Abstract
Background: Delayed graft function (DGF) is a frequent early complication after deceased donor kidney transplantation (DDKT), leading to prolonged hospitalization, increased risk of acute rejection, and reduced graft survival. Reliable and easily measurable preoperative biomarkers for DGF prediction remain limited. This study aimed to evaluate the predictive value of pre-operative Absolute Eosinophil Count (AEC) and Albumin-to-Globulin Ratio (AGR) for DGF in DDKT recipients. Methods: A retrospective analysis was conducted on all DDKT procedures performed at our institution between January 2018 and December 2023. Patients were divided into two groups: Group 1 (DGF) and Group 2 (non-DGF). DGF was defined as the requirement for hemodialysis within the first seven postoperative days. Demographic, clinical, and laboratory data—including pre-operative AEC and AGR—were collected and compared between groups. Statistical analysis was performed using appropriate parametric and nonparametric tests. Receiver operating characteristic (ROC) curves were generated to assess the individual and combined predictive performance of AEC and AGR for DGF. Results: A total of 38 patients underwent DDKT, comprising 27 males (71.05%) and 11 females (28.95%), with a mean age of 43.3 ± 9.41 years. Fifteen patients (39.47%) developed DGF. The mean AEC and AGR were significantly lower in the DGF group compared to the non-DGF group (AEC: 0.20 ± 0.16 vs. 0.40 ± 0.35, p = 0.04; AGR: 1.43 ± 0.22 vs. 1.66 ± 0.39, p = 0.02). ROC analysis demonstrated that both AEC (p = 0.04) and AGR (p = 0.04) were significant predictors of DGF. Combining both parameters resulted in a higher area under the curve (AUC), improved sensitivity, and enhanced negative predictive value (NPV) compared to either marker alone. Conclusions: DGF occurred in nearly two-fifths of DDKT recipients in this cohort. Patients with lower preoperative AEC and AGR were more likely to develop DGF, suggesting that these easily available hematological and biochemical indices can serve as potential preoperative predictors of early graft dysfunction. Future multicentric prospective studies are warranted to validate these findings and explore their integration into DGF risk prediction models.
1. Introduction
Chronic kidney disease (CKD) is a progressive and irreversible condition characterized by the gradual loss of renal function over time [1]. With the global rise in hypertension, diabetes, and aging populations, the incidence of CKD and end-stage renal disease (ESRD) continues to increase, leading to a growing demand for renal replacement therapies. Among the available options, kidney transplantation remains the most effective treatment, offering improved survival and quality of life compared to long-term dialysis. However, the persistent disparity between the number of patients requiring transplantation and the limited availability of living donors has necessitated greater reliance on deceased donor kidney transplantation (DDKT) to bridge this gap. Despite its critical role in expanding access, grafts from deceased donors generally demonstrate inferior short- and long-term outcomes compared with living donor grafts, largely due to ischemia–reperfusion injury and donor-related factors.
Delayed graft function (DGF) is one of the most frequent and clinically significant early complications following DDKT and is typically defined as the requirement for dialysis within the first week after transplantation [2]. This one-week diagnostic window delays early recognition and intervention, potentially compromising graft recovery. DGF has been consistently identified as a strong negative prognostic factor for graft survival, increasing the risk of acute rejection, prolonged hospitalization, and long-term allograft loss [3]. Reported incidence rates of DGF vary widely, ranging from 20% to 29% in most series, with some centers observing rates as high as 50% [4]. Such variability is attributed to differences in donor selection criteria, perioperative management, and recipient profiles. Major risk factors contributing to DGF include ischemia–reperfusion injury, donor source (living versus deceased), cold ischemia time, and recipient comorbidities [5].
Given these complexities, identifying reliable and easily measurable predictors of DGF remains a priority in transplant medicine. Early prediction of graft dysfunction could allow clinicians to adopt timely preventive strategies—such as optimizing hemodynamics, adjusting immunosuppression, or mitigating ischemic injury—to improve early graft recovery. Although several donor and procedural variables have been incorporated into predictive indices, there is increasing interest in recipient-based biomarkers that reflect physiological and immunological readiness for transplantation.
Among these, serum albumin and globulin levels are of particular clinical interest. Serum albumin is a well-established marker of nutritional status and systemic protein reserves, while globulin reflects the burden of chronic inflammation and immune activation. Both parameters are influenced by a range of physiological and pathological processes, including malnutrition, infection, hepatic dysfunction, and chronic inflammation—all of which can affect post-transplant outcomes. The Albumin-to-Globulin Ratio (AGR), derived from these two parameters, provides a more comprehensive assessment of the balance between nutritional and inflammatory states. Low AGR values have been associated with poor outcomes in several medical conditions, including malignancies, cardiovascular disease, and chronic inflammatory disorders [6,7,8]. Reduced AGR has been linked to increased morbidity and mortality among patients with CKD and those undergoing dialysis. However, its specific role in predicting DGF after DDKT remains poorly defined and has not been extensively investigated [9].
The immune–inflammatory milieu of the recipient also plays a critical role in determining graft outcomes. In this context, the absolute eosinophil count (AEC) is gaining attention as a potential immunological biomarker. Eosinophils are multifunctional granulocytes that account for 1–3% of circulating leukocytes and play a dual role in inflammation and immune regulation. Although traditionally associated with allergic reactions and parasitic infections, eosinophils are now recognized as modulators of tissue repair, vascular homeostasis, and immune tolerance. In solid organ transplantation, their role remains controversial—eosinophilia has been correlated with acute graft rejection in liver transplantation [10,11], likely due to their involvement in proinflammatory cytokine release and immune cell recruitment. However, eosinophils may also exert protective effects by releasing interleukin-4 (IL-4) and interleukin-13 (IL-13), promoting regulatory immune responses and limiting ischemia–reperfusion injury. The relationship between preoperative eosinophil levels and post-transplant graft outcomes, particularly in DDKT, has not been thoroughly elucidated.
Given the multifactorial nature of DGF, biomarkers that can integrate metabolic, nutritional, and immune information may provide greater predictive value than traditional parameters alone. Both AEC and AGR are simple, cost-effective, and routinely available measures that reflect distinct but interconnected biological processes—AEC indicates immune and inflammatory balance, and AGR reflects nutritional and systemic inflammatory status. Assessing their relationship with DGF could help identify high-risk recipients before surgery, allowing for preemptive optimization of their clinical condition.
Therefore, the present study was undertaken to evaluate whether preoperative AEC and AGR can predict DGF in recipients undergoing DDKT. By identifying such associations, this study aims to contribute to the development of simple, integrative biomarkers for early risk stratification and improved perioperative management in kidney transplantation.
2. Materials and Methods
A retrospective study was performed in 38 patients who had DDKT in our hospital from January 2018 to December 2023. Demographic details of recipients, pre-operative complete blood count analysis, and renal function tests were obtained. The patients with known hematologic disorders or incomplete medical records were excluded from the study. The patients were classified into two groups based on graft function: group 1 (DGF) and group 2 (non-DGF). The non–DGF group included immediate and slow graft function.
The DGF definition for this study required at least one dialysis within the first seven days of transplantation. Patients with variable delay in graft function and serum creatinine > 3 mg/dL on POD 5 but no dialysis within the first seven days were categorized as SGF and included in the non-DGF group.
All patients underwent hemodialysis on the day before the transplant. Standard surgical technique was followed in all the cases, wherein the kidney was placed extraperitoneally. Vascular anastomosis of the kidney transplant was done to the external iliac vessels in all the patients with an end-to-side technique. The immunosuppression protocol followed consisted of Tacrolimus, Mycophenolate Mofetil, and Prednisolone with induction of Anti-Thymocyte Globulin (ATG) or Basiliximab. AEC and AGR were calculated by Total White blood cell count × Eosinophil %/100 and Serum Albumin/Serum Globulin, respectively. Creatinine clearance ratio 1 (CCR1) was calculated by serum creatinine prior to surgery—serum creatinine on post-op day 1 × 100/serum creatinine prior to surgery. Creatinine clearance ratio 2 (CCR2) was calculated by serum creatinine on post-op day 1—serum creatinine on post-op day 2 × 100/serum creatinine on post-op day 1.
Our study aimed to compare the values of AEC and AGR and their combination between the groups to determine their role in predicting DGF in DDKT. The Institutional Ethics Committee reviewed and approved the study protocol based on the ethical principles of the Declaration of Helsinki.
3. Statistical Analysis
Continuous variables are presented as mean ± standard deviation (SD), and categorical variables as frequencies and percentages. The normality of data distribution was assessed using the Shapiro–Wilk test and visual inspection of Q–Q plots. Although certain variables demonstrated mild deviations from normality, Student’s t-test was considered robust to such deviations owing to the sample size (n = 38). Differences between the two independent groups were evaluated using the independent samples t-test. The equality of variances was assessed using Levene’s test; when the assumption of homogeneity of variance was violated (p < 0.05), Welch’s correction was applied.
We assessed the ability of AEC and AGR to predict outcomes using receiver operating characteristic (ROC) curves. Additionally, we used binary logistic regression to combine both parameters and calculate the area under the curve (AUC), sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV). To categorize the parameters, the Youden index was used to determine the cut-off values. The study compared groups 1 (DGF) and 2 (non-DGF) with several clinical variables, including perioperative blood tests and intraoperative warm and cold ischemic times. The statistical analyses were performed using Jamovi 2.3.28 software, and statistical significance was established by considering a p value of less than 0.05.
4. Results
Thirty-eight patients were included in the study, consisting of 27 males and 11 females, and the mean age of the study population was 43.3 ± 9.41 years. The clinicopathological characteristics of the study population are shown in Table 1. Out of the 38 patients, 15 (39.47%) were found to have DGF, and 23 (60.53%) were identified as having immediate or SGF (Non-DGF).

Table 1.
Descriptive characteristics of the study population.
The mean age of patients was similar between the DGF and Non-DGF groups (43.5 ± 10.3 vs. 43.1 ± 9.05 years, p = 0.91).
The Shapiro–Wilk test indicated that the data for both AGR and AEC deviated from normality (p < 0.05). For AGR, Levene’s test showed homogeneity of variances (p = 0.21); therefore, results from the equal variances assumed Student’s t-test were reported. For AEC, Levene’s test indicated unequal variances (p = 0.01); therefore, Welch’s t-test results were used. AEC (p = 0.04), AGR (p = 0.02), CCR1 (p = 0.007), and CCR 2 (p = <0.001) values were found to be significantly lower in the DGF group (mean ± SD: 0.2 ± 0.16, 1.43 ± 0.22, 22.3 ± 21.8, 12 ± 23.9) compared to the non-DGF group (mean ± SD: 0.4 ± 0.35, 1.66 +/− 0.39, 43.9 ± 23.1, 23.1 ± 31.1), respectively (Figure 1).
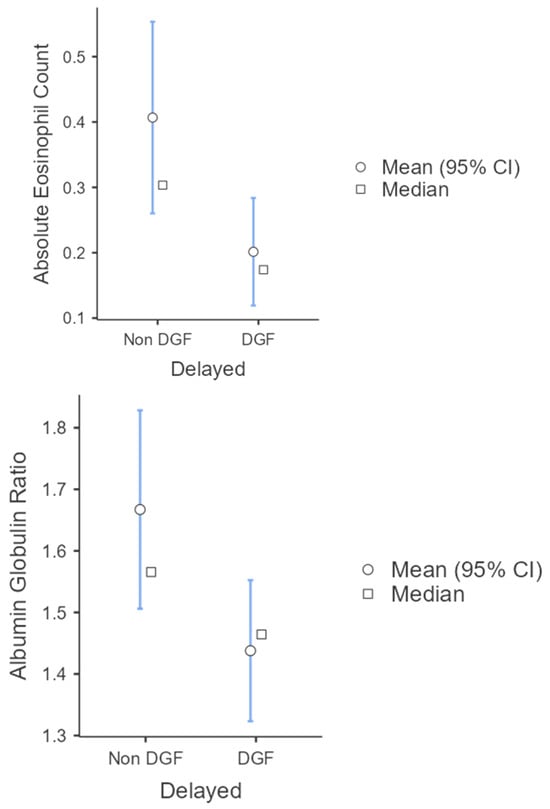
Figure 1.
Descriptive plots for AEC and AGR with statistical analysis done using Student’s t-test. p < 0.05 was considered statistically significant.
In Figure 2 and Figure 3’s ROC curve analysis, optimal cut-off value, sensitivity, specificity, PPV, and NPV values associated with AEC and AGR and their combined parameters in predicting DGF in DDKT are represented in Table 2. The AUC, sensitivity, and NPV are increased significantly by combining both parameters to predict DGF.
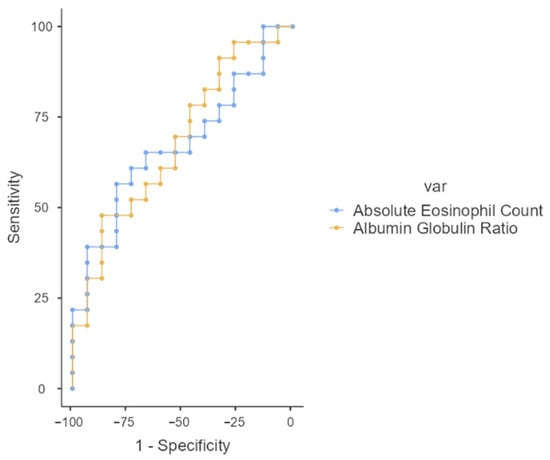
Figure 2.
ROC curve for AEC and AGR as predictors of DGF in DDKT.
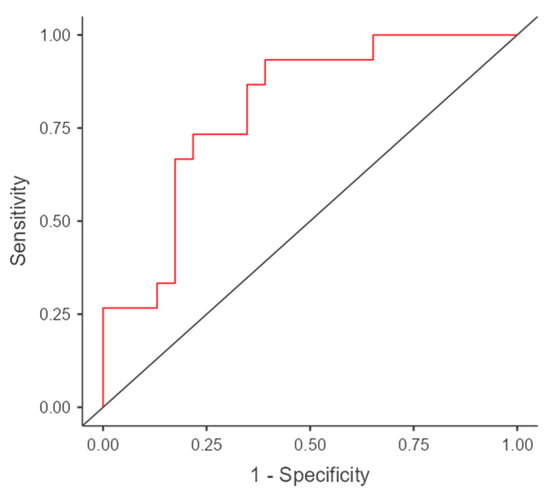
Figure 3.
ROC curve for combined AEC and AGR in the prediction of DGF in DDKT. The red line represents the ROC curve of the model showing the relationship between sensitivity and 1-specificity. The black diagonal line indicates the reference line representing no discrimination (AUC = 0.5).

Table 2.
Best cutoff values at which AEC, AGR, and their combined values predict DGF, sensitivity, specificity, PPV, and NPV.
5. Discussion
Our study aimed to identify the predictors of DGF in DDKT, and we assessed the relationship between AEC, AGR, and graft function in DDKT. These preoperative parameters are routinely analyzed in preoperative evaluation and are cost-effective.
Eosinophils are multifunctional granulocytes representing approximately 1–3% of circulating leukocytes and are increasingly recognized as active participants in immune regulation beyond their traditional roles in allergy and parasitic infection. Recent evidence suggests that eosinophils play a dual role in transplantation, contributing both to graft tolerance and rejection through the release of immunomodulatory cytokines and tissue-repair mediators [12]. They interact with T cells and dendritic cells and secrete cytokines such as interleukin-4 (IL-4), interleukin-13 (IL-13), and transforming growth factor-β (TGF-β), which promote tissue repair, angiogenesis, and immune homeostasis.
In our study, patients who developed DGF after DDKT exhibited significantly lower preoperative AEC compared to those with immediate graft function. This observation aligns with emerging data suggesting that eosinophils may exert protective effects in transplantation by attenuating ischemia–reperfusion injury, facilitating vascular repair, and modulating alloimmune responses. A lower baseline AEC could therefore reflect a diminished capacity for immune regulation and tissue recovery, rendering recipients more susceptible to early graft dysfunction.
Mechanistic insights from recent immunological studies provide further explanation for this association. Th1-polarized eosinophils can upregulate inducible nitric oxide synthase (iNOS) and programmed death-ligand 1 (PD-L1), forming inhibitory synapses with cytotoxic CD8+ T cells. This iNOS/PD-L1-mediated feedback suppresses T-cell activation, curtails excessive inflammation, and promotes an “immunologically quiescent” graft environment. Additionally, eosinophil-derived IL-4 has been shown to stimulate macrophages to produce heparin-binding epidermal growth factor (HB-EGF), enhancing tissue regeneration and recovery in ischemia–reperfusion models. Thus, eosinophils contribute not only to immune tolerance but also to post-transplant repair through cytokine-driven crosstalk with other immune and parenchymal cells [12].
Taken together, these findings suggest that lower preoperative AEC may serve as a marker of impaired immune equilibrium and regenerative potential in transplant recipients. Integrating AEC assessment into preoperative evaluation could help identify patients at increased risk for DGF and provide insight into the broader immunoregulatory mechanisms underlying early graft adaptation in DDKT.
Serum albumin is a well-established predictor of postoperative outcomes and overall mortality [13]. In patients with chronic kidney disease (CKD), lower serum albumin has been shown to independently increase the risk of all-cause mortality [14]. In the setting of renal transplantation, reduced preoperative serum albumin has been associated with a higher incidence of DGF, likely due to increased susceptibility to post-transplant hypoalbuminemia and impaired recovery from ischemia–reperfusion injury [15]. The AGR, which integrates both albumin and globulin components, provides a more comprehensive assessment of a patient’s nutritional and inflammatory status. A low AGR generally reflects chronic inflammation, poor nutritional reserves, hepatic dysfunction, or underlying comorbidities. These conditions can impair endothelial function, delay tissue recovery, and alter immune responses, thereby predisposing to DGF [16].
In our study, recipients who developed DGF after DDKT had significantly lower preoperative AGR compared to those with immediate graft function. This observation underscores the potential of AGR as a surrogate biomarker for the host’s metabolic and inflammatory milieu before transplantation. Our findings differ from those of Dialameh et al., who reported no significant association between AGR and DGF [9]. Several methodological and population-based factors may explain this divergence. Their study included both living and deceased donor transplants, with a predominance of living donors. Since living donor kidneys experience minimal cold ischemia and less ischemia–reperfusion injury, the influence of preoperative inflammatory and nutritional factors such as AGR may be less pronounced. Additionally, variations in donor demographics, recipient comorbidities, perioperative care, and sample size could have contributed to the contrasting outcomes.
Importantly, Dialameh et al. did not specifically stratify their analysis for deceased donor grafts, where ischemic stress and inflammatory activation play a central role in early graft recovery [9]. In contrast, our study focused exclusively on DDKT recipients, making it more sensitive to detecting the impact of preoperative metabolic and inflammatory status on graft outcomes. Taken together, these findings suggest that preoperative AGR may serve as an integrative marker of systemic inflammation, immune activation, and nutritional adequacy, influencing the early recovery of graft function. Further multicentric and mechanistic studies are warranted to validate AGR as a reliable predictor of DGF and to explore whether perioperative correction of hypoalbuminemia or inflammation can improve outcomes in high-risk recipients.
The improved predictive accuracy observed when AEC and AGR were analyzed together—resulting in a higher area under the curve (AUC), improved sensitivity, and enhanced negative predictive value (NPV)—can be explained by the complementary nature of these parameters. While AGR reflects chronic inflammation and nutritional status, AEC reflects immune readiness and regulatory balance. Low AGR signals ongoing inflammation and metabolic depletion, while low AEC suggests impaired immunologic control and reduced ability to limit ischemic and inflammatory injury. By combining these parameters, a more comprehensive assessment of the recipient’s physiological resilience is achieved.
It is also important to contextualize our findings within the framework of established DGF prediction models. Donor-related factors are among the strongest determinants and include advanced donor age, expanded criteria donor status, donation after circulatory death, prolonged cold ischemia time, and comorbidities such as hypertension, diabetes mellitus, and atherosclerosis. These parameters directly affect graft quality and ischemic tolerance by contributing to endothelial injury, microvascular dysfunction, and heightened susceptibility to ischemia–reperfusion injury. Recipient-related factors also play a major role in influencing graft function. Established recipient predictors include pre-existing diabetes, cardiovascular and peripheral vascular diseases, obesity, human leukocyte antigen (HLA) mismatch, and high panel reactive antibody (PRA) levels. Each of these factors contributes to the recipient’s immunological and metabolic stress, thereby affecting early graft recovery and long-term outcomes. Additionally, perioperative factors such as delayed initiation of immunosuppression, hemodynamic instability, intraoperative hypotension, and the use of nephrotoxic drugs may further exacerbate graft dysfunction. Therefore, understanding the intricate interplay between donor, recipient, and perioperative determinants is essential for interpreting the predictive value of hematological and biochemical parameters in our study and for identifying potential avenues to improve early graft outcomes and overall transplant success.
The Kidney Donor Risk Index (KDRI) is a validated and widely used tool that quantifies the relative risk of graft failure associated with donor characteristics. It was developed to improve kidney allocation decisions by providing an objective measure of donor organ quality. The KDRI incorporates multiple donor parameters, including age, cause of death, race, history of hypertension or diabetes, serum creatinine at procurement, height, weight, and whether the donation was after cardiac death (DCD). Each factor is assigned a weight based on its impact on long-term graft survival. A higher KDRI value indicates a greater predicted risk of graft failure and is inversely related to graft survival potential.
The index was later standardized into the Kidney Donor Profile Index (KDPI) to express donor risk as a percentile relative to the national donor pool. This made it easier to interpret for organ allocation systems, with lower KDPI kidneys representing better quality grafts. Studies have demonstrated that kidneys with higher KDRI/KDPI values are more prone to DGF, early graft dysfunction, and reduced long-term survival, especially when combined with prolonged cold ischemia time or suboptimal recipient factors.
Despite its robustness, KDRI focuses solely on donor-related variables and overlooks recipient-specific factors such as immune sensitization, inflammation, and nutritional status, all of which influence post-transplant outcomes. Thus, while KDRI is invaluable in assessing donor organ quality, integrating it with recipient biomarkers like AEC and AGR could provide a more holistic prediction model for DGF and overall transplant success.
Lower urine output after renal transplantation is a well-established clinical indicator of DGF and reflects impaired early graft recovery [17]. Normally, successful engraftment is characterized by prompt diuresis and a rapid decline in serum creatinine, signifying good perfusion and tubular function. Persistently low urine output and a slow decline in creatinine suggest ischemia–reperfusion injury, acute tubular necrosis, or early immune-mediated dysfunction. The creatinine clearance ratio (CCR), measured within the first few postoperative days, offers a more dynamic assessment of graft function than serum creatinine alone. It quantifies renal clearance capacity by comparing postoperative creatinine clearance to expected physiological values. A low CCR (typically <30–40%) on postoperative day 2 has been correlated with DGF and inferior short-term graft outcomes [2,17,18].
CCR reflects both glomerular filtration and tubular function, offering early insight into graft recovery kinetics. Unlike serum creatinine, which can be influenced by factors such as hydration status or muscle mass, CCR provides a functional measure of renal performance. Studies suggest that serial CCR monitoring during the initial postoperative days can help clinicians distinguish transient graft dysfunction from evolving DGF [18]. Integration of CCR with other perioperative markers—such as urine output trends, inflammatory parameters, and donor factors—can improve risk stratification and guide timely therapeutic interventions. Our study found that the creatinine clearance ratio on postoperative days 1 and 2 revealed significantly lower values among the DGF group than the non-DGF group.
Limitations: It is vital to acknowledge the limitations of the current study. Firstly, the study findings cannot be generalized due to the study’s single-center design and small sample size. The results should therefore be interpreted with caution and considered hypothesis-generating. A multicentric, prospective study with follow-ups in the future could address this preliminary observation. Secondly, this study was retrospective in nature and based primarily on recipient data. Detailed donor-related parameters such as donor age, cause of death, and pre-donation creatinine were not consistently available and, therefore, could not be analyzed. The absence of these variables may have influenced the predictive strength of the proposed biomarkers. Thirdly, hematologic indices are easy to measure, but their effectiveness can be affected by various factors. This can lead to different cut-off points, which makes it challenging to compare results across different studies. Therefore, it is essential to consider these indices as one part of a complete clinical evaluation instead of relying solely on them as diagnostic tools.
6. Conclusions
In summary, our study suggests that preoperative AEC and AGR may serve as novel, cost-effective, recipient-derived predictors of DGF in deceased donor kidney transplantation. When integrated with established donor-based parameters and recipient factors, these biomarkers may enhance early identification of high-risk patients. Larger prospective, multicenter studies incorporating both donor and recipient characteristics are warranted to validate these findings and develop unified predictive models for clinical use.
Author Contributions
Conceptualization, A.V.B.K.; Software, A.V.B.K.; Validation, K.V.; Formal analysis, A.V.B.K.; Investigation, A.V.B.K.; Data curation, A.S.; Writing—original draft, A.V.B.K.; Writing—review and editing, K.R.S.; Supervision, A.C., S.P. and P.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical approval for the study was obtained from the institutional ethics committee of Kasturba Medical College and Kasturba Hospital (IEC1: 43/2024, Approval Date: 11 March 2024). The study was conducted adhering to the Declaration of Helsinki.
Informed Consent Statement
The Institutional Ethics Committee of Kasturba Medical College and Kasturba Hospital approved the waiver of consent to publish from study participants due to the retrospective, non-interventional nature of the study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
List of Abbreviations
| DGF | Delayed Graft Function |
| SGF | Slow Graft Function |
| IGF | Immediate Graft Function |
| AEC | Absolute Eosinophil Count |
| AGR | Albumin–Globulin Ratio |
| CCR1 | Creatinine Clearance Ratio 1 |
| CCR2 | Creatinine Clearance Ratio 2 |
| CKD | Chronic Kidney Disease |
| DDKT | Deceased donor kidney transplant |
| ATN | Acute Tubular Injury |
| PPV | Positive Predictive Value |
| NPV | Negative Predictive Value |
| ROC | Receiver Operating Curve |
| AUC | Area Under Curve |
References
- Kovesdy, C.P. Epidemiology of Chronic Kidney Disease: An Update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, E.; Ruiz, J.C.; Piñera, C.; Fernández-Fresnedo, G.; Escallada, R.; Palomar, R.; Cotorruelo, J.G.; Zubimendi, J.A.; Martín De Francisco, A.L.; Arias, M. Creatinine Reduction Ratio on Post-Transplant Day Two as Criterion in Defining Delayed Graft Function. Am. J. Transplant. 2004, 4, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, K.B.; Baar, W.; Silbach, K.; Knörlein, J.; Jänigen, B.; Kaben, J.; Heinrich, S.; Pisarski, P.; Buerkle, H.; Göbel, U. Modifiable Risk Factors for Delayed Graft Function After Deceased Donor Kidney Transplantation. Prog. Transplant. 2019, 29, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Halloran, P.F.; Hunsicker, L.G. Delayed Graft Function: State of the Art, November 10-11, 2000. Summit Meeting, Scottsdale, Arizona, USA. Am. J. Transplant. 2001, 1, 115–120. [Google Scholar] [CrossRef]
- Ponticelli, C.; Reggiani, F.; Moroni, G. Delayed Graft Function in Kidney Transplant: Risk Factors, Consequences and Prevention Strategies. J. Pers. Med. 2022, 12, 1557. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; He, X.; Fang, W.; Zhan, J.; Hong, S.; Qin, T.; Ma, Y.; Sheng, J.; Zhou, N.; Zhao, Y.; et al. Pretreatment Albumin/Globulin Ratio Predicts the Prognosis for Small-Cell Lung Cancer. Medicine 2016, 95, e3097. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Fu, X.; Yuan, X.; Li, J.; Wang, H.; Sun, J.; Wu, J.; Tang, L. Serum Albumin to Globulin Ratio Prior to Treatment as a Potential Non-Invasive Prognostic Indicator for Urological Cancers. Front. Nutr. 2022, 9, 1012181. [Google Scholar] [CrossRef] [PubMed]
- Azab, B.N.; Bhatt, V.R.; Vonfrolio, S.; Bachir, R.; Rubinshteyn, V.; Alkaied, H.; Habeshy, A.; Patel, J.; Picon, A.I.; Bloom, S.W. Value of the Pretreatment Albumin to Globulin Ratio in Predicting Long-Term Mortality in Breast Cancer Patients. Am. J. Surg. 2013, 206, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Dialameh, H.; Menbari Oskouie, I.; Kasaeian, A.; Mousavi, S.H.; Ghaderi, A.; Sharafi, A.; Khalili, A.; Nazarpour, M.J. Albumin-to-Globulin and Neutrophil-to-Lymphocyte Ratios: Potential Prognostic Markers for Delayed Graft Function in Kidney Transplantation. Transl. Res. Urol. 2025, 7, 61–69. [Google Scholar] [CrossRef]
- Wang, G.-Y.; Li, H.; Liu, W.; Zhang, J.; Zhu, H.-B.; Wang, G.-S.; Zhang, Q.; Yang, Y.; Chen, G.-H. Elevated Blood Eosinophil Count Is a Valuable Biomarker for Predicting Late Acute Cellular Rejection after Liver Transplantation. Transplant. Proc. 2013, 45, 1198–1200. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Perálvarez, M.; Germani, G.; Tsochatzis, E.; Rolando, N.; Luong, T.V.; Dhillon, A.P.; Thorburn, D.; O’Beirne, J.; Patch, D.; Burroughs, A.K. Predicting Severity and Clinical Course of Acute Rejection after Liver Transplantation Using Blood Eosinophil Count. Transpl. Int. 2012, 25, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Onyema, O.O.; Guo, Y.; Hata, A.; Kreisel, D.; Gelman, A.E.; Jacobsen, E.A.; Krupnick, A.S. Deciphering the Role of Eosinophils in Solid Organ Transplantation. Am. J. Transplant. 2020, 20, 924–930. [Google Scholar] [CrossRef]
- Goldwasser, P.; Feldman, J. Association of Serum Albumin and Mortality Risk. J. Clin. Epidemiol. 1997, 50, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Greene, T.; Wang, X.; Pereira, A.A.; Marcovina, S.M.; Beck, G.J.; Kusek, J.W.; Collins, A.J.; Levey, A.S.; Sarnak, M.J. C-Reactive Protein and Albumin as Predictors of All-Cause and Cardiovascular Mortality in Chronic Kidney Disease. Kidney Int. 2005, 68, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Molnar, M.Z.; Kovesdy, C.P.; Bunnapradist, S.; Streja, E.; Mehrotra, R.; Krishnan, M.; Nissenson, A.R.; Kalantar-Zadeh, K. Associations of Pretransplant Serum Albumin with Post-Transplant Outcomes in Kidney Transplant Recipients. Am. J. Transplant. 2011, 11, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Soeters, P.B.; Wolfe, R.R.; Shenkin, A. Hypoalbuminemia: Pathogenesis and Clinical Significance. J. Parenter. Enter. Nutr. 2018, 43, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, E.; Miñambres, E.; Ruiz, J.C.; Ballesteros, A.; Piñera, C.; Quintanar, J.; Fernández-Fresnedo, G.; Palomar, R.; Gómez-Alamillo, C.; Arias, M. Prediction of Delayed Graft Function by Means of a Novel Web-Based Calculator: A Single-Center Experience. Am. J. Transplant. 2012, 12, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Govani, M.V.; Kwon, O.; Batiuk, T.D.; Milgrom, M.L.; Filo, R.S. Creatinine Reduction Ratio and 24-Hour Creatinine Excretion on Posttransplant Day Two: Simple and Objective Tools to Define Graft Function. J. Am. Soc. Nephrol. 2002, 13, 1645–1649. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).