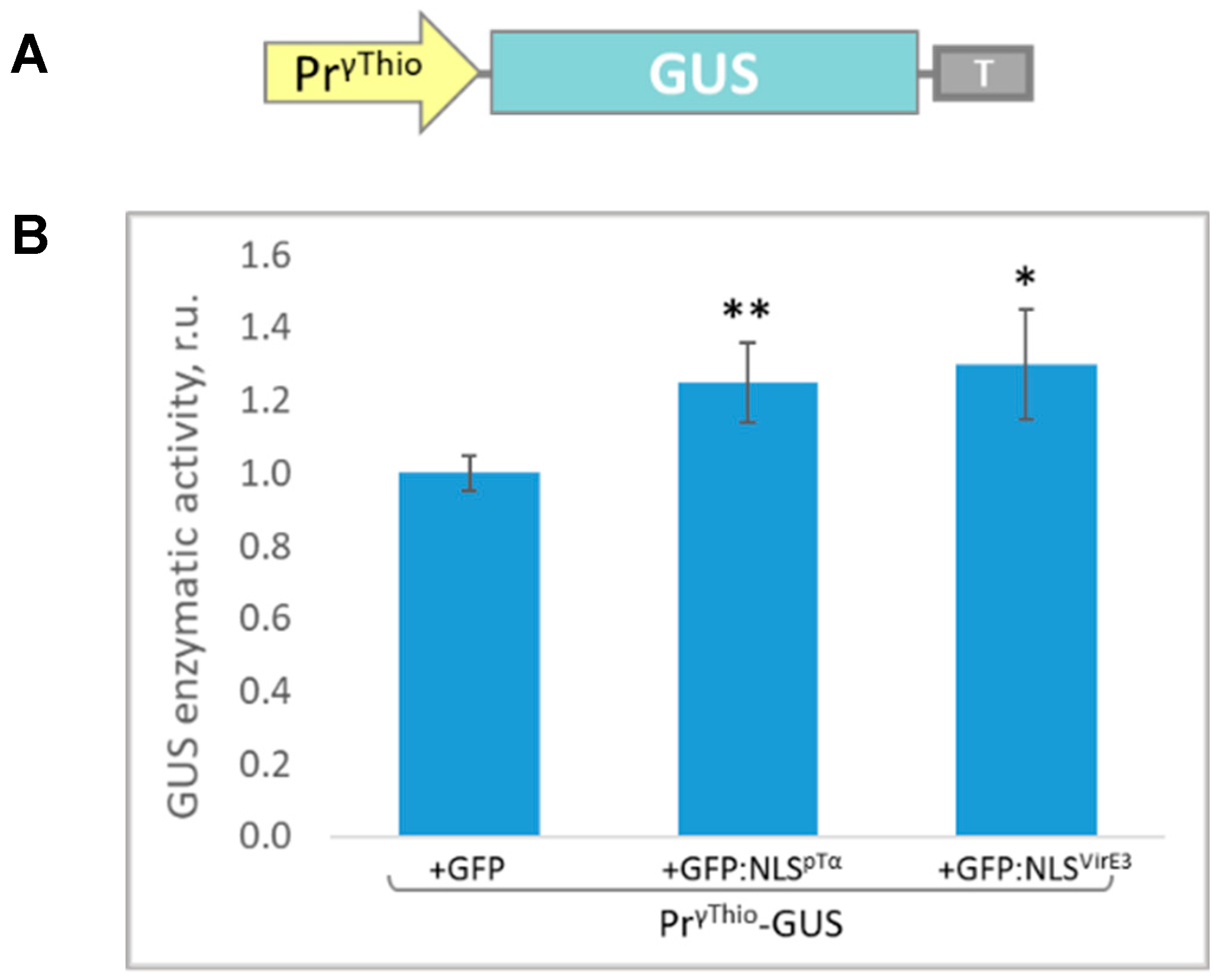
2.3. GUS Activity Measurement
2.4. Quantitative Real-Time PCR (qRT-PCR) Analysis of Transcript Concentrations
3. Results
3.1. A Massive Synthesis of mRFP Fused with a Nuclear Localization Signal Stimulates γ-Thionin mRNA Accumulation
3.2. Isolation of the γ-Thionin Promoter (PrγThio) and Assessment of Its Sensitivity to the Intensive Accumulation of the Model Nuclear Protein
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanford, H.E.; Von Dwingelo, J.; Abu Kwaik, Y. Bacterial Nucleomodulins: A Coevolutionary Adaptation to the Eukaryotic Command Center. PLoS Pathog. 2021, 17, e1009184. [Google Scholar] [CrossRef]
- Le, L.H.M.; Ying, L.; Ferrero, R.L. Nuclear Trafficking of Bacterial Effector Proteins. Cell. Microbiol. 2021, 23, e13320. [Google Scholar] [CrossRef] [PubMed]
- Pelegrini, P.B.; Franco, O.L. Plant γ-Thionins: Novel Insights on the Mechanism of Action of a Multi-Functional Class of Defense Proteins. Int. J. Biochem. Cell Biol. 2005, 37, 2239–2253. [Google Scholar] [CrossRef] [PubMed]
- Sathoff, A.E.; Samac, D.A. Antibacterial Activity of Plant Defensins. Mol. Plant-Microbe Interact. 2019, 32, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Höng, K.; Austerlitz, T.; Bohlmann, T.; Bohlmann, H. The Thionin Family of Antimicrobial Peptides. PLoS ONE 2021, 16, e0254549. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, R.A.; Kavanagh, T.A.; Bevan, M.W. GUS Fusions: Beta-Glucuronidase as a Sensitive and Versatile Gene Fusion Marker in Higher Plants. EMBO J. 1987, 6, 3901–3907. [Google Scholar] [CrossRef] [PubMed]
- Dorokhov, Y.L.; Komarova, T.V.; Petrunia, I.V.; Frolova, O.Y.; Pozdyshev, D.V.; Gleba, Y.Y. Airborne Signals from a Wounded Leaf Facilitate Viral Spreading and Induce Antibacterial Resistance in Neighboring Plants. PLoS Pathog. 2012, 8, e1002640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Bierne, H.; Cossart, P. When Bacteria Target the Nucleus: The Emerging Family of Nucleomodulins. Cell. Microbiol. 2012, 14, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Bierne, H.; Pourpre, R. Bacterial Factors Targeting the Nucleus: The Growing Family of Nucleomodulins. Toxins 2020, 12, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacroix, B.; Vaidya, M.; Tzfira, T.; Citovsky, V. The VirE3 Protein of Agrobacterium Mimics a Host Cell Function Required for Plant Genetic Transformation. EMBO J. 2005, 24, 428–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheshukova, E.; Ershova, N.; Lipskerov, F.; Komarova, T. Nicotiana benthamiana γ-Thionin Synthesis Is Induced in Response to Foreign Nucleus-Targeted Proteins. Biol. Life Sci. Forum 2022, 11, 11. https://doi.org/10.3390/IECPS2021-12006
Sheshukova E, Ershova N, Lipskerov F, Komarova T. Nicotiana benthamiana γ-Thionin Synthesis Is Induced in Response to Foreign Nucleus-Targeted Proteins. Biology and Life Sciences Forum. 2022; 11(1):11. https://doi.org/10.3390/IECPS2021-12006
Chicago/Turabian StyleSheshukova, Ekaterina, Natalia Ershova, Fedor Lipskerov, and Tatiana Komarova. 2022. "Nicotiana benthamiana γ-Thionin Synthesis Is Induced in Response to Foreign Nucleus-Targeted Proteins" Biology and Life Sciences Forum 11, no. 1: 11. https://doi.org/10.3390/IECPS2021-12006
APA StyleSheshukova, E., Ershova, N., Lipskerov, F., & Komarova, T. (2022). Nicotiana benthamiana γ-Thionin Synthesis Is Induced in Response to Foreign Nucleus-Targeted Proteins. Biology and Life Sciences Forum, 11(1), 11. https://doi.org/10.3390/IECPS2021-12006





