Threats and Challenges for Conservation of Meloidae (Coleoptera) in a Global Change Context, Emphasizing the Iberian Peninsula †
Abstract
:1. Introduction
2. Material and Methods
2.1. Literature Study
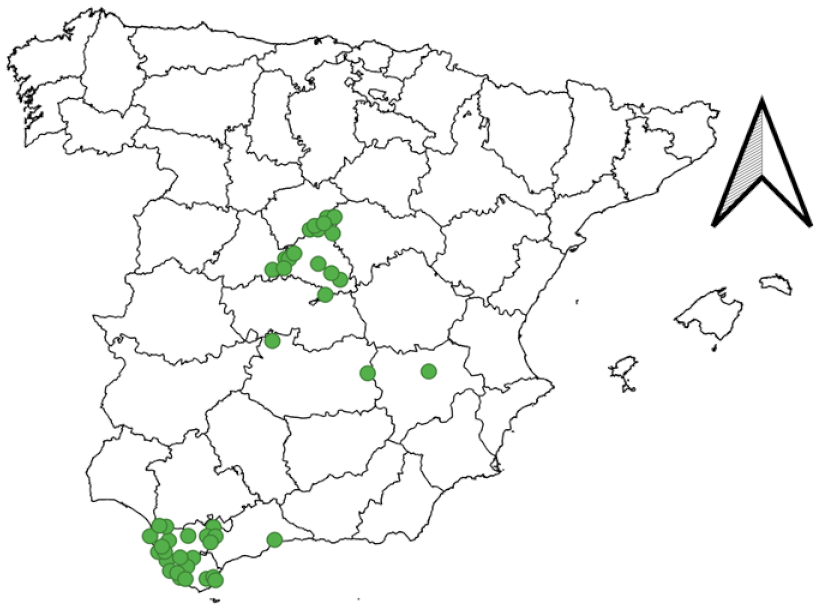
2.2. Sampling Campaigns
3. Results
3.1. Literature Review
3.2. Field Work and Citizen Science Program
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Impact | Species |
|---|---|
| Biocides (species directed) | Berberomeloe insignis [28,29] Berberomeloe payoyo [33] Eurymeloe nanus [25] Meloini tribe [29] Physomeloe corallifer [8] Lampromeloe variegatus [29] Taphromeloe foveolaus [26] |
| Forest fires | Taphromeloe foveolaus [26] |
| Fragmentation | Apalus bimaculatus [19,34] Berberomeloe insignis [28,29] Berberomeloe payoyo [33] Eurymeloe brevicollis [35] Eurymeloe rugosus [35] Lampromeloe variegatus [29] Meloe proscarabaeus [35] Meloe violaceus [35] |
| Genetic impoverishment | Gnathium minimum [20] |
| Global warming | Mylabris nevadensis [27,30] |
| Habitat loss/degradation | Apalus bimaculatus [19,34] Epicauta pensylvanica [21] Eurymeloe brevicollis [35] Eurymeloe nanus [25] Eurymeloe rugosus [35] Meloe proscarabaeus [35] Meloe scabriusculus [18] Meloe uralensis [18] Meloini/Meloinae [35] Micromeloe decorus [18] Meloe violaceus [35] Mylabris nevadensis [26,29] Sitaris rufipennis [9] Taphromeloe foveolaus [31] |
| Host loss | Apalus bimaculatus [19,34] Eurymeloe brevicollis [35] Meloini/Meloinae [29,35] Eurymeloe brevicollis [35] Eurymeloe nanus [25] Eurymeloe rugosus [35] Hycleus uhagonii [8] Meloe proscarabaeus [35] Meloe violaceus [35] Sitaris rufipennis [9] |
| Illegal dumping | Berberomeloe payoyo [33] |
| Inadequate forestry practices/unnecessary reforestation | Berberomeloe insignis [28,29] Eurymeloe nanus [25] Mylabris nevadensis [27,30] Taphromeloe foveolaus [26] |
| Intensive agriculture/damages due to agriculture | Berberomeloe insignis [28,29] Eurymeloe nanus [25] Lampromeloe variegatus [29] Taphromeloe foveolaus [26] |
| Livestock overexploitation | Mylabris nevadensis [27,30] |
| Open-pit mining/quarrying | Berberomeloe insignis [28,29] Eurymeloe nanus [25] |
| Road traffic | Berberomeloe payoyo [33] Berberomeloe sp. [8] Lampromeloe variegatus [29] Taphromeloe foveolaus [26] |
| Soil composition changes/change in land uses | Berberomeloe insignis [28,29] Berberomeloe payoyo [33] Eurymeloe brevicollis [35] Eurymeloe rugosus [35] Meloe proscarabaeus [18,35] Meloe scabriusculus [18] Meloe uralensis [18] Meloe violaceus [35] Micromeloe decorus [18] |
| Water reserve overexploitation | Berberomeloe insignis [28,29] |
| Unsustainable tourism | Mylabris nevadensis [27,30] Taphromeloe foveolaus [26] |
| Urban development | Berberomeloe insignis [28,29] Berberomeloe payoyo [33] Berberomeloe sp. [8] Eurymeloe nanus [8] Eurymeloe tuccia [8] Lampromeloe variegatus [29] Meloini tribe [29] Physomeloe corallifer [8] Taphromeloe foveolaus [26] |
| Unknown reasons, but: observed regression/very restricted range/supposed to be endangered/extinct in some regions | Eurymeloe brevicollis [8] Eurymeloe mediterraneus [8] Euzonitis quadrimaculata [8] Hycleus duodecimpuctatus [8] Lampromeloe cavensis [8] Lampromeloe variegatus [8,29,35,36] Lytta hoppingi [37] Lytta insperata [37] Lytta moesta [37] Lytta molesta [37] Lytta morrisoni [37] Meloe proscarabaeus [8] Meloe violaceus [8] Meloegonius cicatricosus [35] Mylabris amorii [28] Mylabris deferreri [28] Mylabris dejeani [8] Mylabris quadripunctata [8] Mylabris uhagonii [32] Mylabris variabilis [8] Sitaris rufipenis [9] Sitarobrachys thoracica [9] Treiodus ajax [22] Treiodus autumnalis [35] |
References
- Bologna, M.A.; Pinto, J.D. Phylogenetic studies of Meloidae (Coleoptera), with emphasis on the evolution of phoresy. Syst. Entomol. 2001, 26, 33–72. [Google Scholar] [CrossRef]
- Bologna, M.A. Fauna de Italia. XXVIII. Coleoptera Meloidae; Edizioni Calderini: Bologna, Italy, 1991; 541p. [Google Scholar]
- Bologna, M.A. Nuove osservazioni sui predatori dei Meloidae (Coleoptera). Boll.-Assoc. Rom. Entomol. 1983, 38, 63–64. [Google Scholar]
- Bologna, M.A.; Oliverio, M.; Pitzalis, M.; Mariottini, P. Phylogeny and evolutionary history of the blister beetles (Coleoptera, Meloidae). Mol. Phylogenet. Evol. 2008, 48, 679–693. [Google Scholar] [CrossRef] [PubMed]
- García-París, M. Revisión sistemática del género Berberomeloe Bologna, 1988 (Coleoptera, Meloidae) y diagnosis de un endemismo ibérico olvidado. Graellsia 1998, 54, 97–109. Available online: http://graellsia.revistas.csic.es/index.php/graellsia/article/view/347 (accessed on 18 June 2021). [CrossRef] [Green Version]
- Bologna, M.A. Berberomeloe, a new west Mediterranean genus of Lyttini for Meloe majalis Lineé (Coleoptera, Meloidae). Systematics and bionomics. Ital. J. Zool. 1988, 55, 359–366. [Google Scholar] [CrossRef] [Green Version]
- Schachat, S.R.; Labandeira, C.C. Are insects heading toward their first mass extinction? Distinguishing turnover from crises in their fossil record. Ann. Entomol. Soc. Am. 2021, 114, 99–118. [Google Scholar] [CrossRef]
- García-París, M.; Trotta-Moreu, N.; Capote, L. Estado de conocimiento actual y problemas de conservación de los Meloidae (Coleoptera) de la Comunidad de Madrid. Graellsia 2006, 62, 333–370. [Google Scholar] [CrossRef] [Green Version]
- Barea-Azcón, J.M.; Ballesteros-Duperón, E.; Moreno, D. (Eds.) Libro Rojo de los Invertebrados de Andalucía; Consejería de Medio Ambiente, Junta de Andalucía: Sevilla, Spain, 2008; Volume 4, 1438p. [Google Scholar]
- Verdú, J.R.; Numa, C.; Galante, E. (Eds.) Atlas y Libro Rojo de los Invertebrados Amenazados de España (Especies Vulnerables); Dirección General de Medio Natural y Política Forestal, Ministerio de Medio Ambiente, Medio Rural y Marino: Madrid, Spain, 2011; Volume I, 719p. [Google Scholar]
- García París, M. Familia Meloidae. In Iberfauna. El Banco de Datos de la Fauna Ibérica; Digital; Museo Nacional de Ciencias Naturales (CSIC): Madrid, Spain, 2017; Available online: http://iberfauna.mncn.csic.es/showficha.aspx?rank=J&idtax=3617 (accessed on 18 June 2021).
- Sánchez-Vialas, A.; García-París, M.; Ruiz, J.L.; Recuero, E. Patterns of morphological diversification in giant Berberomeloe blister beetles (Coleoptera: Meloidae) reveal an unexpected taxonomic diversity concordant with mtDNA phylogenetic structure. Zool. J. Linn. Soc. 2020, 189, 1249–1312. [Google Scholar] [CrossRef]
- Bologna, M.A.; Cerny, L.; Zubair, A. Meloidae (Coleoptera) of Pakistan and Kashmir with the description of three new species, new faunistic and taxonomic records, and a zoogeographic analysis. Turk. Zool. Derg. 2018, 42, 637–660. [Google Scholar] [CrossRef]
- Černý, L.; Bologna, M.A. A new species of Diaphorocera from Morocco with unclear relationships and a key to the species (Coleoptera, Meloidae, Cerocomini). ZooKeys 2018, 748, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, J.; Pan, Z. New species and new faunistic records of the family Meloidae Gyllenhal, 1810 (Coleoptera: Tenebrionoidea) from China, with a list of meloid species from Xinjiang. J. Asia. Pac. Entomol. 2020, 23, 1144–1150. [Google Scholar] [CrossRef]
- Bickford, D.; Lohman, D.; Sodhi, N.; Ng, P.; Meier, R.; Winker, K.; Ingram, K. Cryptic species as a window on diversity and conservation. Trends Ecol. Evol. 2007, 22, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.T.; Packer, L. The environmental basis of North American species richness patterns among Epicauta (Coleoptera: Meloidae). Biodivers. Conserv. 1999, 8, 617–628. [Google Scholar] [CrossRef]
- Cizek, L.; Hauck, D.; Pokluda, P. Contrasting needs of grassland dwellers: Habitat preferences of endangered steppe beetles (Coleoptera). J. Insect Conserv. 2012, 16, 281–293. [Google Scholar] [CrossRef]
- Ahlbäck, L.; Berggren, Å. The effect of landscape structure and habitat composition on the presence of the threatened parasitic sand-living beetle Apalus bimaculatus (Coleoptera: Meloidae). Can. Entomol. 2012, 145, 626–638. [Google Scholar] [CrossRef]
- Marschalek, D.A.; Berres, M.E. Genetic population structure of the blister beetle, Gnathium minimum: Core and peripheral populations. J. Hered. 2014, 105, 878–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marschalek, D.A.; Ogden, H.V.; Wolcott, D.M. A Marking Study of the Black Blister Beetle, Epicauta pensylvanica (Degeer) (Coleoptera: Meloidae), Demonstrates a Preference for a Restored Tallgrass Prairie. J. Kans. Entomol. Soc. 2020, 92, 639–648. [Google Scholar] [CrossRef]
- Pinto, J.D. A New Meloe Linnaeus (Coleoptera: Meloidae, Meloinae) from Southern California Chaparral: A Rare and Endangered Blister Beetle or Simply Secretive? Coleopt. Bull. 1998, 52, 378–385. Available online: www.jstor.org/stable/4009345 (accessed on 18 June 2021).
- Moreno, I.P.; Moreno, A.F.S.M.; Irurzun, J.I.R. Aportaciones corológicas y faunísticas sobre meloidos ibérico (Coleoptera: Meloidae). Bol. SEA 2003, 33, 195–217. [Google Scholar]
- García-París, M.; Ruíz, J.L. Berberomeloe insignis (Charpentier, 1818). In Libro Rojo de los Invertebrados de Andalucía; Barea-Azcón, J.M., Ballesteros-Duperón, E., Moreno, D., Eds.; Consejería de Medio Ambiente, Junta de Andalucía: Sevilla, Spain, 2008; Volume 3, pp. 1020–1029. [Google Scholar]
- Ruíz, J.L.; García-París, M. Meloe (Eurymeloe) nanus Lucas, 1849. In Libro Rojo de los Invertebrados de Andalucía; Barea-Azcón, J.M., Ballesteros-Duperón, E., Moreno, D., Eds.; Consejería de Medio Ambiente, Junta de Andalucía: Sevilla, Spain, 2008; Volume 3, pp. 1030–1037. [Google Scholar]
- Ruíz, J.L.; García-París, M. Meloe (Taphromeloe) foveolatus Guérin de Méneville, 1842. In Libro Rojo de los Invertebrados de Andalucía; Barea-Azcón, J.M., Ballesteros-Duperón, E., Moreno, D., Eds.; Consejería de Medio Ambiente, Junta de Andalucía: Sevilla, Spain, 2008; Volume 3, pp. 1038–1044. [Google Scholar]
- García-París, M.; Ruíz, J.L. Mylabris (Micrabris) nevadensis (Escalera, 1915). In Libro Rojo de los Invertebrados de Andalucía; Barea-Azcón, J.M., Ballesteros-Duperón, E., Moreno, D., Eds.; Consejería de Medio Ambiente, Junta de Andalucía: Sevilla, Spain, 2008; Volume 3, pp. 1045–1051. [Google Scholar]
- García-París, M.; Ruíz, J.L. Berberomeloe insignis (Charpentier, 1818). In Atlas y Libro Rojo de los Invertebrados Amenazados de España (Especies Vulnerables); Verdú, J.R., Numa, C., Galante, E., Eds.; Dirección General de Medio Natural y Política Forestal, Ministerio de Medio Ambiente, Medio Rural y Marino: Madrid, Spain, 2011; Volume I, pp. 285–294. [Google Scholar]
- García-París, M.; Ruíz, J.L. Meloe (Lampromeloe) variegatus Donovan, 1793. In Atlas y Libro Rojo de los Invertebrados Amenazados de España (Especies Vulnerables); Verdú, J.R., Numa, C., Galante, E., Eds.; Dirección General de Medio Natural y Política Forestal, Ministerio de Medio Ambiente, Medio Rural y Marino: Madrid, Spain, 2011; Volume I, pp. 295–302. [Google Scholar]
- García-París, M.; Ruíz, J.L. Mylabris (Micrabris) nevadensis (Escalera, 1915). In Atlas y Libro Rojo de los Invertebrados Amenazados de España (Especies Vulnerables); Verdú, J.R., Numa, C., Galante, E., Eds.; Dirección General de Medio Natural y Política Forestal, Ministerio de Medio Ambiente, Medio Rural y Marino: Madrid, Spain, 2011; Volume I, pp. 303–308. [Google Scholar]
- García-París, M.; Ruíz, J.L. Meloe (Taphromeloe) foveolatus Guérin de Méneville, 1842. In Atlas y Libro Rojo de los Invertebrados Amenazados de España (Especies en Peligro Crítico y en Peligro); Verdú, J.R., Galante, E., Eds.; Dirección General de Medio Natural y Política Forestal, Ministerio de Medio Ambiente, Medio Rural y Marino: Madrid, Spain, 2009; pp. 121–124. [Google Scholar]
- García-París, M.; Ruíz, J.L. Mylabris uhagonii Martínez y Sáez, 1873. In Atlas y Libro Rojo de los Invertebrados Amenazados de España (Especies en Peligro Crítico y en Peligro); Verdú, J.R., Galante, E., Eds.; Dirección General de Medio Natural y Política Forestal, Ministerio de Medio Ambiente, Medio Rural y Marino: Madrid, Spain, 2009; pp. 125–129. [Google Scholar]
- Cortés-Fossati, F. Un primer acercamiento al estado de conservación de las poblaciones de Berberomeloe majalis (Linnaeus, 1758) (Coleoptera: Meloidae) en la provincia de Cádiz (España). Observaciones de campo y percepciones del mundo rural sobre el estado de la especie. Bol. SEA 2018, 62, 327–331. [Google Scholar]
- Ahlbäck, L.; Sallmén, N.; Hedin, Å.; Berggren, Å. Translocation of a sand-associated blister beetle due to urban development in Uppsala, Sweden. In Global Reintroduction Perspectives: 2018. Case Studies from around the Globe; Soorae, P.S., Ed.; IUCN/SSC Reintroduction Specialist Group: Gland, Switzerland; Environment Agency: Abu Dhabi, United Arab Emirates, 2018; pp. 1–6. [Google Scholar] [CrossRef]
- BugLife—The Invertebrate Conservation Trust. Oil Beetle Hunt. Available online: https://www.buglife.org.uk/get-involved/surveys/oil-beetle-hunt/ (accessed on 18 June 2021).
- UK Oil Beetle Recording. Oil Beetle Scheme. Available online: https://www.coleoptera.org.uk/meloidae/home (accessed on 18 June 2021).
- State of California Natural Resources Agency. Special of Special Concern. Department of Fish and Wildlife, Biogeographic Data Branch, California Natural Diversity Database. Available online: https://wildlife.ca.gov/Conservation/SSC (accessed on 11 April 2022).
| Species Affected | Impact | Environment Type |
|---|---|---|
| Berberomeloe payoyo | Illegal dumping | Natural/suburban |
| Berberomeloe payoyo | Fragmentation | Suburban |
| Berberomeloe payoyo | Quarrying | Suburban |
| Berberomeloe payoyo | Phytosanitary treat. | Crops |
| Berberomeloe payoyo | Road traffic | Natural/suburban |
| Berberomeloe payoyo | Soil overexploitation | Crops |
| Berberomeloe payoyo | Urban development | Natural/suburban |
| Berberomeloe comunero | Fragmentation | Natural/suburban |
| Berberomeloe majalis | Human transit | Natural |
| Hycleus sp. | Fragmentation | Natural |
| Mylabris sp. | Illegal dumping | Natural |
| Meloe tuccia | Fragmentation | Natural/suburban |
| Physomeloe corallifer | Human transit | Natural |
| Physomeloe corallifer | Fragmentation | Suburban |
| Physomeloe corallifer | Road traffic | Natural |
| Physomeloe corallifer | Urban development | Natural/suburban |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés-Fossati, F. Threats and Challenges for Conservation of Meloidae (Coleoptera) in a Global Change Context, Emphasizing the Iberian Peninsula. Biol. Life Sci. Forum 2022, 10, 2. https://doi.org/10.3390/IECE-10495
Cortés-Fossati F. Threats and Challenges for Conservation of Meloidae (Coleoptera) in a Global Change Context, Emphasizing the Iberian Peninsula. Biology and Life Sciences Forum. 2022; 10(1):2. https://doi.org/10.3390/IECE-10495
Chicago/Turabian StyleCortés-Fossati, Fernando. 2022. "Threats and Challenges for Conservation of Meloidae (Coleoptera) in a Global Change Context, Emphasizing the Iberian Peninsula" Biology and Life Sciences Forum 10, no. 1: 2. https://doi.org/10.3390/IECE-10495
APA StyleCortés-Fossati, F. (2022). Threats and Challenges for Conservation of Meloidae (Coleoptera) in a Global Change Context, Emphasizing the Iberian Peninsula. Biology and Life Sciences Forum, 10(1), 2. https://doi.org/10.3390/IECE-10495






