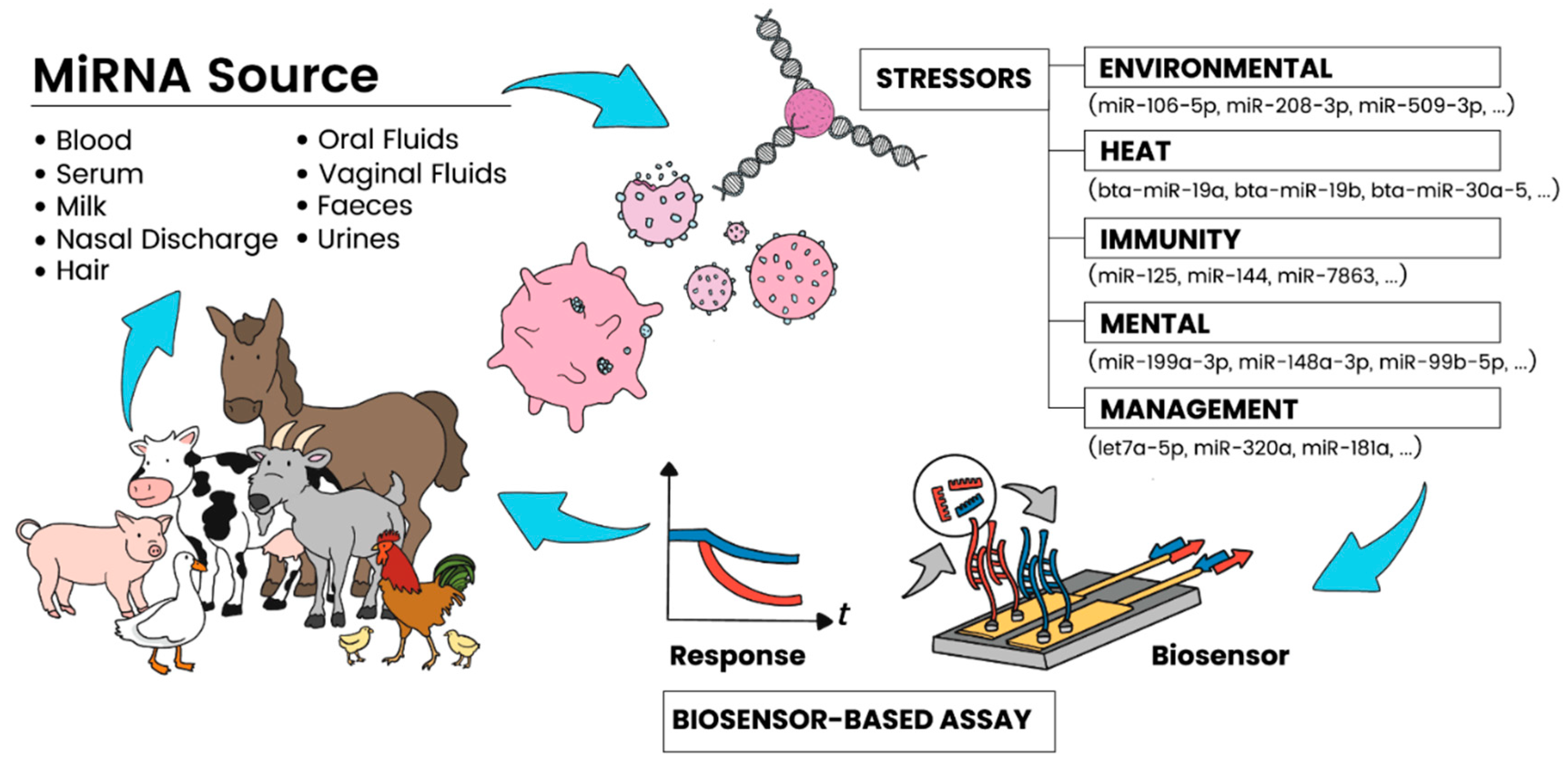
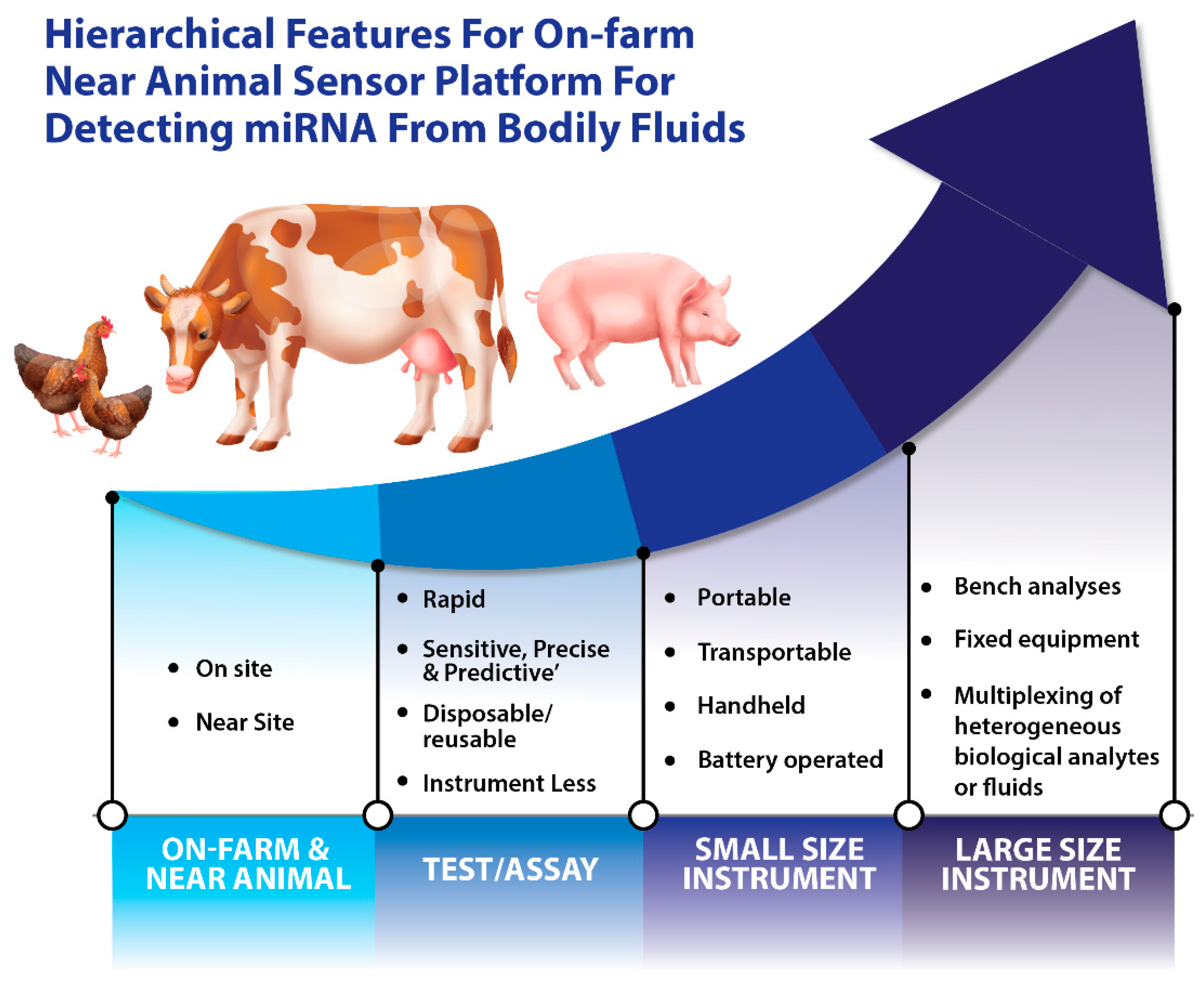
Abstract
Globally, the livestock sector represents a $1.4 trillion market employing at least 1.3 billion people. However, the farming of animals for food raises complex questions regarding livestock diseases and their potential impacts on both human health and national and international export trade markets. Early disease detection in livestock allows for targeted treatments, decreasing the antibiotics use, and for advancements in precision veterinary medicine. MicroRNA (miRNA)-driven signaling cascades play a crucial role in the context of farm animal disease diagnostics and prediction, and their proper understanding remains a challenge. Many studies have shown a link between circulating miRNAs and diseases in livestock, including paratuberculosis, foot and mouth disease, and various metabolic diseases. Important information regarding the stage, pathogenesis, and prognosis of a disease may therefore be acquired through the detection and analysis of a small number of miRNAs. Currently, there are no on-farm sensing tools available to detect miRNAs from the bodily fluids of livestock. This review is aimed at demonstrating that circulating miRNAs are powerful biomarkers of disease in livestock and at describing the potential of sensor technologies for their rapid detection. I provide an overview of the recent developments of miRNA sensing and the current bottlenecks in the realization of the sensors for detecting miRNAs as the target analytes for the identification of various livestock diseases. Due to the nascent stages of this research, the exploitation of miRNA as a biomarker opens up ways to move from reactive to predictive possibilities in disease detection via sensor platforms in modern digital livestock farming.
1. MicroRNAs: Small Molecules, High Impact
The past two decades have seen a revolution in the development of rapid, low-cost, and high-specificity molecular biology techniques for characterizing and quantitating DNA, RNA, and proteins. Within cells, numerous species of RNA molecules exist. These include messenger RNA (mRNA), ribosomal RNA (rRNA), and transfer RNA (tRNA). RNAs can be further classified as either coding (cRNA) or non-coding RNA (ncRNA) [1]. There are also two types of ncRNAs, namely, housekeeping ncRNAs (tRNA and rRNA), and regulatory ncRNAs. ncRNAs are typically classified based on their size. Long ncRNAs (lncRNAs) are at least 200 nucleotides long. In contrast, ncRNAs contain typically fewer than 200 nucleotides. MicroRNAs (miRNA) are a species of regulatory ncRNAs. Others in this category include small interference RNAs (siRNAs), long non-coding RNAs (lncRNAs), circular RNAs (circRNAs), piwi-associated RNAs (piRNAs), and tRNAs [2]. Most recently, there has been significant interest surrounding the role of non-coding RNAs as active modulators of protein-coding gene functions. MicroRNAs (miRNAs) comprise a family of endogenously expressed, small (∼22 nucleotides in length), non-coding transcripts. The binding of miRNAs to complementary sequences in the 3’-untranslated regions of target mRNAs results in the posttranscriptional regulation of both gene expression and protein translation via the inhibition of translation initiation and elongation [3]. miRNAs are capable of regulating the expression of genes involved in various biological processes, including signal transduction [4], cell cycle [5], differentiation [6], proliferation [7], and apoptosis [8]. Endogenous mRNAs contain multiple target sites for several miRNAs [9]. miRNAs are able to regulate most protein-coding transcripts, with the most recent estimates suggesting that at least 60% of gene expression is regulated by miRNAs [10]. In some cases, miRNAs are known to target a large number (100 to 200) of different mRNAs [11]. Endogenous transcription factors are known to be able to repress and enhance the expression of miRNA depending on the availability of a particular factor [12]. In the context of disease states, miRNA genetic variability has been hypothesized to play a role in the susceptibility of livestock to diseases [13].
2. Role of miRNAs in Livestock Diseases and Conditions
Extracellular miRNAs have been found in the peripheral blood of a number of animal species, including bovines [14], poultry [15], and pigs [16]. As a result of their high stability in the body fluids of livestock, there is significant interest in the use of circulating miRNA as biomarkers for both the detection and the monitoring of disease progression in animals [17,18]. Expression-profiling studies conducted in domestic livestock have determined that miRNAs have distinct spatial (e.g., cell and tissue) and temporal expression patterns [19]. The aberrant expression of miRNAs can impair the regulation of several cellular functions and genetic networks. Studies have shown a link between circulating miRNAs and diseases in livestock, including paratuberculosis [20], foot and mouth disease [21], and various metabolic diseases [22]. As such, important information regarding the stage, pathogenesis, and prognosis of a disease may be obtained through the detection and analysis of a small number of miRNAs. miRNAs have been detected in several bodily fluids of animals, including plasma, serum, and milk. Typically, miRNAs are released into extracellular environments in protein complexes or within micro vesicles or exosomes [23]. There is increasing evidence that specific miRNAs are altered in various disease states in livestock [24]. Similarly, for many livestock diseases, miRNAs have been found to play important roles in predicting the health status, resilience, and even the mental function. Correlations between multiple pathogens, including bovine viral diarrhea virus, Mycoplasma bovis, and Staphylococcus aureus, and miRNAs with differential expression have been reported in cattle.
2.1. Immunity
By optimizing the differentiation and function of immune cells, a role for miRNAs in bovine immunity has been proposed [25]. miRNAs have been hypothesized to play a vital role in bovine immunity by influencing immune cell differentiation and function. The differential expression of miRNAs has been reported in bovines in response to infection by pathogens. These include Mycoplasma bovis, bovine viral diarrhea virus, and Staphylococcus aureus [26,27,28].
2.2. Mycobacterium Avium ssp. Paratuberculosis
Changes in the levels of circulating miRNAs have been identified in the serum [20], whole blood [29], and ileal tissues [30] of cattle infected with Mycobacterium avium ssp. paratuberculosis (MAP). MAP-infected cattle display progressive granulomatous inflammation of the ileum giving rise to a condition known as Johne’s Disease. This disease causes diarrhea, weight loss, and even death in animals and results in considerable losses to the agriculture industry world-wide every year.
2.3. Foot and Mouth Disease
This is a contagious viral disease that highly affects cloven-hoofed animals (those with divided hoofs), including cattle, sheep, goats, pigs, deer, buffalo, and camels.
2.4. Heat Stress in Livestock
The detection of specific miRNAs in the blood of cows is an important tool for assessing the responses of animals to heat stress. Elevated environmental temperatures can negatively affect the health and productivity of animals. In a study by Lee et al. [9] blood samples from cows were collected to check the expression of both mRNA and miRNA in different environmental conditions. A total of 11 miRNAs (bta-miR-19a, bta-miR-30a-5p, bta-miR-19b, and several from the bta-miR-2284 family) were differentially expressed in both pregnant and non-pregnant cows under heat stress conditions. Another study of the effect of heat stress on pregnant cows showed that the miRNAs bta-miR-146b, bta-miR-29d-3p, bta-miR-1246, and bta-miR-20b that specifically target progesterone biosynthesis (StAR) and the function of corpus luteum-related genes (CCL11, XCL) were differentially expressed [31,32,33]. Similarly, a study by Li et al. [34] showed that in heat-stressed Holstein cattle, 20 miRNAs had higher levels of expression in their mammary tissues. The seven differentially expressed candidate miRNAs were bta-miR-21-5p, bta-miR-29c, bta-miR-2285 t, bta-miR-146b, bta-miR-145, bta-miR-133a, and bta-miR-99a-5p.
2.5. Tumorigenesis and Cancer
Recent studies have highlighted the important role that abnormal miRNA expression plays in tumorigenesis and in the pathogenesis of various cancers [33]. In livestock, cancer is a highly problematic disease owing to its high rate of mortality and expensive treatments, which often have only limited success. The earlier detection of tumors and the estimation of correct tumor subtypes can significantly aid in the determination of appropriate treatment regimens that can help lower the time and incurred risks in treating infected animals [34]. Additionally, if appropriate treatment is started before the disease reaches an advanced stage, the cost of the risks can be curtailed. miRNAs may therefore have a significant diagnostic value for detecting and possibly predicting certain cancers.
2.6. Pregnancy and Lactation
In bovines, miRNAs are known to be present in the mammary glands; however, they are not present in the glands of animals who are either non-pregnant or non-lactating [35]. In milk, miRNAs are present in exosomes and are released into the body fluids by different types of cells. miRNAs contribute to the regulation of follicular and luteal development along with the endometrial function. In the mammary glands themselves, miRNAs play important roles in several cellular processes. The expression of miR-10a, miR-33b, miR-15b, miR-16, miR-21, miR-31, miR-181a, miR-145, miR-146b, miR-223, miR-205, miR-221, and miR-155, has been studied -30 days prepartum, 7 days postpartum, and 30 days postpartum [36]. When analyzed, all miRNAs apart from miR-31 showed increases in their expression between -30 days prepartum and 7 days post-partum. Similarly, the expression of miR-221 was shown to further increase 30 days postpartum, which corresponded to early lactation. As a result, a role for miR-221 in the control of endothelial cell proliferation and/or angiogenesis was proposed. In contrast, miR-223 was found to be decreased at early lactation, which suggests that it may play a role in the mammary response to pathogens in the period following parturition. miR-31, a known inhibitor of cyclin gene expression which is hormonally regulated, was found to be expressed at higher levels at timepoints corresponding to early lactation when compared to -30 days prepartum. Similarly, the upregulation of miR-33b during early lactation may play a role in lipogenesis in the mammary tissue. The availability and detection of miRNAs in cattle milk also depicts the role of miRNAs in the gastrointestinal system and their potential role in modulating the immune system. One of the most critical miRNAs, microRNA-145 (mir-145), is a potent tumor suppressor [37] that regulates multiple cellular pathways, including those involved in regulating heat stress in the mammary tissues. MicroRNA-145 has been shown to be downregulated in many types of cancer. It regulates several cellular processes, including entry into the cell cycle, proliferation, and apoptosis. Additionally, mir-145 has been shown to play a critical role in cellular invasion through the targeting of multiple oncogenes [38].
Using small RNA sequencing and RT-qPCR, Ioannidis et al. [15] were able to identify 92 miRNAs that showed higher expression in the plasma compared with paired blood cell samples (n = 4 cows). Interestingly, three miRNAs—miR-133a (muscle), miR-122 (liver), and miR-215 (intestine)—were found to be enriched in tissues, while miR-802 which reportedly regulates insulin sensitivity and lipid metabolism, a key parameter in the context of post-partum negative energy balance in dairy cows, was highly enriched specifically in the liver (Table 1). Another significant role of miRNAs in livestock is in pregnancy diagnosis, most notably as they act as biomarkers for early pregnancy detection. Circulating miRNA signatures of early pregnancy in cattle have been determined. MiRNAs have been shown to regulate various biological functions, including ovarian function, embryonic development, uterine receptivity, and placental function [39].

Table 1.
MiRNAs as indicators of physiological, behavioral, and biological functions of livestock.
2.7. Endometritis
This is a significant reproductive disorder in dairy cattle. The disorder tends to result in reduced fertility and milk production. The endometrium of cows serves to act as a barrier to the pathogen invasion of uterine tissues. By controlling the inflammatory immune response, endometrial epithelial cells are able to mount a response for resisting pathogen invasion [42]. miR-21-3p has been suggested to play an important role in promoting the viability and proliferation of the epithelial tissue in the mammary glands of dairy cows [48].
3. Challenges to MiRNA Detection
The on-field detection of miRNA for disease recognition is vital to ensuring not only the future safety of our food supply but also the economic viability of farmers across the world. In light of the recent emergence of new epidemic- and pandemic-causing diseases, the need for tools for rapidly identifying infections will be crucial in halting cross-species disease transmission. To be able to understand the role that miRNA plays in the pathogenesis of disease in livestock, sensor tools and sensing platforms (Figure 1) for detecting the levels of expression of these molecules become essential. However, significant challenges remain in the reliable sensing of miRNA from blood and other bodily fluids or target analytes of livestock because of their small size, low abundance in samples, and high sequence similarity. A critical factor is their susceptibility to degradation.
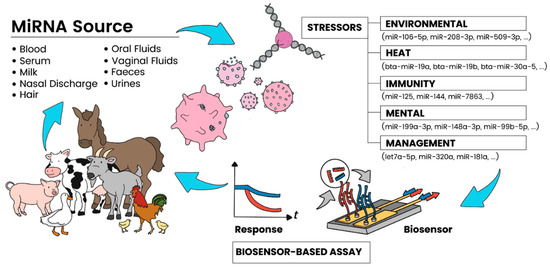
Figure 1.
Schematic showing the various sources of miRNA targets in livestock and their relationship to causal agents, as well as the potential of sensing approaches for rapid, on-farm applications.
4. MiRNA Research: Technological Advancements
Since the discovery of miRNAs, research into their detection and characterization has given rise to a rather exciting field. miRBase (version 22) [49] provides data for 38,589 pre-miRNAs from 271 organisms, including livestock. For a better understanding of miRNA functions and to fully understand the complete repertoire of their biological roles, new sensing platforms and sensor technologies are required. Traditional methods for miRNA detection include quantitative polymerase chain reaction (qPCR) [50], microarray analysis [51], and northern blotting [52]. However, these methods for miRNA detection and analysis are not capable of meeting the current needs such as real-time data transmission, high transmission speed, and on-farm deployment. Additional drawbacks of the existing sensing methods include the relatively high cost of equipment and reagents, time-consuming protocols, their limited use with different input samples (e.g., blood versus feces), and their low detection sensitivity. The hierarchical features of on-farm and near-animal sensor platforms for detecting miRNAs from animal bodily fluids can be seen in Figure 2.
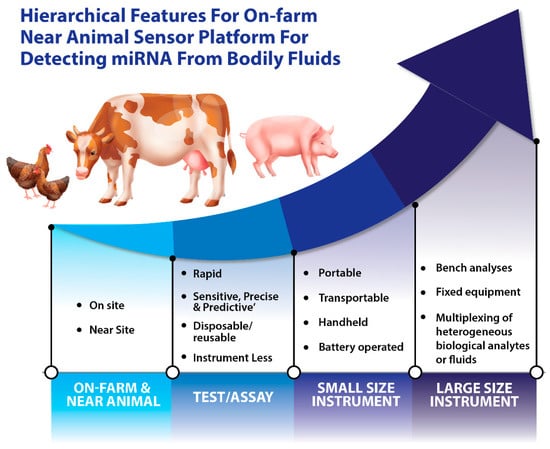
Figure 2.
Hierarchical features of on-farm and near-animal sensor platforms for detecting miRNAs from bodily fluids.
4.1. Next-Generation Sequencing (NGS)
NGS is a valuable tool for investigating the complexity of the miRNA transcriptome. NGS is a unique method most commonly used in animal science and veterinary research [53]. The use of high-throughput NGS technologies in livestock research has enabled not only developments in metagenomics and genome-wide association studies but also an elucidation of the role of miRNAs in livestock health. A novel NGS approach was used to profile the expression of miRNAs in primary bovine mammary epithelial cells following infection with Streptococcus uberis, a causative agent of bovine mastitis [54]. An analysis of over 450 million sequencing reads identified 21 miRNAs as differentially expressed post-infection with S. uberis. For example, the miRNA bta-let-7 was upregulated at early time-points post-infection (4- and 6 h). The let-7 family is known to play a role in the immune response.
4.2. RNA Sequencing (RNA-Seq)
This is one of the major technology advancements in the study of ncRNA species, such as miRNA. RNA-Seq offers researchers the ability to identify change(s) in the levels of expression of RNA species that cannot currently be detected using PCR, microarray-, or Northern blotting-based platforms. However, RNA-Seq is costly and requires specialized software and hardware (i.e., because of intensive computing power). Importantly, RNA-Seq is capable of identifying transcript variants of miRNA or miRNA isomers (“isomiRs”). IsomiRs are responsible for the large diversity of miRNA sequence variants (“isomiRs”), which result from the processing or post-transcriptional modification of miRNAs. For example, changes to the seed sequence (nucleotides 2–8), including shifted start positions of a miRNA, can result in the redirection of targeting to a different set of target RNAs.
4.3. MinION and GridION
These related technologies were developed by Oxford Nanopore Technologies for the direct electronic analysis of proteins, RNA, DNA, and single molecules. MinION is focused on the identification of DNA bases by measuring the changes in electrical conductivity generated as DNA strands pass through a biological pore [55,56]. The GridION technology allows the simple scaling of MinION and Flongle Flow Cells. With GridION, users can simultaneously run up to five flow cells, enabling the generation of as much as 150 Gb sequence data in a single run. These tools offer applications in portable sensing and were extensively used in field diagnosis during the African swine fever outbreak in China in 2020 [57]. A future application of this technology is “crush-side genotyping,” which has been specifically designed for the real-time, on-farm genotyping of livestock. MinION sequencing has been used to functionally characterize S. suis bacteria in pigs. These bacteria are important pathogens for pigs and have recently developed antimicrobial resistance. Whole-genome generation and characterization profiling, including miRNA sequences, were successfully carried out using the MinION technology.
4.4. Ion Torrent Sequencing
Established in 2010, the protocol for Ion Torrent technology is simple, requiring a four-step workflow: library construction, template preparation, sequencing, and analysis. Ion Torrent sequencing is not based on dye-labeled oligonucleotides or any expensive optics. Istead, it uses measurements of H+ ions released during base incorporation and it is uniquely suited for amplicon sequencing. The sequencing method was employed to identify differentially expressed microRNAs in the Sahiwal [58] and Frieswal breeds of cattle during heat stress [59]. In the latter study, out of a total of 420 miRNAs investigated, several were identified as heat shock responsive genes, especially members of the heat shock protein family. Of the 420, a total of 65 miRNAs were differentially expressed during the peak summer temperatures, including bta-miR-2898, which is known to target HSPB8 (heat shock protein 22) [59]. Ion sequencing has also been used to determine the profiles of expression of host and viral miRNAs in pigs with Aujeszky’s disease virus (suid herpesvirus type 1 [SuHV-1]) [44]. The data showed that miR-206, miR-133b, miR-133a, and miR-378 were differentially expressed in animals that were infected with the virus.
4.5. Electrochemical Sensing
Approaches to electrochemical sensing include amperometric and potentiometric, voltammetric, impedimetric, conductometric, and field-effect transistor-based biosensors [60,61]. Amperometry-based sensors detect analytes by quantifying the current at a constant applied potential. The measurement of the current is then related to the concentration of the target analyte. In contrast, voltametric measurements measure the current as the potential is raised at a given rate. The labeling and electrocatalytic amplification of miRNAs offers a promising approach to their detection. Gao et al. developed a technique for the sensing of nucleic acids with a silicon nanowire field effect transistor biosensor [24]. Using this technique, Gao et al. successfully detected miRNA in animal cells. A similar technique using hybridized miRNA-templated deposition of a polymer film and electrochemical impedance spectroscopic detection was able to detect miRNA at concentrations ranging from 5.0 fM to 2.0 pM, with a detection limit of 2.0 fM [62,63].
A similar sensor developed by Yin et al. [22] used a label-free free, electrochemical-based biosensor to detect miRNA. The detection method employed dendritic gold nanostructures in combination with a graphene nanosheets-modified glassy carbon electrode. Using thiol-modified locked-nucleic acid (LNA) hairpin molecular beacons (MB), nucleic acids were successfully captured. The duplex formed from the hybridization with the target miRNA was then probed based on the complementarity to the distal terminus of the capture probe. Modification with biotin-coated gold nanoparticles and detection using streptavidin-modified horseradish peroxidase permitted the chemical oxidation of the hydro-quinone to benzoquinone following the addition of hydrogen peroxide. The biosensor was able to detect miRNAs at a concentration as low as 0.06 pM.
4.6. Loop-Mediated Isothermal Amplification (LAMP)
The LAMP techniques offer the advantage that they can be performed without the need for a precise control of temperature cycling (compared with PCR) [56,57]. The technique is well suited for detecting short RNA sequences, such as miRNAs [64]. As a result of the relatively mild reaction conditions, LAMP enables the detection of miRNA in single cells, which can be vital to determining the biological reactions to infection in lineage-specific cells. Ge et al. used a novel and ultrasensitive detection platform for microRNA detection. This method works by combining tetrahedron-structured, DNA nanostructure probes in conjunction with an HCR amplification [65]. MicroRNAs could be successfully detected at concentrations as low as 10 aM (corresponding to 600 microRNAs in a 100 μL sample). This method was able to improve the detection limits by three orders of magnitude when compared with super-sandwich amplification [66]. SDA assays allow the exponential amplification of miRNA. The process is miRNA-initiated through a Klenow fragment polymerase, a nicking enzyme (such as Nt.AlwI), and two primers. During the process, the miRNA target triggers two cycles consisting of nicking, polymerization, and displacement. As a result of exponential miRNA amplification, the dsDNA products that are formed can be measured at concentrations as low as 16 zmol of the target miRNA, using SYBR Green I real-time PCR, and within 90 min [67].
A microRNA detection method that was developed by Gines et al. [33] uses isothermal amplification chemistry. This method was built around molecular programming concepts using a DNA circuit capable of converting, thresholding, amplifying, and reporting the presence of specific microRNAs within samples. The method is extremely sensitive and could detect specific miRNAs with absolute target quantification even at femtomolar concentrations. Importantly, the method can reliably suppress nonspecific amplifications that are typically encountered with other exponential amplification reactions. Most recently, the label-free detection of miRNA was performed using surface-enhanced Raman spectroscopy (SERS). This surface-sensitive technology is based on the phenomenon that molecules boost Raman scattering adsorbed on the surface of plasmonic metals or nanostructures of plasmonic metals, owing to the strong electromagnetic coupling generated in the vicinity. This technique is highly effective for the detection of nucleic acids because of its distinct benefits of high stability, strong specificity, and low background noise [68,69]. In a SERS study by Li et al., titanium ions were used as aggregating agents that were subsequently employed to induce the aggregation of silver nanoparticles. As a result of this aggregation, “hot spots” were formed, allowing for fingerprint information on the miRNAs to be obtained.
Finally, nanobiosensors made of graphene oxide and a DN- binding dye have been shown as possible sensing approaches for the detection of multi-miRNAs [70]. In that study, the assay was fluorescence-based and used an isothermal hybridization chain reaction. SYBR Green was used as the signal, while graphene oxide was used as the fluorescence quencher. Fluorescence spectrophotometry was used for the detection and quantification of various targets miRNA. This novel graphene nanobiosensor was able to achieve a limit of detection for miRNAs from 0.05 to 5 nM—a range which is often considered to be ideal for detecting candidate biomarkers. A similar sensor involving a sandwich hybridization of a capture probe immobilized on a magnetic bead and a reporter probe assembled on gold nanoparticles with a miRNA target was developed by Wen et al. [70]. As a result of the gold nanoparticle-catalyzed enhancement of silver staining, miRNAs could be detected in less than 70 min at a lower-level detection limit of 15 fM.
4.7. Surface Plasmon Resonance
The ultrasensitive detection of miRNAs has been accomplished using a number of SPR techniques [71,72]. A label-free, antimonene-based SPR sensor was demonstrated by Xue et al. for miRNA detection [73]. The detection limit for miRNAs reached 10 aM. Specifically, miRNA-21, whose upregulation is associated with numerous types of cancers along with miRNA-155, which plays a significant role in the immune response, could be detected at levels 2.3–10,000 times higher than those of existing miRNA sensors. SPR has been merged with orthogonal signal amplification to facilitate the direct determination of sub-fM concentrations of miRNAs [74]. Orthogonal signal amplification uses an in-plane and vertical signal amplification strategy to add additional mass on a target sample initially at the surficial direction and subsequently upwards from the surface. Using this technique, investigators were able to reach a detection limit and quantification limit of 0.56 and 5 fM for miR-NA-15a. This represented a 107-fold improvement of sensitivity compared to other SPR methods. miRNA-15a plays a critical role in gene regulation and tumor suppression.
Investigations on the function of miRNAs and their interaction with genes in the modulation of macronutrients in animals showed that dietary macronutrients and the expression of specific genes and miRNA were directly related to the metabolism of these compounds [75,76]. Extracellular miRNAs were found to be particularly promising markers of metabolic changes. In fact, circulating miR-935 was identified as a potential biomarker in individuals who responded differentially to weight loss interventions induced by energy restriction. This raises the possibility of using this type of miRNA in sensors to monitor health-related changes in the metabolism of animals based on their diets.
The potential use of miRNAs as biomarkers to evaluate the stress of livestock and improve their welfare was recently evaluated by Miretti et al. [55]. Cells actively secrete these highly stable molecules into the extracellular environment where they can be easily collected, from saliva, blood, exosomes, or milk. In one study on pigs, the levels of expression of certain salivary miRNAs were more abundant in animals that had been exposed to high levels of pain, making them good candidates for potential biomarkers for pain. In another study, miR-914b-5p showed promise as a candidate biomarker for the intestinal health of piglets during weaning. Both cattle and swine are highly sensitive to heat stress, which has been correlated with the levels of other miRNA molecules. The authors stressed that accurately identifying and validating miRNAs and their target genes is indispensable to develop gene-based breeding strategies. Other miRNAs are upregulated following hypoxia and represent potential biomarkers for immune responses to inflammatory stimuli. These studies raise the possibility that further research on these regulatory molecules will enable their use in biosensors to monitor the stress levels of livestock and therefore improve their welfare.
Given the importance of detecting miRNAs as markers of a wide range of physiological conditions, researchers have been developing new techniques to enhance the sensitivity of the methods used to detect miRNAs and measure their concentrations in bodily fluids and tissues. Current cutting-edge techniques include probes that utilize the principles of fluorescence resonance energy transfer (FRET) and bioluminescent resonance energy transfer (BRET).
Progress in utilizing BRET techniques was elucidated by Li et al. [32], who developed a paper-based system that was highly stable at room temperature and could detect femtomolar concentrations of miRNAs. Chen et al [16] reviewed the developments in the use of new fluorescent hybridization probes to improve the sensitivity of existing probes. Using nucleic acid probes coupled with gold nanoparticles, miRNAs could be detected at concentrations of 1.5 femtomolars with the FRET technique. The ability to measure such low concentrations will enable the detection of trace amounts of these molecules in real samples and could also distinguish homologous miRNAs. These results bode well for the future use of these techniques in biosensors to quantify key miRNAs that are associated with the effects of stress on animals.
5. Conclusions
The livestock industry faces significant challenges in managing disease in animals. Specific types of circulating miRNAs have been correlated with specific diseases or other conditions, such as pregnancy. The accurate quantification of these molecules is paramount for their routine use in disease detection and can enable the early diagnosis of critical health conditions. A goal in animal care is to have biosensors that monitor the changes in hormones and miRNAs that mirror an animal’s condition (outstanding questions). Recent research has identified techniques that can identify specific miRNAs at femtomolar concentrations. These include fluorescence resonance energy transfer (FRET) and bioluminescent resonance energy transfer (BRET). Ideally, these techniques will be adapted for use in biosensors that will revolutionize the well-being of livestock and therefore the livestock industry.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An Overview of MicroRNAs: Biology, Functions, Therapeutics, and Analysis Methods. J. Cell. Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef]
- Baptista, B.; Riscado, M.; Queiroz, J.A.; Pichon, C.; Sousa, F. Non-coding RNAs: Emerging from the discovery to therapeutic applications. Biochem. Pharmacol. 2021, 189, 114469. [Google Scholar] [CrossRef] [PubMed]
- Fabian, M.R.; Sonenberg, N.; Filipowicz, W. Regulation of MRNA Translation and Stability by MicroRNAs. Annu. Rev. Biochem. 2010, 79, 351–379. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Deng, H.; Shen, W.; Ren, Y. A Label-Free Biosensor for Electrochemical Detection of Femtomolar MicroRNAs. Anal. Chem. 2013, 85, 1624–1630. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Lv, M.; Li, J.; Yu, R.; Jiang, J. A Ligation-Based Loop-Mediated Isothermal Amplification (Ligation-LAMP) Strategy for Highly Selective MicroRNA Detection. Chem. Commun. 2016, 52, 12721–12724. [Google Scholar] [CrossRef] [PubMed]
- Rahaie, M.; Noroozi, S.K. A Nanobiosensor Based on Graphene Oxide and DNA Binding Dye for Multi-MicroRNAs Detection. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Li, Z.; Moore, P.S.; Monaghan, A.P.; Chang, Y.; Nichols, M.; John, B. A Sensitive Non-Radioactive Northern Blot Method to Detect Small RNAs. Nucleic Acids Res. 2010, 38, e98. [Google Scholar] [CrossRef]
- Liang, G.; Malmuthuge, N.; Guan, Y.; Ren, Y.; Griebel, P.J.; Guan, L.L. Altered MicroRNA Expression and Pre-MRNA Splicing Events Reveal New Mechanisms Associated with Early Stage Mycobacterium Avium Subspecies Paratuberculosis Infection. Sci. Rep. 2016, 6, 24964. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, S.; Son, J.; Lim, H.; Kim, E.; Kim, D.; Ha, S.; Hur, T.; Lee, S.; Choi, I. Analysis of Circulating-MicroRNA Expression in Lactating Holstein Cows under Summer Heat Stress. PLoS ONE 2020, 15, e0231125. [Google Scholar] [CrossRef]
- Casas, E.; Cai, G.; Kuehn, L.A.; Register, K.B.; McDaneld, T.G.; Neill, J.D. Association of MicroRNAs with Antibody Response to Mycoplasma Bovis in Beef Cattle. PLoS ONE 2016, 11, e0161651. [Google Scholar] [CrossRef]
- Schanzenbach, C.I.; Kirchner, B.; Ulbrich, S.E.; Pfaffl, M.W. Can Milk Cell or Skim Milk MiRNAs Be Used as Biomarkers for Early Pregnancy Detection in Cattle? PLoS ONE 2017, 12, e0172220. [Google Scholar] [CrossRef] [PubMed]
- Otto, T.; Candido, S.V.; Pilarz, M.S.; Sicinska, E.; Bronson, R.T.; Bowden, M.; Lachowicz, I.A.; Mulry, K.; Fassl, A.; Han, R.C.; et al. Cell Cycle-Targeting MicroRNAs Promote Differentiation by Enforcing Cell-Cycle Exit. Proc. Nat. Acad. Sci. USA 2017, 114, 10660–10665. [Google Scholar] [CrossRef]
- Li, Q.; Yang, C.; Du, J.; Zhang, B.; He, Y.; Hu, Q.; Li, M.; Zhang, Y.; Wang, C.; Zhong, J. Characterization of MiRNA Profiles in the Mammary Tissue of Dairy Cattle in Response to Heat Stress. BMC Genom. 2018, 19, 975. [Google Scholar] [CrossRef] [PubMed]
- Correia, C.N.; Nalpas, N.C.; McLoughlin, K.E.; Browne, J.A.; Gordon, S.V.; MacHugh, D.E.; Shaughnessy, R.G. Circulating MicroRNAs as Potential Biomarkers of Infectious Disease. Front. Immunol. 2017, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.; Donadeu, F.X. Circulating MiRNA Signatures of Early Pregnancy in Cattle. BMC Genom. 2016, 17, 184. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, C. Coding or Noncoding, the Converging Concepts of RNAs. Front. Genet. 2019, 10, 496. [Google Scholar] [CrossRef]
- Ioannidis, J.; Donadeu, F.X. Comprehensive Analysis of Blood Cells and Plasma Identifies Tissue-Specific MiRNAs as Potential Novel Circulating Biomarkers in Cattle. BMC Genom. 2018, 19, 243. [Google Scholar] [CrossRef]
- Chen, M.; Yao, Y.L.; Yang, Y.; Zhu, M.; Tang, Y.; Liu, S.; Li, K.; Tang, Z. Comprehensive Profiles of MRNAs and MiRNAs Reveal Molecular Characteristics of Multiple Organ Physiologies and Development in Pigs. Front. Genet. 2019, 10, 756. [Google Scholar] [CrossRef]
- Gupta, S.K.; Maclean, P.H.; Ganesh, S.; Shu, D.; Buddle, B.M.; Wedlock, D.N.; Heiser, A. Detection of MicroRNA in Cattle Serum and Their Potential Use to Diagnose Severity of Johne’s Disease. J. Dairy Sci. 2018, 101, 10259–10270. [Google Scholar] [CrossRef]
- Muroya, S.; Shibata, M.; Hayashi, M.; Oe, M.; Ojima, K. Differences in Circulating MicroRNAs between Grazing and Grain-Fed Wagyu Cattle Are Associated with Altered Expression of Intramuscular MicroRNA, the Potential Target PTEN, and Lipogenic Genes. PLoS ONE 2016, 11, e0162496. [Google Scholar] [CrossRef]
- Sengar, G.S.; Deb, R.; Singh, U.; Raja, T.V.; Kant, R.; Sajjanar, B.; Alex, R.; Alyethodi, R.R.; Kumar, A.; Kumar, S.; et al. Differential Expression of MicroRNAs Associated with Thermal Stress in Frieswal (Bos Taurus x Bos Indicus) Crossbred Dairy Cattle. Cell Stress Chaperon. 2018, 23, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhou, Y.; Zhang, H.; Meng, X.; Ai, S. Electrochemical Determination of MicroRNA-21 Based on Graphene, LNA Integrated Molecular Beacon, AuNPs and Biotin Multifunctional Bio Bar Codes and Enzymatic Assay System. Biosens. Bioelectron. 2012, 33, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Labib, M.; Berezovski, M.V. Electrochemical Sensing of MicroRNAs: Avenues and Paradigms. Biosens. Bioelectron. 2015, 68, 83–94. [Google Scholar] [CrossRef]
- Gao, A.; Lu, N.; Wang, Y.; Dai, P.; Li, T.; Gao, X.; Wang, Y.; Fan, C. Enhanced Sensing of Nucleic Acids with Silicon Nanowire Field Effect Transistor Biosensors. Nano Lett. 2012, 12, 5262–5268. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Qiao, Z.; He, X.; Yu, Y.; Lei, Y.; Tang, J.; Shi, H.; He, D.; Wang, K. Enzyme-Free Amplified Detection of MiRNA Based on Target-Catalyzed Hairpin Assembly and DNA-Stabilized Fluorescent Silver Nanoclusters. Analyst 2020, 145, 5194–5199. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and Exosomal MicroRNA: Trafficking, Sorting, and Function. Genom. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef]
- Shi, C.; Liu, Q.; Ma, C.; Zhong, W. Exponential Strand-Displacement Amplification for Detection of MicroRNAs. Anal. Chem. 2014, 86, 336–339. [Google Scholar] [CrossRef]
- Cammaerts, S.; Strazisar, M.; De Rijk, P.; Del Favero, J. Genetic Variants in MicroRNA Genes: Impact on MicroRNA Expression, Function, and Disease. Front. Genet. 2015, 6. [Google Scholar] [CrossRef]
- Horikawa, A.; Ogasawara, H.; Okada, K.; Kobayashi, M.; Muroya, S.; Hojito, M. Grazing-Induced Changes in Muscle MicroRNA-206 and -208b Expression in Association with Myogenic Gene Expression in Cattle. Anim. Sci. J. 2015, 86, 952–960. [Google Scholar] [CrossRef]
- Basagoudanavar, S.H.; Hosamani, M.; Tamil Selvan, R.P.; Sreenivasa, B.P.; Sanyal, A.; Venkataramanan, R. Host Serum MicroRNA Profiling during the Early Stage of Foot-and-Mouth Disease Virus Infection. Arch. Virol. 2018, 163, 2055–2063. [Google Scholar] [CrossRef]
- Sengar, G.S.; Deb, R.; Singh, U.; Junghare, V.; Hazra, S.; Raja, T.V.; Alex, R.; Kumar, A.; Alyethodi, R.R.; Kant, R.; et al. Identification of Differentially Expressed MicroRNAs in Sahiwal (Bos Indicus) Breed of Cattle during Thermal Stress. Cell Stress Chaperon. 2018, 23, 1019–1032. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Zhang, K.; Li, J. Isothermal Amplification for MicroRNA Detection: From the Test Tube to the Cell. Accounts Chem. Res. 2017, 50, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Gines, G.; Menezes, R.; Nara, K.; Kirstetter, A.-S.; Taly, V.; Rondelez, Y. Isothermal Digital Detection of MicroRNAs Using Background-Free Molecular Circuit. Sci. Adv. 2020, 6, eaay5952. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xia, L.; Zhou, Q.; Wang, L.; Chen, D.; Gao, X.; Li, Y. Label-Free Detection of MiRNA Using Surface-Enhanced Raman Spectroscopy. Anal. Chem. 2020, 92, 12769–12773. [Google Scholar] [CrossRef]
- Persano, S.; Guevara, M.L.; Wolfram, J.; Blanco, E.; Shen, H.; Ferrari, M.; Pompa, P.P. Label-Free Isothermal Amplification Assay for Specific and Highly Sensitive Colorimetric MiRNA Detection. ACS Omega 2016, 1, 448–455. [Google Scholar] [CrossRef]
- Rosa, A.; Ballarino, M. Long Noncoding RNA Regulation of Pluripotency. Stem Cells Int. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Yao, S. MicroRNA Biogenesis and Their Functions in Regulating Stem Cell Potency and Differentiation. Biol. Proced. Online 2016, 18, 8. [Google Scholar] [CrossRef]
- Li, W.; Ruan, K. MicroRNA Detection by Microarray. Anal. Bioanal. Chem. 2009, 394, 1117–1124. [Google Scholar] [CrossRef]
- Ouyang, T.; Liu, Z.; Han, Z.; Ge, Q. MicroRNA Detection Specificity: Recent Advances and Future Perspective. Anal. Chem. 2019, 91, 3179–3186. [Google Scholar] [CrossRef]
- Ye, D.; Shen, Z.; Zhou, S. Function of microRNA-145 and mechanisms underlying its role in malignant tumor diagnosis and treatment. Cancer Manag. Res. 2019, 11, 969–979. [Google Scholar] [CrossRef]
- Taxis, T.M. MicroRNA Expression and Implications for Infectious Diseases in Livestock. CAB Rev. Perspect. Agric. Veter. Sci. Nutr. Nat. Resour. 2017, 2017, 12. [Google Scholar] [CrossRef]
- Wang, M.; Moisá, S.; Khan, M.J.; Wang, J.; Bu, D.; Loor, J.J. MicroRNA Expression Patterns in the Bovine Mammary Gland Are Affected by Stage of Lactation. J. Dairy Sci. 2012, 95, 6529–6535. [Google Scholar] [CrossRef] [PubMed]
- Lenkala, D.; LaCroix, B.; Gamazon, E.R.; Geeleher, P.; Im, H.K.; Huang, R.S. The Impact of MicroRNA Expression on Cellular Proliferation. Hum. Genet. 2014, 133, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-Z.; Chen, Y.; Guan, L.L. MicroRNA Expression Profiles across Blood and Different Tissues in Cattle. Sci. Data 2019, 6, 190013. [Google Scholar] [CrossRef] [PubMed]
- Timoneda, O.; Núñez-Hernández, F.; Balcells, I.; Muñoz, M.; Castelló, A.; Vera, G.; Pérez, L.J.; Egea, R.; Mir, G.; Córdoba, S.; et al. The Role of Viral and Host MicroRNAs in the Aujeszky’s Disease Virus during the Infection Process. PLoS ONE 2014, 9, e86965. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, Z.; Wang, L.; Jiao, B.; Yang, H.; Wang, X. MiR-21-3p Centric Regulatory Network in Dairy Cow Mammary Epithelial Cell Proliferation. J. Agric. Food Chem. 2019, 67, 11137–11147. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Sharma, N.; Singh, A.K.; Gera, M.; Pulicherla, K.K.; Jeong, D.K. Transformation of Animal Genomics by Next-Generation Sequencing Technologies: A Decade of Challenges and Their Impact on Genetic Architecture. Criti. Rev. Biotechnol. 2018, 38, 1157–1175. [Google Scholar] [CrossRef] [PubMed]
- Schnall-Levin, M.; Rissland, O.S.; Johnston, W.K.; Perrimon, N.; Bartel, D.P.; Berger, B. Unusually Effective MicroRNA Targeting within Repeat-Rich Coding Regions of Mammalian MRNAs. Genome Res. 2011, 21, 1395–1403. [Google Scholar] [CrossRef]
- Sun, J.; Aswath, K.; Schroeder, S.G.; Lippolis, J.D.; Reinhardt, T.A.; Sonstegard, T.S. MicroRNA Expression Profiles of Bovine Milk Exosomes in Response to Staphylococcus Aureus Infection. BMC Genom. 2015, 16, 806. [Google Scholar] [CrossRef]
- Barbu, M.G.; Condrat, C.E.; Thompson, D.C.; Bugnar, O.L.; Cretoiu, D.; Toader, O.D.; Suciu, N.; Voinea, S.C. MicroRNA Involvement in Signaling Pathways During Viral Infection. Front. Cell Dev. Biol. 2020, 8, 143. [Google Scholar] [CrossRef] [PubMed]
- Kuosmanen, S.M.; Kansanen, E.; Sihvola, V.; Levonen, A.-L. MicroRNA Profiling Reveals Distinct Profiles for Tissue-Derived and Cultured Endothelial Cells. Sci. Rep. 2017, 7, 10943. [Google Scholar] [CrossRef] [PubMed]
- Lawless, N.; Reinhardt, T.A.; Bryan, K.; Baker, M.; Pesch, B.; Zimmerman, D.; Zuelke, K.; Sonstegard, T.; O’Farrelly, C.; Lippolis, J.D.; et al. MicroRNA Regulation of Bovine Monocyte Inflammatory and Metabolic Networks in an in Vivo Infection Model. G3 Genes|Genomes|Genetics 2014, 4, 957–971. [Google Scholar] [CrossRef] [PubMed]
- Oladejo, A.O.; Li, Y.; Wu, X.; Imam, B.H.; Shen, W.; Ding, X.Z.; Wang, S.; Yan, Z. MicroRNAome: Potential and Veritable Immunomolecular Therapeutic and Diagnostic Baseline for Lingering Bovine Endometritis. Front. Veter. Sci. 2020, 7, 614054. [Google Scholar] [CrossRef]
- Sato, F.; Tsuchiya, S.; Meltzer, S.J.; Shimizu, K. MicroRNAs and Epigenetics. FEBS J. 2011, 278, 1598–1609. [Google Scholar] [CrossRef]
- Miretti, S.; Lecchi, C.; Ceciliani, F.; Baratta, M. MicroRNAs as Biomarkers for Animal Health and Welfare in Livestock. Front. Veter. Sci. 2020, 7, 578193. [Google Scholar] [CrossRef]
- Friedman, R.C.; Farh, K.K.-H.; Burge, C.B.; Bartel, D.P. Most Mammalian MRNAs Are Conserved Targets of MicroRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef]
- Jia, L.; Jiang, M.; Wu, K.; Hu, J.; Wang, Y.; Quan, W.; Hao, M.; Liu, H.; Wei, H.; Fan, W.; et al. Nanopore Sequencing of African Swine Fever Virus. Sci. China Life Sci. 2020, 63, 160–164. [Google Scholar] [CrossRef]
- Deiuliis, J.A. MicroRNAs as Regulators of Metabolic Disease: Pathophysiologic Significance and Emerging Role as Biomarkers and Therapeutics. Int. J. Obes. 2016, 40, 88–101. [Google Scholar] [CrossRef]
- Cortez, M.A.; Bueso-Ramos, C.; Ferdin, J.; Lopez-Berestein, G.; Sood, A.K.; Calin, G.A. MicroRNAs in Body Fluids—The Mix of Hormones and Biomarkers. Nat. Rev. Clin. Oncol. 2011, 8, 467–477. [Google Scholar] [CrossRef]
- Shirjang, S.; Mansoori, B.; Asghari, S.; Duijf, P.H.G.; Mohammadi, A.; Gjerstorff, M.; Baradaran, B. MicroRNAs in Cancer Cell Death Pathways: Apoptosis and Necroptosis. Free Radic. Biol. Med. 2019, 139, 1–15. [Google Scholar] [CrossRef]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. MiRBase: From MicroRNA Sequences to Function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef] [PubMed]
- Leti, F.; DiStefano, J.K. MiRNA Quantification Method Using Quantitative Polymerase Chain Reaction in Conjunction with C q Method. In Methods in Molecular Biology; DiStefano, J., Ed.; Humana Press: New York, NY, USA, 2018; Volume 1706, pp. 257–265. [Google Scholar]
- Zheng, C.Y.; Zou, X.; Lin, H.J.; Zhao, B.C.; Zhang, M.L.; Luo, C.H.; Fu, S.X. MiRNA-185 Regulates the VEGFA Signaling Pathway in Dairy Cows with Retained Fetal Membranes. Theriogenology 2018, 110, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.-X.; Guo, T.; Lu, H.; Yue, L.; Li, Y.; Jin, D.; Zhang, G.-J.; Yang, F. Nanostructuring Synergetic Base-Stacking Effect: An Enhanced Versatile Sandwich Sensor Enables Ultrasensitive Detection of MicroRNAs in Blood. ACS Sens. 2020, 5, 2514–2522. [Google Scholar] [CrossRef]
- Lawless, N.; Foroushani, A.B.K.; McCabe, M.S.; O’Farrelly, C.; Lynn, D.J. Next Generation Sequencing Reveals the Expression of a Unique MiRNA Profile in Response to a Gram-Positive Bacterial Infection. PLoS ONE 2013, 8, e57543. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Nie, Q.; Zhang, X.; Nolan, L.K.; Lamont, S.J. Novel MicroRNA Involved in Host Response to Avian Pathogenic Escherichia Coli Identified by Deep Sequencing and Integration Analysis. Infect. Immun. 2017, 85, e00688-16. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Giordano, F.; Ning, Z. Oxford Nanopore MinION Sequencing and Genome Assembly. Genom. Proteom. Bioinform. 2016, 14, 265–279. [Google Scholar] [CrossRef]
- Stenfeldt, C.; Arzt, J.; Smoliga, G.; LaRocco, M.; Gutkoska, J.; Lawrence, P. Proof-of-Concept Study: Profile of Circulating MicroRNAs in Bovine Serum Harvested during Acute and Persistent FMDV Infection. Virol. J. 2017, 14, 71. [Google Scholar] [CrossRef][Green Version]
- Okotrub, K.A.; Surovtsev, N.V.; Semeshin, V.F.; Omelyanchuk, L.V. Raman Spectroscopy for DNA Quantification in Cell Nucleus. Cytom. Part A 2015, 87, 68–73. [Google Scholar] [CrossRef]
- Wen, Q.; Liang, X.; Pan, H.; Li, J.; Zhang, Y.; Zhu, W.; Long, Z. Rapid and Sensitive Electrochemical Detection of MicroRNAs by Gold Nanoparticle-Catalyzed Silver Enhancement. Analyst 2020, 145, 7893–7897. [Google Scholar] [CrossRef]
- Gebert, L.F.R.; MacRae, I.J. Regulation of MicroRNA Function in Animals. Nat. Rev. Mol. Cell Biol. 2019, 20, 21–37. [Google Scholar] [CrossRef]
- Malvisi, M.; Palazzo, F.; Morandi, N.; Lazzari, B.; Williams, J.L.; Pagnacco, G.; Minozzi, G. Responses of Bovine Innate Immunity to Mycobacterium Avium Subsp. Paratuberculosis Infection Revealed by Changes in Gene Expression and Levels of MicroRNA. PLoS ONE 2016, 11, e0164461. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Liang, W.; Li, Y.; Sun, Y.; Xiang, Y.; Zhang, Y.; Dai, Z.; Duo, Y.; Wu, L.; Qi, K.; et al. Ultrasensitive Detection of MiRNA with an Antimonene-Based Surface Plasmon Resonance Sensor. Nat. Commun. 2019, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Lecchi, C.; Zamarian, V.; Gini, C.; Avanzini, C.; Polloni, A.; Rota Nodari, S.; Ceciliani, F. Salivary MicroRNAs Are Potential Biomarkers for the Accurate and Precise Identification of Inflammatory Response after Tail Docking and Castration in Piglets. J. Anim. Sci. 2020, 98, skaa153. [Google Scholar] [CrossRef]
- Hu, F.; Xu, J.; Chen, Y. Surface Plasmon Resonance Imaging Detection of Sub-Femtomolar MicroRNA. Anal. Chem. 2017, 89, 10071–10077. [Google Scholar] [CrossRef]
- Sohel, M.M.H. Macronutrient modulation of mRNA and microRNA function in animals: A review. Anim. Nutr. 2020, 6, 258–268. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).