A Two-Filter Adaptation to Achieve Enhanced Hemodialysis Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Bench Model
2.2. Clinical Evaluation
3. Results
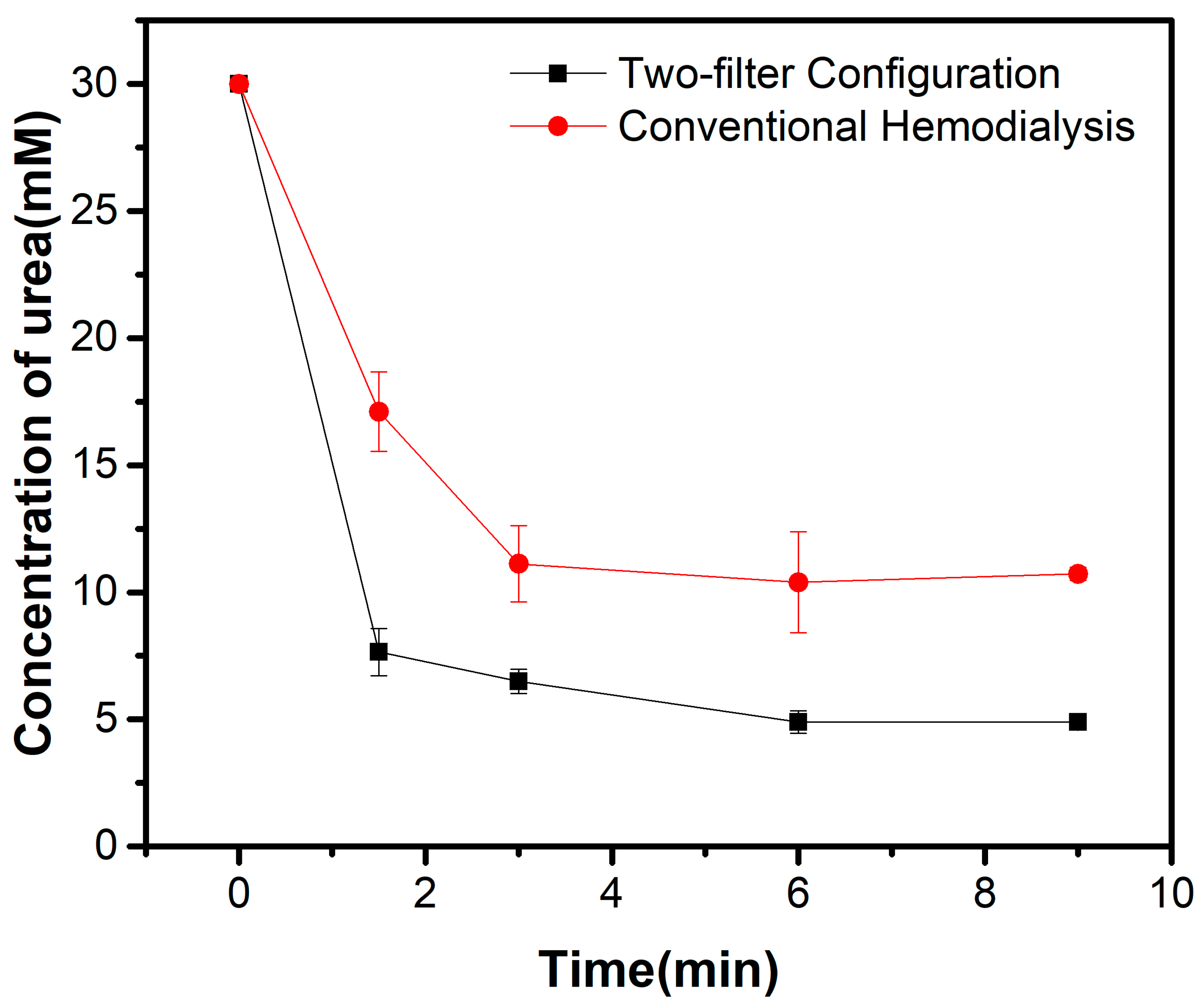
3.1. Bench Model Performance
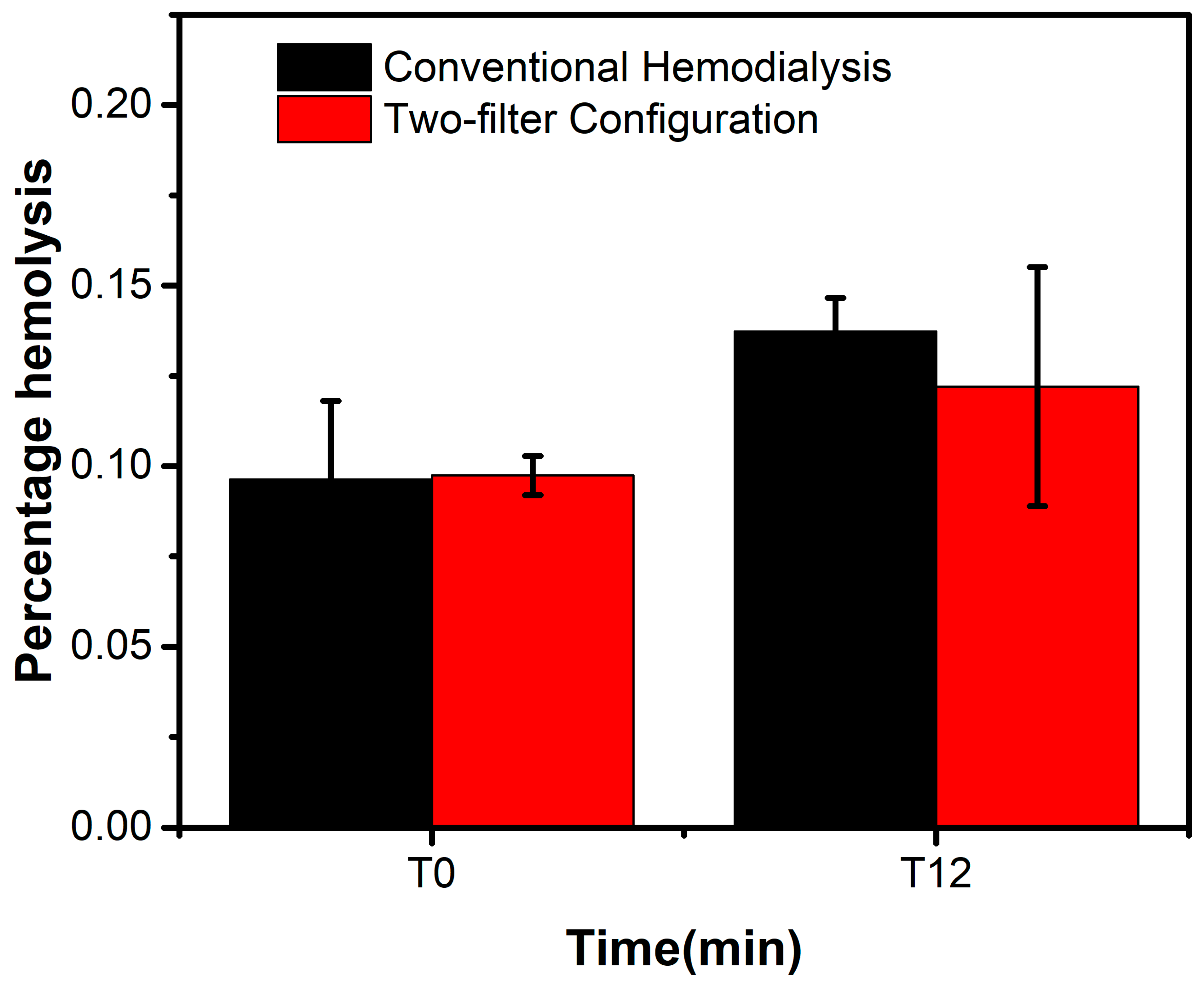
3.2. Clinical Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gottschalk, C.W.; Fellner, S.K. History of the science of dialysis. Am. J. Nephrol. 1997, 17, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Kolff, W.J.; Berk, H.T.; ter Welle, M.; van der Ley, A.J.; van Dijk, E.C.; van Noordwijk, J. The artificial kidney: A dialyser with a great area. 1944. J. Am. Soc. Nephrol. 1997, 8, 1959–1965. [Google Scholar] [CrossRef] [PubMed]
- Karkar, A. Advances in Hemodialysis Techniques. In Hemodialysis; IntechOpen: Rijeka, UK, 2013. [Google Scholar] [CrossRef][Green Version]
- Pisitkun, T.; Eiam-Ong, S.; Tiranathanagul, K.; Sakunsrijinda, C.; Manotham, K.; Hanvivatvong, O.; Suntaranuson, P.; Praditpornsilpa, K.; Chusil, S.; Tungsanga, K. Convective-controlled double high flux hemodiafiltration: A novel blood purification modality. Int. J. Artif. Organs 2004, 27, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.A. Development of a Microfluidic Hemodialysis Device Equipped with an Antifouling Nanoporous Dialysis Membrane. Master’s Thesis, University of Waterloo, Waterloo, ON, Canada, 2021; pp. 30–33. [Google Scholar]
- Ledebo, I. Principles and Practice of Hemofiltration and Hemodiafiltration. Artif. Organs 1998, 22, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Tattersall, J. Clearance of Beta-2-Microglobulin and Middle Molecules in Haemodiafiltration. Contrib. Nephrol. 2007, 158, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Canova, C.; Mancini, E.; Deppisch, R.; Beck, W. Protein Loss in On-Line Hemofiltration. Blood Purif. 2004, 22, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Blackshear, P.L., Jr.; Dorman, F.D.; Steinbach, J.H. Some mechanical effects that influence hemolysis. ASAIO J. 1965, 11, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Lawson, D.S.; Ing, R.; Cheifetz, I.M.; Walczak, R.; Craig, D.; Schulman, S.; Kern, F.; Shearer, I.R.; Lodge, A.; Jaggers, J. Hemolytic characteristics of three commercially available centrifugal blood pumps. Pediatr. Crit. Care Med. 2005, 6, 573–577. [Google Scholar] [CrossRef]
- Tharmaraj, D.; Kerr, P.G. Haemolysis in haemodialysis. Nephrology 2017, 22, 838–847. [Google Scholar] [CrossRef] [PubMed]
- National Kidney Foundation. KDOQI Clinical Practice Guideline for Hemodialysis Adequacy: 2015 update. Am. J. Kidney Dis. 2015, 66, 884–930. [Google Scholar] [CrossRef] [PubMed]
- Pyart, R.; Magadi, W.; Steenkamp, R.; Davenport, A. UK Renal Registry 20th Annual Report: Chapter 6 Adequacy of Haemodialysis in UK Adult Patients in 2016: National and Centre-specific Analyses. Nephron 2018, 139 (Suppl. S1), 151–164. [Google Scholar] [CrossRef]
- Liang, K.V.; Zhang, J.H.; Palevsky, P.M. Urea reduction ratio may be a simpler approach for measurement of adequacy of intermittent hemodialysis in acute kidney injury. BMC Nephrol. 2019, 20, 82. [Google Scholar] [CrossRef]
- Lindley, E.; Keane, D. Are haemodialysis patients at risk of excessive dehydration? Ren. Soc. Australas. J. 2013, 9, 110–111. [Google Scholar]
- ELISIO TM-H Dialyzer Brochure; Nipro Medical Corporation: Bridgewater, NJ, USA, 2014. Available online: https://nipro-group.picturepark.com/v/DuZeNGdg/21DF4A9BEA78247426995C2E23BFF17E297A33D8.pdf (accessed on 30 June 2024).
- Külz, M.; Nederlof, B.; Schneider, H. In vitro and in vivo evaluation of a new dialyzer. Nephrol. Dial. Transplant. 2002, 17, 1475–1479. [Google Scholar] [CrossRef] [PubMed]
- Lowrie, E.G.; Chertow, G.M.; Lew, N.L.; Lazarus, J.M.; Owen, W.F. The urea {clearance × dialysis time} product (Kt) as an outcome-based measure of hemodialysis dose. Kidney Int. 1999, 56, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Kim-Shapiro, D.B.; Lee, J.; Gladwin, M.T. Storage lesion: Role of red blood cell breakdown. Transfusion 2011, 51, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Storr, M.; Ward, R.A. Membrane innovation: Closer to native kidneys. Nephrol. Dial. Transplant. 2018, 33 (Suppl. S3), iii22–iii27. [Google Scholar] [CrossRef] [PubMed]
- Schepers, E.; Glorieux, G.; Eloot, S.; Hulko, M.; Boschetti-De-Fierro, A.; Beck, W.; Krause, B.; Van Biesen, W. Assessment of the association between increasing membrane pore size and endotoxin permeability using a novel experimental dialysis simulation set-up. BMC Nephrol. 2018, 19, 1. [Google Scholar] [CrossRef] [PubMed]
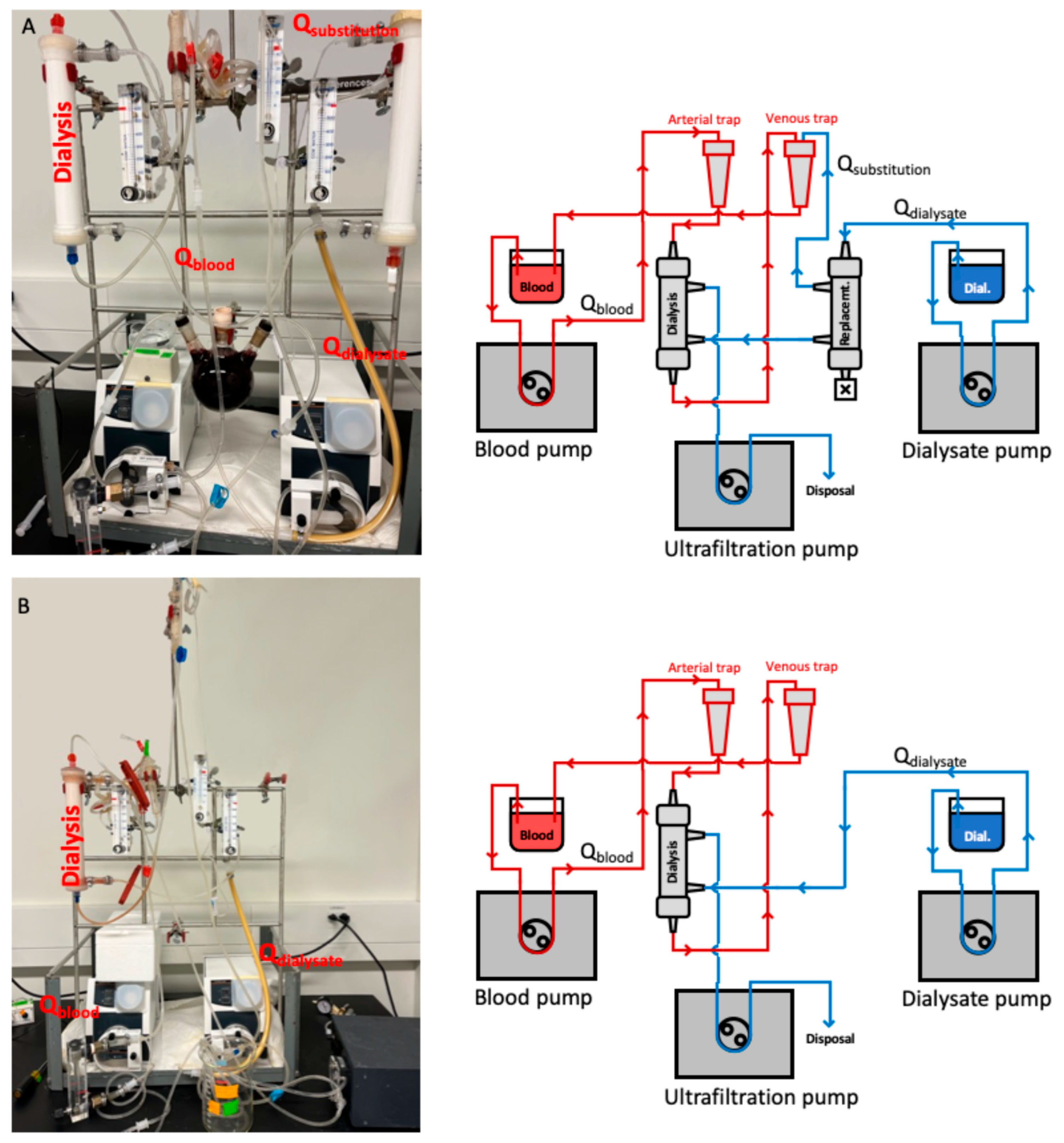

| Parameter | Value |
|---|---|
| Dialysate flow rate (QD) | 600 mL/min |
| Blood flow rate (QB) | 400 mL/min |
| Substitution rate (QS) | 44 mL/min |
| Ultrafiltration rate (Quf) | 8.3 mL/min |
| Performance | Conventional HD | Two-Filter Configuration | p-Value * |
|---|---|---|---|
| Urea Clearance | 165.41 ± 22.38 mL/min | 254.90 ± 12.17 mL/min | 0.003 |
| Albumin Loss | 0.23 ± 0.025 µg/mL | 0.06 ± 0.036 µg/mL | 0.014 |
| Percentage Change in Hemolysis | 12.26 ± 16.7% | 8.87 ± 9.15% | 0.78 |
| Conventional HD | Conventional Hemodiafiltration (HDF) | Two-Filter Configuration | |
|---|---|---|---|
| Kt/V | 1.60 | 1.70 | 1.82 |
| URR (%) | 70 | 75 | 80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, K.; Li, P.; Ausri, I.; Cañizares, B.; Vasconez, C.; Guo, Z.; Tang, X. A Two-Filter Adaptation to Achieve Enhanced Hemodialysis Performance. Kidney Dial. 2025, 5, 52. https://doi.org/10.3390/kidneydial5040052
Chu K, Li P, Ausri I, Cañizares B, Vasconez C, Guo Z, Tang X. A Two-Filter Adaptation to Achieve Enhanced Hemodialysis Performance. Kidney and Dialysis. 2025; 5(4):52. https://doi.org/10.3390/kidneydial5040052
Chicago/Turabian StyleChu, Kyle, Pei Li, Irfani Ausri, Bernardo Cañizares, Cesar Vasconez, Zilei Guo, and Xiaowu (Shirley) Tang. 2025. "A Two-Filter Adaptation to Achieve Enhanced Hemodialysis Performance" Kidney and Dialysis 5, no. 4: 52. https://doi.org/10.3390/kidneydial5040052
APA StyleChu, K., Li, P., Ausri, I., Cañizares, B., Vasconez, C., Guo, Z., & Tang, X. (2025). A Two-Filter Adaptation to Achieve Enhanced Hemodialysis Performance. Kidney and Dialysis, 5(4), 52. https://doi.org/10.3390/kidneydial5040052






