1. Introduction
Diabetic ketoacidosis (DKA) is a common presentation in acute care. Non-diabetic forms may also occur in adults who are starving, pregnant, who consume excess alcohol, or as a side effect of certain medications when compounded by dehydration or fluid restriction [
1]. The global burden of diabetes mellitus (DM) continues to grow, contributing to the increasing prevalence of kidney failure [
2], a trend mirrored in the United Kingdom (UK) [
3]. For patients receiving dialysis, DM management presents a unique challenge, due to complications [
4], including DKA [
5]. Although comprehensive guidelines exist for DKA management in the general population, evidence supporting their direct application to individuals with kidney failure is scarce [
6,
7]. Factors such as impaired renal insulin clearance, altered fluid and electrolyte homeostasis, and the absence of osmotic diuresis, render standard DKA protocols suboptimal and potentially harmful [
8,
9]. The clinical harm from misapplication of these guidelines can lead to treatment induced fluid overload, profound hypoglycaemia, and severe electrolyte derangements [
8,
9]. Diabetic ketoacidosis (DKA) arises as a result of insulin deficiency, leading to hyperglycaemia, dehydration, and electrolyte imbalance. In the presence of normal renal function, this results in hypovolaemia and reduced total body potassium, despite high blood levels. In kidney failure or on dialysis, the presentation may be altered and harder to recognize due to changes in fluid retention and the effects of dialysis. Peritoneal dialysis may exacerbate hyperglycaemia via dialysate glucose. Net volume status depends on ultrafiltration and prescription. Haemodialysis may temporarily correct blood glucose and potassium, masking diagnostic features of DKA. Kidney transplant recipients also face increased risk of diabetes and DKA due to medications and other factors [
8].
This narrative review combines published literature and bedside experience to propose a practical approach to managing DKA in dialysis patients. The unique metabolic features that distinguish this population are explored, to delineate the importance of identifying fluid volume status, in guiding individualized strategies for fluid, insulin, and electrolyte management [
8]. The human factors and real-world challenges to clinical staff inherent in deviating from familiar protocols are also explored, emphasizing the need for interdisciplinary collaboration and the development of context-specific guidelines (
Table 1). This manuscript aimed to identify common themes about diabetes ketoacidosis management in patients diagnosed with kidney failure undergoing renal dialysis, by integrating physiological principles, clinical practice, expert reviews and clinical guidelines to equip clinicians with the knowledge and insights required to navigate this complex emergency safely and effectively, improving patient outcomes.
2. Methods
This manuscript presents a narrative review; a synthesis approach suited to exploring complex clinical topics where randomised controlled trials may be scarce or ethically challenging. Our objective was to provide a comprehensive overview of diabetic ketoacidosis management in dialysis patients, integrating physiological principles with practical clinical considerations. Search was conducted through A search strategy was applied using PubMed® (n = 10), Embase® (n = 7) and Google® Scholar™ (n = 28), for articles published between January 2004–June 2024. A combination of keywords and Medical Subject Headings (MeSH) terms, including “diabetic ketoacidosis”, “DKA”, “kidney failure”, “dialysis”, “anuria”, “insulin kinetics”, “fluid balance”, and “cerebral oedema.”
Adults with kidney failure on dialysis; included observational studies, reviews, guidelines, consensus statements, and physiological papers. Case reports used only for illustration. Excluded paediatric and non-English publications where translation was not feasible. Articles used were dialysis-specific physiology, guideline statements, and observational cohorts; single case reports were weighted low. The initial search yielded 45 records, of which 27 were retained for final synthesis Nephrology-led authorship prioritised safety and pragmatic applicability; contradictory recommendations were deliberately sought and integrated.
3. Pathophysiological Nexus: Metabolic Alterations in Dialysis-Associated DKA
DKA is characterised by hyperglycaemia, metabolic acidosis, and ketonaemia and their underlying drivers and physiological responses profoundly modified in kidney failure [
10].
Firstly, the reduced renal insulin clearance in kidney failure patients prolongs the half-life of exogenous insulin [
8,
11,
12,
13]. This means that insulin administered to a dialysis patient will persist in the circulation for longer than in an individual with normal renal function, necessitating lower doses and a heightened vigilance for hypoglycaemia. Compounding this, is the phenomenon of uraemicinsulin resistance; a complex interplay of post-receptor defects and circulating uraemictoxins that impair the action of insulin at the cellular level [
8,
9,
14]. This resistance can lead to seemingly paradoxical insulin requirements; while overall insulin clearance is reduced, the degree of resistance can vary, making insulin requirements non-linear and difficult to predict [
8,
9,
14]. This delicate balance between reduced clearance and variable resistance underscores why standard insulin protocols frequently overshoot their therapeutic targets in this population [
15].
The kidneys also contribute to glucose homeostasis through gluconeogenesis. In kidney failure, this capacity is reduced, predisposing patients to hypoglycaemia during insulin therapy, particularly if caloric intake is low or insulin is escalated rapidly [
15]. In contrast, preserved renal gluconeogenesis in individuals with healthy kidneys provides a degree of protection against insulin-induced hypoglycaemia [
16].
Perhaps the most striking physiological divergence in DKA management for dialysis patients is the lack of osmotic diuresis [
4,
9,
14]. In typical DKA, hyperglycaemia drives a profound osmotic diuresis, leading to significant fluid and electrolyte losses [
17], necessitating aggressive fluid resuscitation to prevent hypovolaemic shock. However, anuric dialysis patients, by definition, cannot excrete urine. Consequently, they do not experience these fluid losses, rendering standard aggressive fluid protocols both unnecessary and actively dangerous [
18]. Administering large volumes of intravenous fluids to an anuric patient will lead to volume overload, precipitating life-threatening pulmonary oedema and exacerbating hypertension. This fundamental difference mandates a complete paradigm shift in fluid management in this group.
Another critical consideration is increased extracellular potassium (K
+). While DKA in the general population often presents with total body potassium depletion despite normal or elevated serum potassium (due to acidosis-induced extracellular shift), dialysis patients often have elevated total body potassium stores due to impaired renal excretion, although exceptions occur with malnutrition or catabolism [
8,
9,
18]. The acidosis and insulin deficiency of DKA will further drive potassium out of cells, leading to profound and potentially life-threatening hyperkalaemia. Unlike patients with normal renal function who can clear excess potassium once insulin therapy is initiated, dialysis patients lack this compensatory mechanism, making hyperkalaemia a persistent and dangerous threat [
15].
Finally, the volume status variability in dialysis patients is a constant challenge. Unlike the predictable hypovolemia of typical DKA, dialysis patients can present as hypovolaemic (e.g., due to severe vomiting or diarrhoea), euvolaemic, or, most commonly, hypervolemic (due to missed dialysis sessions, excessive interdialytic weight gain, or fluid shifts during DKA). Accurately assessing their true volume status is paramount to guiding the approach to fluid therapy. This dynamic and often precarious fluid balance requires meticulous clinical assessment and continuous reassessment, moving beyond a conventional approach to fluid resuscitation [
8,
9].
Clinical note: Rapid correction of hyperglycaemia, particularly if combined with haemodialysis, can precipitate cerebral oedema due to abrupt osmotic shifts. This risk underscores the importance of gradual correction and careful modality choice.
4. Volume Phenotyping and Fluid Strategy
Accurate assessment of volume status is arguably the single most critical determinant of safe and effective DKA management in dialysis patients. Unlike the predictable hypovolaemia of typical DKA, dialysis patients may be hypovolaemic, euvolaemic, or more commonly hypervolaemic; accurate assessment of this true phenotype is paramount and dictates the approach to fluid therapy. Their fluid status is heterogeneous, ranging from profound dehydration to life-threatening fluid overload. Failure to correctly identify a patient’s volume status can lead to treatment induced harm, particularly from injudicious fluid administration [
19]. Clinicians must therefore be observant for subtle cues and integrate multiple data points to make informed decisions.
As previously noted, dialysis patients with DKA may be categorized into three volume-based types: hypovolaemic, euvolaemic, and hypervolemic [
8,
20]. Hypovolemia, though less common than in the general DKA population, may occur, particularly in patients experiencing significant extrarenal fluid losses such as protracted vomiting or severe diarrhoea. Patients may present with signs of dehydration, orthostatic hypotension, or even overt shock. Conversely, many dialysis patients, especially those who have missed dialysis sessions or have significant interdialytic weight gain, will present in a euvolaemic or, more frequently, a hypervolemic state [
11,
14,
20]. The osmotic shift of water from the intracellular to the extracellular compartment due to hyperglycaemia can transiently mask true volume status, but as glucose levels fall with insulin therapy, this fluid can shift back, unmasking or exacerbating fluid overload. Pulmonary oedema, elevated jugular venous pressure, and peripheral oedema are classic manifestations [
11,
14,
20]. In typical DKA, hyperglycaemia drives osmotic diuresis with profound fluid loss; however, anuric dialysis patients cannot excrete urine, and aggressive fluid protocols may precipitate pulmonary oedema. Missed dialysis sessions, interdialytic weight gain, or osmotic shifts unmasked during glucose correction are common hypervolaemic triggers.
To guide decision-making, a structured approach is essential: take a detailed history (dialysis attendance, interdialytic weight gain, vomiting/diarrhoea), perform a focused examination, and use ancillary investigations judiciously. Volume status should be rephenotyped every 2–4 h, as osmotic shifts can alter congestion and haemodynamics during therapy.
Table 2 provides a framework for phenotype-specific strategies [
8,
20].
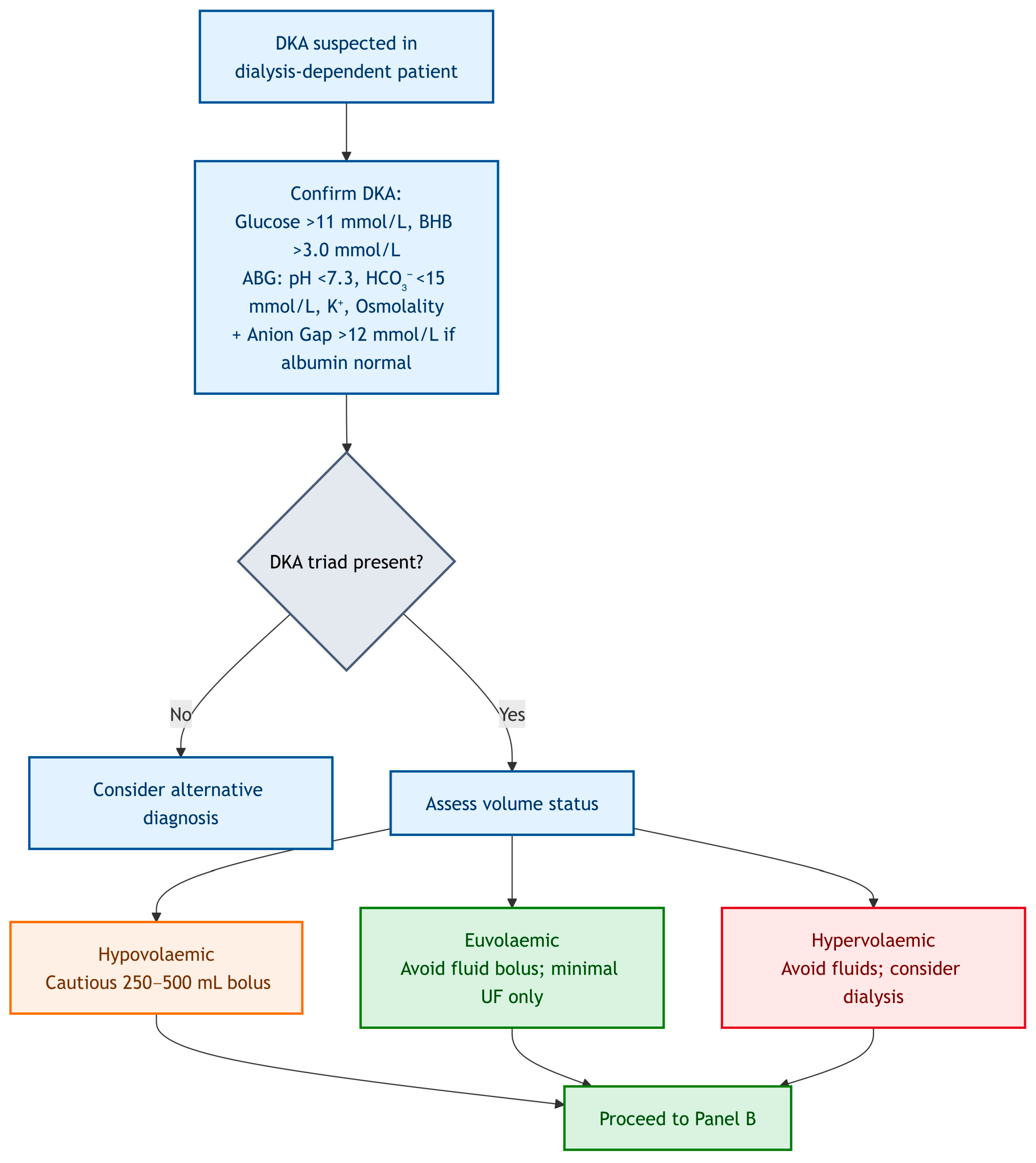
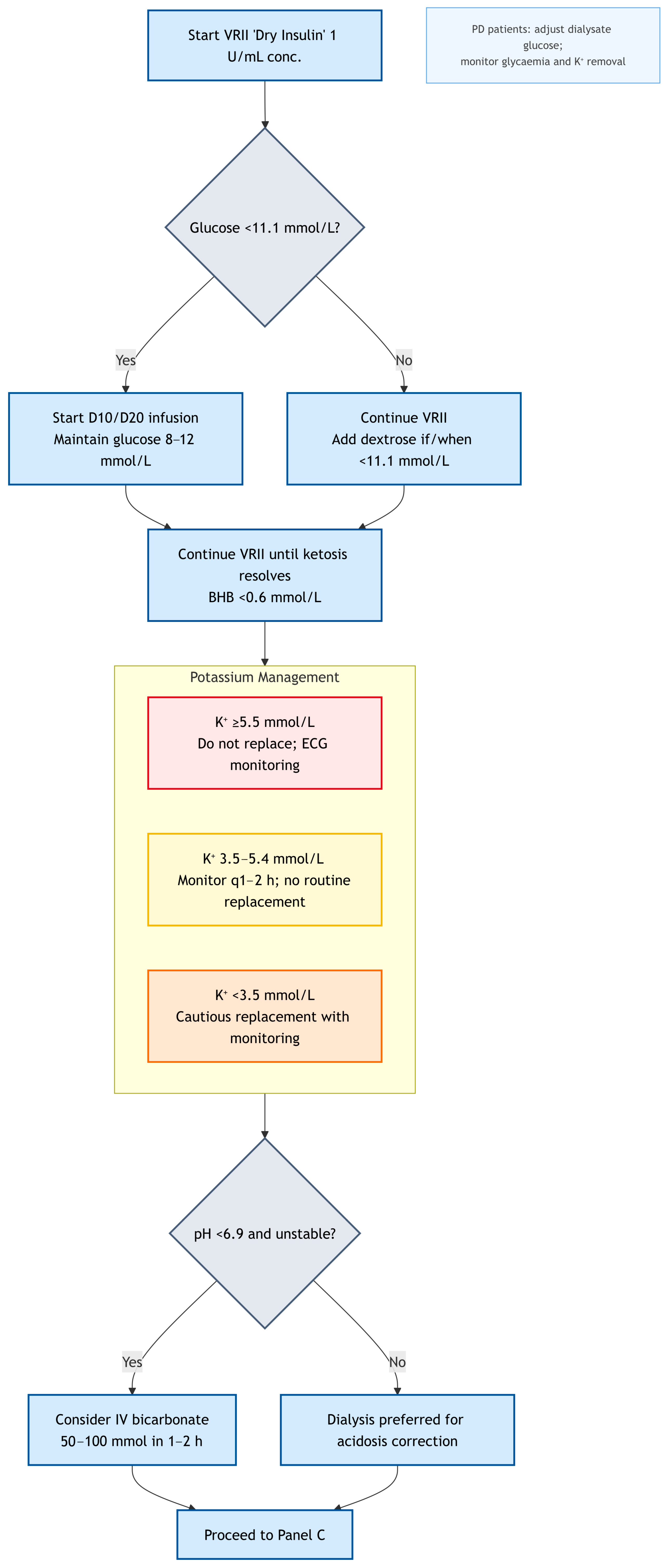
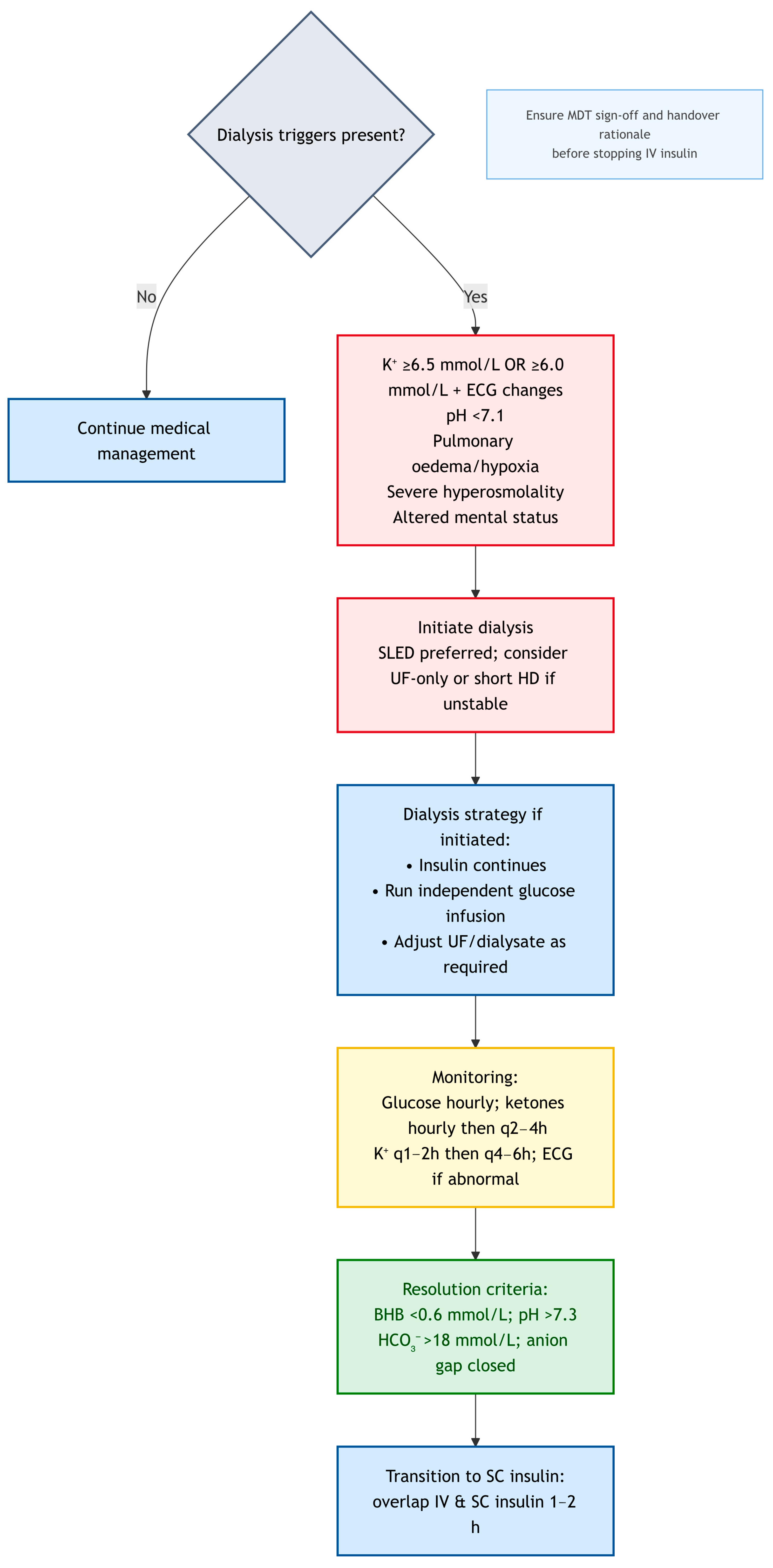
Navigating the complexities of DKA in dialysis patients, especially during on-call hours, benefits from a clear, actionable framework. The algorithm presented was designed to guide clinicians through the initial assessment and management decisions, emphasizing the critical deviations from standard DKA protocols. It is not a substitute for clinical judgment or specialist consultation but serves as a cognitive aid for rapid, safe intervention. For clinical clarity, we propose management in this cohort should always begin with: (i) early measurement of β-hydroxybutyrate (BHB), potassium, and serum osmolality; (ii) recognition that volume status is not uniform but phenotype-specific; and (iii) framing insulin as a tool for ketosis resolution, not just glucose control. This structured approach; while demanding, it can significantly reduce the risk of treatment induced complications and optimizes outcomes for this highly vulnerable patient population. Always remember; think physiology, assess phenotype, and consult early. To guide clinicians through the complexities of DKA management in dialysis-dependent patients, we propose a structured diagnostic and management algorithm. For clarity and readability, the pathway has been divided into three linked panels, each addressing a distinct stage of care.
Figure 1 illustrates the diagnostic confirmation and volume phenotyping step;
Figure 2 presents the tailored insulin, glucose, potassium, and acidosis management strategy; and
Figure 3 summarises dialysis triggers, modality choice, and transition to resolution.
Figure 1,
Figure 2 and
Figure 3 operationalise the text targets: VRII titrated to ketone clearance, glucose maintained at 8–12 mmol/L, conservative fluids by phenotype, and electrolyte/dialysis triggers as stated.
A patient with DKA and kidney failure who presented with several days of intractable vomiting and minimal oral intake. Despite being anuric, this patient may genuinely be hypovolaemic and require careful fluid resuscitation.
By contrast, a patient who missed their last two dialysis sessions and presented with DKA and new-onset dyspnoea is almost certainly hypervolemic, and additional fluid would be detrimental.
The key is to move beyond the aggressive fluid resuscitation seen in typical DKA and instead adopt an individualized approach based upon assessed fluid status. To truly appreciate the complexities and apply the principles discussed, it is invaluable to consider the following vignettes which are adopted from real-world clinical scenarios with an added educational value. These cases highlight the importance of meticulous assessment, individualized management, and the human factors that influence decision-making.
Scenario 1 (an anuric patient with unexplained hyperglycaemia and mild acidosis): A 68-year-old man with type 2 diabetes and kidney failure on haemodialysis presented with 2 days of weakness, nausea, and reduced appetite. He missed his last dialysis. Glucose was 28 mmol/L, pH 7.28, HCO3− 16 mmol/L, anion gap 18, and ketones strongly positive. He was anuric, with mild peripheral oedema and JVP 8 cm; lungs were clear. Clinical challenge: Standard reflex to give fluid boluses would be harmful. Application: He is euvolaemic–mildly hypervolaemic. Fluids should be withheld; insulin (dry VRII) is the primary intervention. Potassium must be monitored closely, with replacement usually contraindicated. Continuous ECG monitoring is essential when K+ ≥ 6.0 mmol/L or changing rapidly. Early nephrology input is required for possible dialysis if acidosis or hyperkalaemia worsen. Human factors: Override the bias toward fluids; align the team on a no-fluid strategy.
Scenario 2 (a hypovolaemic dialysis patient with DKA): A 55-year-old female with Type 1 Diabetes Mellitus and kidney failure on haemodialysis presents with severe abdominal pain, intractable vomiting, and diarrhoea for 12 h. She was usually dialyzed three times a week but missed her last session due to illness. Her blood glucose was 35 mmol/L, pH 7.15, bicarbonate 10 mmol/L, anion gap 22 mmol/L, and ketones are strongly positive. She was hypotensive (BP 80/50 mmHg) and tachycardic (HR 110 bpm). Her JVP was flat, and skin turgor was poor. She reported significant weight loss since her last dialysis. Clinical Challenge: This patient presents with clear signs of hypovolaemic shock in the setting of DKA. While she is a dialysis patient, her significant extrarenal fluid losses (vomiting and diarrhoea) have rendered her genuinely hypovolaemic. The key here is to recognise that not all dialysis patients are hypervolemic, and a blanket fluid restriction would be harmful. Application of Principles: Volume phenotyping correctly identifies her as hypovolaemic. Cautious, small boluses of balanced electrolyte solution (e.g., 250 mL) should be administered, with frequent reassessment of haemodynamic parameters. The goal is to restore perfusion, not necessarily to achieve euvolemia. Insulin therapy (VRII) should be initiated concurrently. Potassium will need close monitoring, and given her fluid losses, she might be at higher risk of hypokalaemia once insulin starts to shift potassium intracellularly. Early and frequent communication with nephrology is vital to guide fluid replacement and plan for urgent dialysis once she is haemodynamically stable. Human Factors: Overcoming the ingrained fear of fluid administration in dialysis patients is crucial. The team must trust their clinical assessment of hypovolemia and act decisively, while remaining cautious and reassessing frequently. This scenario highlights the importance of individualized care over rigid protocols.
Scenario 3 (a DKA patient on peritoneal dialysis): A 45-year-old male with Type 2 Diabetes Mellitus and kidney failure on continuous ambulatory peritoneal dialysis (CAPD) presented with DKA. His blood glucose was 30 mmol/L, pH 7.20, Bicarbonate 14 mmol/L, anion gap 20 mmol/L, and ketones were positive. He was euvolaemic. He typically uses a 2.5% w/v Glucose dialysate. Clinical Challenge: Managing DKA in PD patients requires careful consideration of the dialysate. The glucose in the dialysate can contribute to hyperglycaemia, and rapid changes can lead to osmotic shifts. Application of Principles: Insulin therapy (VRII) is initiated. PD can generally be continued, but the dialysate glucose concentration may need adjustment. Initially, lower glucose concentrations (e.g., 1.5% or even icodextrin) might be considered to avoid exacerbating hyperglycaemia. As DKA resolves and blood glucose falls, treat hypoglycaemia first with intravenous glucose while continuing insulin for ketone clearance; only if hypoglycaemia recurs despite this should the PD prescription be cautiously adjusted (e.g., modestly higher dialysate glucose or shorter dwells) with close monitoring. Frequent monitoring of blood glucose and potassium is essential, as PD can also remove potassium. The patient’s fluid balance via PD exchanges needs to be meticulously tracked. Human Factors: This scenario emphasizes the need for specialized knowledge of PD management in acute settings. Collaboration with the PD nursing team and nephrology is paramount to ensure appropriate dialysate prescription and exchange schedules.
5. Insulin Management
5.1. Physiological Rationale for Insulin Adjustment in Dialysis
Insulin therapy is the cornerstone of DKA management, driving glucose into cells, suppressing ketogenesis, and correcting acidosis [
6,
21,
22]. However, in dialysis patients, the approach to insulin administration requires significant modification from standard protocols [
11]. Clinicians must re-evaluate their approach, recognizing the unique kinetics and challenges posed by kidney failure. This is not merely about adjusting doses; it is about understanding a fundamental decoupling of glucose and insulin dynamics [
8,
9].
As previously discussed, the reduced renal insulin clearance in Kidney failure prolongs the half-life of insulin. This necessitates lower starting doses and slower titration of insulin infusions compared to patients with normal renal function. To apply standard insulin protocols, can rapidly lead to profound and protracted hypoglycaemia. Conversely, uraemicinsulin resistance can sometimes necessitate higher doses, creating a delicate and often unpredictable balance. This is where meticulous, individualized titration becomes an art, not just a science [
10,
14,
22].
5.2. VRII (“Dry Insulin”) Preparation and Strategy
For DKA in dialysis patients, a continuous variable rate insulin infusion (VRII) is the preferred method of insulin delivery. This allows for precise titration based on frequent glucose monitoring and clinical response. Standard VRII protocols, however, often involve significant volumes of diluent, which are unsuitable for fluid-restricted patients. This introduces the concept of “dry insulin”, a critical adaptation for this cohort [
23].
‘Dry insulin’ refers to the preparation of low-volume syringe-pump infusions (e.g., 1 unit/mL made by diluting 50 units of soluble insulin in 50 mL of 0.9% sodium chloride) to minimise the amount of carrier fluid required.
Dry insulin is a pragmatic solution to a complex problem, allowing clinicians to continue insulin therapy until ketosis has fully resolved, while independently managing glucose to avoid hypoglycaemia. This enables glucose-insulin decoupling—a critical concept in dialysis DKA. The Joint British Diabetes Societies (JBDS) guidance for diabetes management in adults on dialysis [
6] is consistent with this approach, emphasising careful insulin titration and minimal fluid administration [
23,
24]. This ketone-titrated infusion approach intentionally diverges from the fixed-rate insulin infusion (FRIII) used in standard DKA, reflecting altered insulin kinetics and fluid constraints in dialysis.
5.3. Practical Recommendations: Dosing, Monitoring & Transition
5.3.1. Initial Dosing and Titration in Dialysis
Starting Dose: We suggest a starting insulin infusion rate of 0.02–0.05 units/kg/hour (lower than the 0.1 units/kg/hour used in preserved renal function) to account for reduced insulin clearance and to minimise rapid hypoglycaemia. JBDS guidance [
6] advises considering a lower infusion rate when glucose is falling rapidly, although it does not specify a numeric starting dose.
Titration: Insulin infusion rates should be adjusted based on BHB and hourly capillary blood glucose (CBG) trends, aiming for a BHB fall of ≥0.5 mmol/L/h and a glucose decline of ~2–3 mmol/L/h. Avoid glucose falls >5 mmol/L/h due to risk of cerebral oedema, especially in patients with neurological vulnerability.
Glucose-Insulin Decoupling: It is crucial to remember that in dialysis patients, glucose levels may not fall as rapidly as in non-dialysis patients, even with adequate insulin. This is due to impaired gluconeogenesis and the absence of renal glucose excretion. Therefore, insulin should be continued until ketosis is resolved, even if glucose levels normalize.
Glucose infusions (e.g., 10% or 20%—to maintain glucose 8–12 mmol/L) should be initiated when glucose falls below 11.1 mmol/L to prevent hypoglycaemia while continuing insulin to clear ketones. These are dialysis-adapted thresholds to minimise fluid and hypoglycaemia risk; continue insulin until ketosis clears (e.g., β-hydroxybutyrate < 0.6 mmol/L). In dialysis patients, this requires careful management of the glucose infusions to avoid fluid overload (
Figure 4).
Safety note (lines & infusions): Use Glucose 10% via a large-bore peripheral line. For Glucose 20–50%, use central access for continuous infusion; if central access is unavailable, give only small, carefully monitored peripheral boluses with frequent site checks. Count all infused glucose toward net fluid balance. Count all infused glucose toward net fluid balance and document on the fluid chart/SOP.
5.3.2. Considerations for Insulin Administration
Insulin Resistance: In some cases, particularly with severe acidosis or significant uraemia, patients may exhibit increased insulin resistance. If glucose levels are not responding to the initial insulin infusion, gradual increases in the infusion rate may be necessary, with close monitoring for hypoglycaemia [
8,
9,
21].
Subcutaneous Insulin Transition: Once DKA has resolved (pH > 7.3, bicarbonate > 18 mmol/L, anion gap closed, and BHB < 0.6 mmol/L), the patient may be transitioned to subcutaneous insulin. This transition should occur with an overlap of at least 1–2 h between the insulin infusion and the first subcutaneous dose to prevent rebound hyperglycaemia [
6,
23].
Practical Notes: It is believed insulin should not be discontinued during dialysis; if pre-dialysis glucose is ≤11 mmol/L, begin glucose before dialysis to maintain glucose levels between 8 and 12 mmol/L during treatment. Avoid “sliding-scale” insulin regimens that adjust solely to glucose. Use a continuous intravenous insulin infusion (VRII) titrated to ketone clearance and acidosis resolution, with separate glucose to maintain 8–12 mmol/L. Frequent monitoring is mandatory (Glucose: hourly, BHB: hourly until a clear downtrend, then every 2–4 h, Potassium: every 1–2 h during initiation)
5.3.3. Glucose–Insulin Decoupling and Glucose Administration
As insulin begins to resolve ketosis, blood glucose levels will fall. In patients with normal renal function, this is typically managed by adding glucose to the intravenous fluids once glucose reaches a certain threshold (e.g., <11.1 mmol/L). This prevents hypoglycaemia while allowing insulin to continue clearing ketones. In dialysis patients, this strategy requires adaptation [
15].
Once glucose falls below 11.1 mmol/L, the focus shifts from aggressive glucose correction to maintaining euglycaemia while allowing insulin to complete ketosis resolution. At this point, a low-volume Glucose 10% or 20% infusion will prevent hypoglycaemia while allowing continued insulin administration to complete ketosis resolution—without compromising fluid restriction. This is where the concept of glucose-insulin decoupling becomes critical: insulin is still needed to clear ketones, even if glucose levels are normalizing. The choice of glucose concentration (10% or 20%) depends on the patient’s fluid status and the rate of glucose decline. Higher concentrations (e.g., 20% glucose) are preferred to minimize fluid volume. This is a delicate balance, requiring frequent glucose monitoring and proactive adjustment of glucose infusions to prevent both hypoglycaemia and fluid overload [
15].
Table 3 summarises glucose–insulin decoupling and glucose administration. It is proposed that by adopting this tailored approach to insulin management, clinicians can effectively treat DKA in dialysis patients while mitigating the risks of hypoglycaemia and fluid overload. The emphasis remains on meticulous monitoring, individualized adjustments, and avoiding rigid, one-size-fits-all protocols.
Clinician Insight: We’re not just chasing glucose numbers; we’re chasing ketones. But you can’t chase ketones if your patient has seizures from hypoglycaemia or is drowning from fluid overload. It’s a tightrope walk [
25].
6. Electrolytes and Acidosis Management
6.1. Potassium Management
In dialysis-associated DKA, it is proposed that potassium should be managed using a ‘conservative until proven necessary’ principle, recognising that total body stores are usually elevated. In typical DKA, despite often presenting with normal or even elevated serum potassium, patients are profoundly total body potassium-depleted due to urinary losses and intracellular shifts. This necessitates aggressive potassium replacement once insulin therapy is initiated. However, for dialysis patients, the landscape is fundamentally different [
13]. Dialysis patients inherently have elevated total body potassium stores due to their inability to excrete potassium. The acidosis of DKA, coupled with insulin deficiency, drives potassium out of the intracellular compartment, leading to a further rise in serum potassium. This can result in severe, life-threatening hyperkalaemia, manifesting as characteristic electrocardiogram (ECG) changes (e.g., peaked T waves, widened QRS, sinuswave pattern). Ultimately, this may precipitate cardiac arrest if not rapidly corrected (
Table 4).
Insulin plays a crucial role in shifting potassium back into the intracellular space by stimulating the sodium/potassium+-ATPase pump. As insulin therapy progresses and acidosis resolves, serum potassium levels will naturally decline. Close and frequent monitoring of serum potassium (e.g., hourly or every two hours initially) is paramount. If hypokalaemia develops (serum K+ < 3.5 mmol/L), cautious replacement may be necessary.
6.2. Acid–Base Management
Insulin corrects ketoacidosis; reserve bicarbonate for extremis—for example, PH < 6.9 with haemodynamic compromise and when dialysis is not immediately available. Bicarbonate administration should always be considered a temporising measure, not definitive therapy. Dialysis remains the safest and most effective way to correct acidosis in this population [
4]. In dialysis patients, the impaired renal buffering capacity means that the kidneys cannot effectively excrete the acid load. While insulin will reduce acid production, the clearance of accumulated acid relies on dialysis. Therefore, indications for urgent dialysis are critical considerations in this population [
8,
9].
Dialysis should be considered early if any of the criteria in
Table 5 are met. When dialysis triggers are met, urgent escalation to nephrology, critical care, and endocrinology is advised to prevent delays and fragmented care. Urgent haemodialysis can rapidly correct both hyperkalaemia and acidosis, providing a rapid and effective means to stabilize the patient. This should be considered early when these life-threatening complications arise, rather than waiting for conventional DKA therapies to slowly take effect. The decision to dialyze should be made in close consultation with a nephrologist [
4,
9,
10].
6.3. Phosphate and Magnesium
We believe derangements in phosphate and magnesium are less predictable in dialysis-associated DKA than in patients with preserved renal function, but they can still contribute significantly to morbidity. Insulin therapy may precipitate hypophosphataemia, even when pre-dialysis phosphate levels are normal. Hypomagnesaemia may worsen arrhythmias and impair potassium repletion.
Clinical insight: if Phosphate—is <0.5 mmol/L and clinical symptoms (e.g., muscle weakness, haemolysis, ventilatory failure), replacement should be cautious and ideally coordinated with dialysis scheduling. If magnesium <0.5–0.6 mmol/L or symptomatic (e.g., tetany, arrhythmias), account for dialysate Mg2+ to avoid overcorrection. Magnesium should be monitored daily and replaced cautiously in light of the dialysate composition.
6.4. Electrolyte-Specific Recommendations
Table 6 summarises the key principles for electrolyte management in the dialysis-associated DKA population.
7. Dialysis Considerations in DKA Management
7.1. Role of Dialysis in DKA
For patients already on dialysis, the modality itself becomes an integral part of DKA management, offering both therapeutic advantages and potential complications [
4]. The judicious use of dialysis, particularly haemodialysis, can be lifesaving in specific scenarios, but it must be approached with caution to avoid treatment-induced complications.
One of the most significant risks associated with dialysis during DKA, especially in patients with very high glucose levels, is the potential for cerebral oedema due to rapid osmotic shifts [
4]. As insulin therapy corrects hyperglycaemia, the extracellular osmolality decreases. If this drop is too rapid, water can shift from the extracellular space into the brain cells, leading to cerebral oedema. This risk is amplified during haemodialysis, which can rapidly remove solutes from the blood.
Therefore, when haemodialysis is indicated, it is often prudent to consider shorter or ultrafiltration-only sessions to minimize rapid osmotic gradients, particularly in the initial phases of DKA management [
8]. The goal is to correct life-threatening electrolyte imbalances (e.g., severe hyperkalaemia) or fluid overload (e.g., pulmonary oedema) without inducing neurological complications.
7.2. Sustained Low-Efficiency Dialysis (SLED)
It is proposed that SLED offers a safer alternative for many patients, providing gradual solute and acid removal with reduced risk of osmotic shifts when compared to other types of dialysis (
Table 7). Expert-opinion target; centre protocol should prevail. SLED typically limits the fall in serum osmolality to ~3–8 mOsm/kg/h; if osmometry is unavailable, clinicians may use a glucose fall ≤ 3 mmol/L/h as a practical proxy, recognising this is an expert-opinion target and that centre protocols should prevail. Dialysate potassium should be tailored to the pre-dialysis serum K+ and ECG findings. Bicarbonate levels should be kept moderate (28–32 mmol/L) to avoid abrupt shifts. Ultrafiltration should be guided by the documented volume phenotype: minimal UF if euvolaemic and cautious net-negative if hypervolaemic. Insulin infusions must continue during SLED, with independent glucose infusions (to maintain glucose at 8–12 mmol/L). If pre-dialysis glucose is ≤11 mmol/L, glucose should be started before the session. Anticoagulation should be regional citrate or low-dose heparin, to avoid over-anticoagulation in the DKA context. Where SLED is unavailable, conventional haemodialysis may still be used, starting with short or UF-only sessions, then escalating to full treatments when safe. Where available, centre protocol and dialysis-team judgement should prevail to balance metabolic correction against risks of rapid osmotic shifts.
7.3. Peritoneal Dialysis (PD)
For patients on peritoneal dialysis (PD), the management of DKA requires careful attention to the dialysate composition [
4]. PD can generally be continued, but the glucose concentration in the dialysate must be adjusted to avoid exacerbating hyperglycaemia, while lower-glucose or icodextrin solutions can help with volume control. Conversely, as DKA resolves and glucose levels fall, recurrent hypoglycaemia should first be managed with intravenous glucose. If it persists despite this, cautious adjustment of the PD prescription (e.g., modestly higher dialysate glucose or shorter dwells) may be considered with close glucose and ketone monitoring. Frequent monitoring of blood glucose and potassium is essential, as PD can also contribute to potassium removal, albeit more slowly than haemodialysis. The choice of PD solution (e.g., icodextrin vs. glucose-based) and its glucose concentration should be carefully considered in the context of the patient’s DKA status. Insulin requirements should be adjusted to account for dialysate glucose absorption, and glucose should be checked every 1–2 h during high-glucose exchanges.
When to escalate: If β-hydroxybutyrate fails to fall by ≥ 0.5 mmol/L every 4–6 h or remains > 3 mmol/L at 6–12 h despite adequate insulin, or if pH remains < 7.2, escalate to haemodialysis (HD) or SLED. Documenting the PD prescription (fill volume, dwell time, exchange frequency) alongside the insulin/glucose plan ensures interdisciplinary team alignment.
Supporting Evidence: In a study by Chen, X., & Ma, T. (2014), of the 55 patients treated with continuous renal replacement therapy (CRRT), 30 patients (54.5%) had a 30-day renal recovery rate, whereas of the 52 patients treated with sustained low-efficiency daily diafiltration with hemofiltration (SLEDD), 61.5% (
n = 32) had a 30-day renal recovery rate. SLED offered a similar renal recovery outcome, a lower heparin dosage, a shorter therapy time, and lower hospitalisation costs than CRRT. While SLED may reduce osmotic shifts compared with conventional haemodialysis, this inference is extrapolated from related populations rather than trials in dialysis-dependent DKA; centre-specific practice should guide use [
12].
Clinical insight: When escalation beyond PD is necessary, SLED may be preferable where available to limit osmotic shifts; parameters should be tailored to local protocols, with insulin continued and glucose administered independently as needed [
12].
7.4. Continuous Renal Replacement Therapy (CRRT)
Continuous renal replacement therapy (CRRT) may be used in intensive care settings if SLED is unavailable. Continuous Veno venous haemodiafiltration (CVVHDF) with modest effluent doses (20–25 mL/kg/h) is preferred to avoid abrupt osmotic shifts. Dialysate bicarbonate should be maintained at 28–32 mmol/L. Monitoring should include hourly glucose, β-hydroxybutyrate every 2–4 h, and serum osmolality every 4–6 h. Insulin infusion must continue uninterrupted, supported by independent glucose titration to maintain glucose between 8 and 12 mmol/L. In summary, dialysis, particularly haemodialysis, serves as a powerful tool for correcting severe electrolyte derangements and fluid overload in DKA patients with kidney failure. However, its application must be strategic, considering the risks of osmotic shifts and aiming for targeted interventions rather than aggressive solute removal. Close collaboration between the nephrology and critical care teams is paramount to optimize the timing and modality of dialysis (
Table 8).
8. Human Factors and System-Based Challenges
Beyond the intricate physiological and pharmacological considerations, the successful management of DKA in dialysis patients is profoundly influenced by human factors and the inherent challenges of real-world clinical practice [
4]. This is not merely a matter of applying a different algorithm; it demands a shift in cognitive approach and a heightened awareness of potential pitfalls.
Table 1 in the Introduction summarises the principal cognitive barriers; here we expand on their practical implications and the safeguards needed at the bedside.
One of the most significant human factors is the cognitive load arising from deviating from standard protocols. The use of evidence-based guidelines can be perceived to suggest that there is a degree of certainty in the best way to respond to illness. To make a decision to deviate from what is perceived as ‘established Practice’ or ‘the gold standard’, even for good clinical reasons, brings a degree of cognitive dissonance [
26] especially for junior staff [
27].
Clinicians, particularly those in acute care settings, are often trained to respond to DKA with a well-rehearsed, aggressive fluid and insulin protocol [
6]. This ingrained response, while appropriate for the majority of DKA cases, becomes a liability in dialysis patients. The mental effort required to consciously override this default, to question the immediate impulse to administer large fluid boluses, and to adopt a counter-intuitive approach can be substantial. This risk is compounded by the fast-paced, high-pressure environment of emergency departments and intensive care units, where time for nuanced reflection may be limited. The pressure to act quickly, coupled with the cognitive strain of managing a critically ill patient, can inadvertently lead to reliance on familiar, albeit inappropriate, protocols. This phenomenon, often termed cognitive tunnelling or confirmation bias, can lead clinicians to seek information that confirms their initial, standard DKA management plan, while overlooking contradictory cues specific to kidney failure [
8]. Explicit system prompts—such as EHR alerts, bedside checklists, or phenotype ‘gates’ before fluid orders—can reduce this risk and make deviation from standard protocols feel less like a dangerous departure.
Closely related is the risk of overconfidence in “cookbook” guidelines. While guidelines are invaluable for standardizing care and improving outcomes, rigid application without critical appraisal can be detrimental in atypical presentations. The very clarity and simplicity that make standard DKA protocols effective in the general population can foster a false sense of security, leading clinicians to overlook the unique physiological context of dialysis patients. This highlights the need for continuous education that emphasizes not just what to do, but why the approach differs in this specific group. True expertise lies not in rote memorization but in the ability to adapt and innovate within established frameworks, understanding the underlying physiological principles that necessitate deviation.
Effective interdisciplinary communication is not merely beneficial but critical. The management of DKA in dialysis patients often involves a complex interplay between nephrology, endocrinology, and critical care teams. Each specialty brings a unique perspective and expertise: nephrologists understand the nuances of fluid and electrolyte balance in kidney failure, endocrinologists are experts in diabetes and insulin kinetics, and intensivists manage acute critical illness. A breakdown in communication, a failure to establish a shared mental model, or a lack of clarity in roles and responsibilities can lead to conflicting orders, delayed interventions, or treatment-induced harm. Regular, proactive communication, including early consultation with nephrology, is paramount. This extends to clear handovers between shifts, ensuring that the rationale for individualized management decisions are consistently communicated and understood by all members of the care team.
This complexity underscores the importance of local Standard Operating Procedures (SOPs) and staff education. In the absence of widely adopted, specific national or international guidelines for DKA in dialysis patients [
4], individual institutions must develop tailored protocols, and ideally, publish and share them through interested organisations to contribute to the evolution of national and international guidelines in the future. These local SOPs should be clear, concise, and readily accessible, providing practical guidance on fluid management, insulin titration, electrolyte correction, and indications for urgent dialysis, coupled with details of where to seek expert guidance. Crucially, these protocols must be accompanied by robust, ongoing staff education and training programs for all healthcare professionals involved in the care of these patients, from junior doctors and nurses to senior consultants. Simulation-based training can be particularly effective in reinforcing these specialized management strategies, allowing clinicians to practice decision-making in a safe environment and experience the consequences of applying standard protocols inappropriately. Two additional safeguards are worth noting: (1) Line safety—Glucose 10% may be infused peripherally, but Glucose 20%–Glucose 50% require central access if continuous or can be given as boluses if central access is not available; embedding this into nursing SOPs prevents phlebitis and extravasation and (2) Monitoring pragmatism — where point-of-care BHB is unavailable, 2-hourly BHB checks are acceptable provided hourly glucose checks and ECG monitoring (if K
+ ≥ 6.0 mmol/L) are maintained.
Finally, fostering a culture of “if unsure, ask” is vital. The rarity of DKA in dialysis patients, coupled with its unique management challenges, means that even experienced clinicians may encounter situations where they feel uncertain. Creating an environment where asking for help or a second opinion is encouraged, rather than perceived as a weakness, is fundamental to patient safety. This collaborative ethos ensures that the collective expertise of the healthcare team is leveraged to navigate these high-stakes clinical scenarios. It also requires leadership to actively promote psychological safety, where concerns can be raised without fear of judgment or reprisal.
In essence, managing DKA in dialysis patients is as much about managing human cognition and team dynamics as it is about managing physiology. It requires humility, a willingness to deviate from the familiar, and a commitment to continuous learning and interdisciplinary collaboration. Embedding these safeguards into SOPs and auditing QI metrics such as hypoglycaemia rate, inappropriate fluid bolus use, or delays to dialysis further strengthens system resilience.
9. Practice Guidance (Consensus-Informed, Physiology-Led)
Given the unique physiological challenges of DKA in dialysis patients, standard DKA protocols often require significant modification. This section provides consensus-informed, physiology-led practice guidance, highlighting key areas of divergence from general population guidelines and offering suggested stances for individualized management (
Table 9). While this guidance remains consensus-driven rather than evidence-based, its value lies in providing clinicians with a structured “translation layer” between standard protocols and the realities of dialysis-associated DKA. Clear tabular comparisons help reduce cognitive overload in emergencies, enabling rapid but physiologically aligned decision-making. Local adaptation with iterative audit (e.g., rates of hypoglycaemia, inappropriate fluid boluses) is strongly recommended to validate and refine these practices.
10. Critical Appraisal of Evidence
It is crucial to acknowledge the inherent limitations of the available evidence base for managing DKA in dialysis patients [
4]. Unlike many areas of acute medicine, the literature on this specific cohort is predominantly composed of case series, retrospective reviews, and expert commentary. High-level randomized controlled trial data, which typically form the bedrock of evidence-based guidelines, are conspicuously absent. This scarcity is understandable given the relative rarity of DKA in this specific patient population and the ethical complexities of conducting large-scale interventional trials in critically ill, vulnerable individuals [
4].
However, the absence of randomized controlled trials do not equate to an absence of knowledge [
4,
8,
9,
11,
15,
18]. The consistency of physiological rationale and the recurring patterns of reported complications across various sources allow for a cautious yet robust synthesis of practice recommendations. The approach in this review has been to integrate these diverse sources of evidence, drawing upon established physiological principles, observed clinical outcomes, and the collective experience of expert clinicians. This narrative review aims only to provide a practical, evidence-informed framework derived from the best available literature. Clinicians must interpret these recommendations within the context of these limitations, prioritizing individualized patient assessment and clinical judgment.
11. Conclusions & Future Directions
Diabetic ketoacidosis in patients with renal disease on dialysis represents a clinical scenario that fundamentally challenges the conventional paradigms of DKA management. As this narrative review has underscored, the unique physiological alterations inherent to kidney failure standard DKA protocols are ineffective and dangerous. The risks of treatment-induced fluid overload, profound hypoglycaemia, and severe electrolyte derangements are ever-present if a one-size-fits-all approach is used.
This review proposes a physiology-based, individualized framework rooted in several core clinical principles:
Volume Phenotyping: Tailoring fluid therapy to the patient’s true volume status—hypovolaemic, euvolaemic, or hypervolaemic—is critical to avoid iatrogenic fluid overload or under-resuscitation.
Glucose–Insulin Decoupling: The use of “dry insulin” within a variable rate insulin infusion (VRII) protocol allows for effective resolution of ketosis while titrating glucose independently, minimizing unnecessary fluid administration.
Electrolyte and Acid–Base Management: Potassium replacement must be cautious and guided by renal function, with avoidance of reflexive supplementation in anuric patients. Dialysis should be used judiciously to manage refractory hyperkalaemia, acidosis, and hyperosmolality.
Dialysis Modality Consideration: When required, modalities such as SLED or Peritoneal Dialysis (PD) may offer safer, slower correction of solute and volume derangements in haemodynamically unstable patients.
Beyond physiology, this review also brings attention to systems-level and human factors that can determine outcomes. These include the cognitive burden clinicians face when deviating from familiar protocols, the overconfidence risks posed by rigid guideline adherence, and the necessity of real-time interdisciplinary communication between nephrology, endocrinology, critical care, and nursing teams. To address these challenges, institutions should:
Develop renal-specific DKA management pathways explicitly incorporating volume phenotyping, insulin–glucose decoupling, and dialysis decision-making.
Integrate simulation-based training and continuous education to reduce cognitive bias, reinforce clinical reasoning, and empower clinicians to adapt protocols based on physiology.
Establish audit frameworks to monitor for iatrogenic complications such as inappropriate fluid administration, hypoglycaemia, and delayed dialysis initiation.
Despite the practical framework proposed, current evidence remains limited. Most recommendations are based on expert consensus and case series rather than prospective trials. Therefore, future research should prioritize the following:
Prospective registries capturing DKA outcomes in dialysis patients across different modalities;
Pharmacokinetic studies evaluating insulin dynamics during SLED, HD, and PD;
Pragmatic trials comparing physiology-based (phenotype-driven) protocols versus standard care;
Development of PD-specific DKA protocols that reflect the modality’s unique fluid and solute profiles;
Clinical integration of advanced monitoring tools, such as VEXUS ultrasound for volume assessment and continuous glucose monitoring;
Evaluating the safety of novel glucose-lowering agents in dialysis populations, including SGLT2 inhibitors;
Embedding patient perspectives, health equity, and implementation science to ensure that physiology-informed models translate into practical, accessible, and patient-centered care.
The critical importance of volume status as the cornerstone of fluid management, advocating for a shift from aggressive resuscitation to a highly individualized, physiology-driven strategy. The concept of “dry insulin” within a variable rate insulin infusion (VRII) framework emerges as a vital tool, allowing for effective ketosis resolution without precipitating fluid overload. Also, the nuanced approach to potassium and acidosis correction, recognizing the unique electrolyte landscape of kidney failure is paramount to prevent life-threatening complications.
Finally this review has highlighted the profound impact of human factors on clinical outcomes. The cognitive burden of deviating from ingrained protocols, the risk of overconfidence with “cookbook” guidelines, and the absolute necessity of seamless interdisciplinary communication are challenges that demand proactive strategies. Fostering a culture of continuous education, simulation-based training, and psychological safety—where clinicians feel empowered to question and consult—is essential to navigate these complex scenarios safely. The effective management of DKA in dialysis patients is a testament to the art and science of medicine.