1. Introduction
Both end-stage renal disease and hemodialysis are becoming more prevalent. Central venous catheters are used by the majority of patients to begin acute hemodialysis. Although the number of central venous catheters is lower in chronic hemodialysis than in acute hemodialysis, this difference is still significant. Despite the considerable efforts made to increase the prevalence of autogenous fistula in patients on hemodialysis, tunneled cuffed catheters are still an important access modality and used in a high percentage of the hemodialysis population [
1]. The use of tunnel hemodialysis catheters can lead to a variety of complications, including central venous stenosis, thrombosis, and infections. The most significant of these complications is catheter-related bloodstream infection, which leads to significant morbidity and mortality. Common complications associated with the use of hemodialysis central venous catheters include bloodstream infections, exit-site infections, and tunnel infections [
2,
3].
Potential risk factors for CLABSI include underlying disease (such as lower hemoglobin levels, lower serum albumin levels, diabetes mellitus, and peripheral atherosclerosis), the method of catheter insertion, the site and duration of catheter insertion, poor personal hygiene, occlusive transparent dressing, moisture around the exit-site,
Staphylococcus aureus nasal colonization, contiguous infections, contamination of dialysate or equipment, inadequate water treatment, dialyzer reuse, higher total intravenous iron dose, increased recombinant human erythropoietin dose, and recent hospitalization or surgery [
2,
4].
Studies have been performed to evaluate risk factors for CLABSIs. The risk of infection is increased by several factors, including prolonged catheter use, age, diabetes mellitus, previous CLABSI, and immunosuppression. The use of central venous catheters outside of regular dialysis facilities poses another significant risk because of differences in catheter care between centers.
Our study aim is to determine the clinical and microbiological factors associated with vascular catheter loss in hemodialysis patients who develop positive blood cultures and, additionally, to identify the microbiological spectrum of organisms responsible for bloodstream infection in this patient population. This study provides a foundation for future initiatives to improve quality, and serves as a benchmark and performance indicator for our institution.
2. Methods
2.1. Study Design and Setting
A retrospective observational study was conducted over five years (January 2019–December 2023) across outpatient hemodialysis centers under the Ministry of National Guard Health Affairs, Saudi Arabia. Participating centers included Jeddah, Makkah, Medina, North Riyadh, and South Riyadh.
2.2. Study Objectives
The primary objective was to identify factors associated with tunneled hemodialysis catheter loss due to bloodstream infection. The secondary objectives were to describe microbial patterns and determine annual rates of bloodstream infection (BSI) and central line-associated bloodstream infection (CLABSI).
2.3. Definitions
A central line-associated bloodstream infection (CLABSI) was defined according to the Centers for Disease Control and Prevention (CDC) criteria as a laboratory-confirmed bloodstream infection in a patient with a central venous catheter in place for more than 48 h before the date of the positive blood culture, with no other identifiable source of infection. All patients included in this study had tunneled central venous catheters.
2.4. Study Population
Adult hemodialysis patients (≥15 years old) regularly enrolled in the participating outpatient dialysis centers during the study period were included. Patients with temporary hemodialysis catheters, tourists, and those with contaminated blood cultures were excluded.
2.5. Data Collection
Patient data were extracted from the electronic medical records (Best Care system) and recorded in an anonymized Excel database. The following information was collected:
Demographics: Age, sex, height, weight, body mass index (BMI);
Clinical parameters: Comorbidities, dialysis vintage, and vascular access type;
Laboratory data: Peripheral and central blood cultures;
Clinical outcomes: Hospitalization, catheter loss, and 30-day mortality (within four weeks of bacteremia diagnosis).
From an initial pool of 482 samples, 13 duplicates and 32 contaminated samples were excluded, resulting in 437 confirmed bacteremia episodes among 432 unique patients.
2.6. Calculation of Infection Rates
Annual rates of bloodstream infection (BSI) and central line-associated bloodstream infection (CLABSI) were calculated according to the Centers for Disease Control and Prevention (CDC) National Healthcare Safety Network (NHSN) Dialysis Event Surveillance Protocol.
The NHSN expresses dialysis event rates per 100 patient-months, stratified by vascular access type. Rates were determined by dividing the number of dialysis events by the total number of patient-months and multiplying by 100:
This formula was applied to calculate the following:
Patient-months were defined as the number of patients with a tunneled catheter present in the dialysis facility during the first two working days of each month, summed across the study period. BSI events and CLABSI events were identified according to CDC criteria.
2.7. Sampling Method
A non-probability consecutive sampling technique was used to include all eligible patients with positive blood cultures during the study period.
2.8. Statistical Analysis
Data were analyzed using IBM SPSS Statistics for Windows, Version 20.0 (IBM Corp., Armonk, NY, USA).
Categorical variables were summarized as frequencies and percentages, while continuous variables were expressed as mean ± standard deviation (SD).
Associations between vascular access loss (dependent variable) and independent variables (age, sex, BMI, comorbidities, exit-site infection, tunnel infection, other infection sources, immunosuppressive medication use, pathogen type [Gram-positive vs. Gram-negative], and mortality) were assessed using Chi-square or Fisher’s exact tests.
Binary logistic regression was used to identify predictors significantly associated with catheter loss.
Multivariate logistic regression analysis was performed to determine independent predictors of vascular access loss due to bloodstream infection.
2.9. Ethical Considerations
Patient confidentiality was maintained by de-identifying all records. Written informed consent was obtained, and data access was restricted to the principal investigator and coinvestigators. The study protocol was approved by the Institutional Review Board of the Ministry of National Guard Health Affairs.
3. Results
3.1. Clinical Demographics and Variables
The clinical and demographic characteristics of the hemodialysis patients were analyzed via descriptive statistics. Throughout the study period from 2019 to 2023, we documented a total of 482 cases across five dialysis units affiliated with the Ministry of National Guard Health Affairs in Saudi Arabia. Among these cases, the laboratory identified 32 samples as contaminated, and we discovered that 13 patients had duplicate blood cultures conducted within a 21-day timeframe. In total, we recorded 437 episodes of bacteremia involving 432 patients. Notably, 405 of these cases were associated with central line blood cultures, whereas 367 pertained to peripheral blood cultures. We also collected paired samples (both central and peripheral blood cultures) from 335 patients. The study population comprised 267 male patients (61.1%) and 170 female patients (38.9%), with two-thirds of the individuals falling within the 46–70 years of age. Patients were categorized into four BMI (body mass index) groups, as detailed in
Table 1. Additionally, our findings revealed that 60% of patients had diabetes mellitus, 88% had hypertension, and approximately one-fifth had a history of ischemic heart disease.
The vascular access types included central venous catheters (CVCs) at 85.8%, arteriovenous fistulas (AVFs) at 6.17%, arteriovenous grafts (AVGs) at 1.14%, and dual access, which comprised both CVCs and fistulas, at 6.9%. The data indicated a higher prevalence of positive blood cultures associated with central venous catheters [
Table 1]. Over the course of a five-year study, the incidence of bloodstream infections (BSI) was recorded at 0.67 per 100 patient-months, while the central line-associated bloodstream infection (CLABSI) rate was 1.55 per 100 patient-months [
Figure 1].
Dependent and independent variables were analyzed using Chi-square test and Fisher’s exact test. The findings revealed that the likelihood of losing vascular access was significantly lower in the presence of Gram-positive pathogens (p = 0.01). In contrast, the loss of access was significantly higher with Gram-negative pathogens (p = 0.036). Additionally, the analysis highlighted a strong association between tunnel infections and vascular access loss (p < 0.001). Patients with a high BMI (>30) also demonstrated a greater risk of losing vascular access (p = 0.04). However, factors such as age, exit site infection, comorbidities, and the use of immunosuppressive medications were not statistically significant.
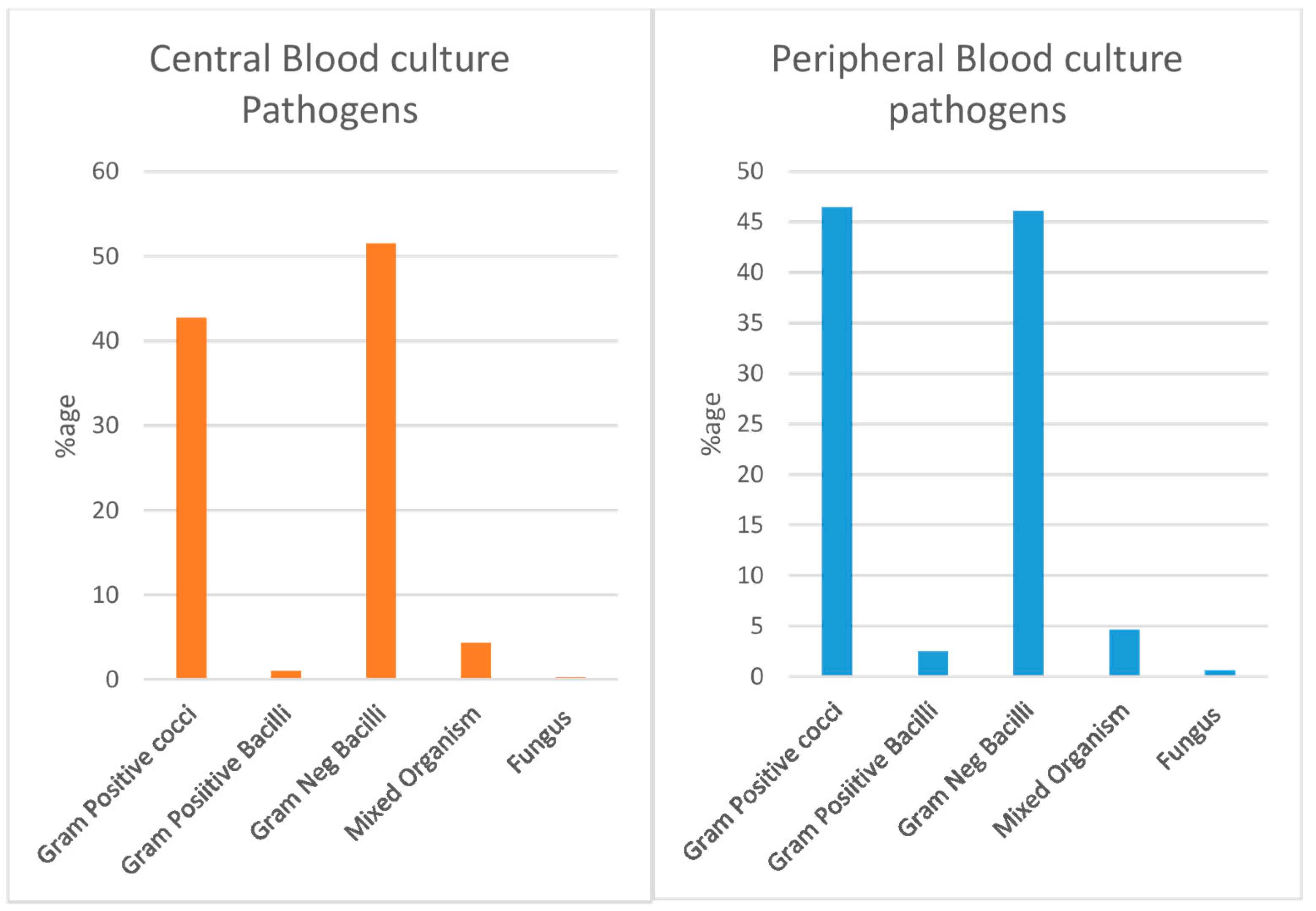
3.2. Microbial Organism Pattern
Blood cultures from 386 patients revealed 169 cases with Gram-positive bacteria and 200 cases with Gram-negative bacteria. There were also 16 cases with mixed organisms and one case involving fungi. Among the Gram-positive cocci,
Staphylococcus aureus, especially methicillin-susceptible strains (MSSA), was the most common. It was followed by MRSA and
Staphylococcus epidermidis. Among the Gram-negative bacteria,
Enterobacter cloacae was the most common, followed by
Klebsiella pneumoniae and
Pseudomonas aeruginosa. The pattern of bacteria found in peripheral blood samples closely matched those in central blood cultures. The data on the percentage of microorganisms in both blood sample types are shown in
Figure 2.
In 263 cases, paired blood culture was taken which revealed 48% Gram-positive pathogens, 48% cases with Gram-negative pathogens and approximately 4% cases with mixed pathogens.
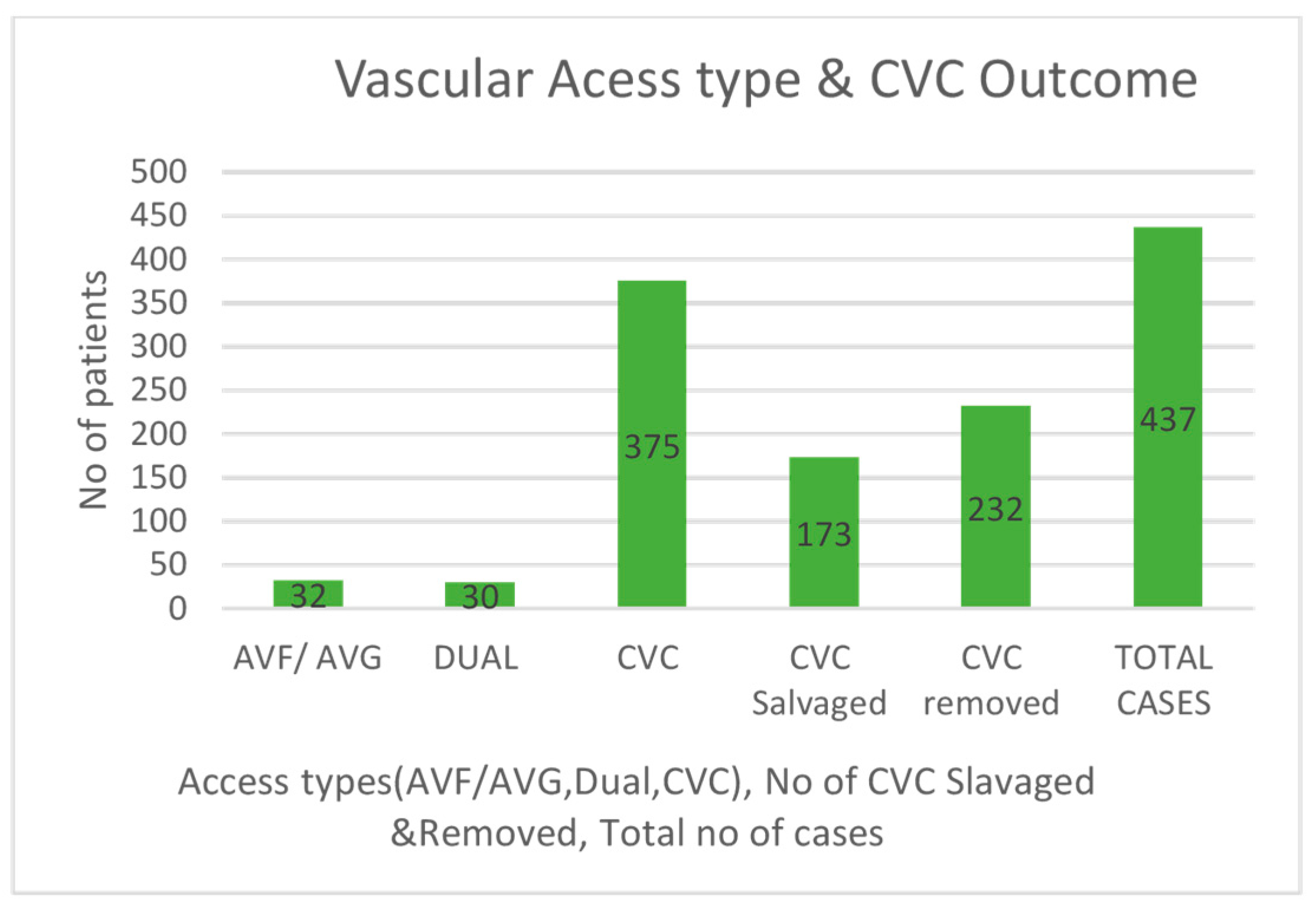
3.3. Vascular Access Analysis
There are three types of vascular access: central venous catheter (CVC), arteriovenous fistula (AVF), and dual access. The study revealed 375 positive blood cultures in patients with CVC, 32 in those with AVF or grafts, and 30 in dual access patients. Patients with CVC needed more hospital treatments and had a significantly greater rate of loss of access (
p < 0.001). CVCs were removed in 232 cases due to infections, leading to loss of access, whereas 173 cases managed to have their access saved (see
Figure 3). For AVF access, there was a high and statistically significant chance of developing other infections (
p < 0.001). At the 4-week follow-up, five patients had died, but these deaths did not reach statistical significance.
3.4. Bivariate Logistic Regression
Binary logistic regression was used to examine whether the predictors had a significant effect on the loss of vascular access of the patients [
Table 2].
Compared with patients with no Gram-positive infections, those with Gram-positive infections had lower odds of losing vascular access (OR = 0.61, 95% CI = 0.42–0.89, p = 0.011). However, patients infected with Gram-negative bacteria had a significant 1.50-fold greater odds of losing vascular access (OR = 1.50, 95% CI 1.02–2.19, p = 0.036). Tunnel infection was found to be 3.66 times more likely to lead to loss of vascular access among patients, whereas other foci of infection were significantly less likely to lead to loss of vascular access in both circumstances. Patients with left AVF vascular access type were significantly less likely to have loss of vascular access than patients with other access types. Compared with those with lower BMIs, those with higher BMIs were significantly more likely to have loss of vascular access.
3.5. Multivariate Logistic Regression
Multivariate logistic regression was conducted to assess the independent predictors of vascular loss due to bloodstream infection. The dependent variable was loss of vascular access, for which yes was coded as 1 for an event [
Table 2].
The overall model was statistically significant, as indicated by the Omnibus test of model coefficients p < 0.001. Negelkerke R2 specifies that 31% of the results can be explained by the predictors. Among all the independent variables, tunnel infection was associated with greater odds of losing vascular access, whereas other factors associated with infection of the left AVF were associated with lower odds of losing vascular access, which was a statistically significant contribution to the model.
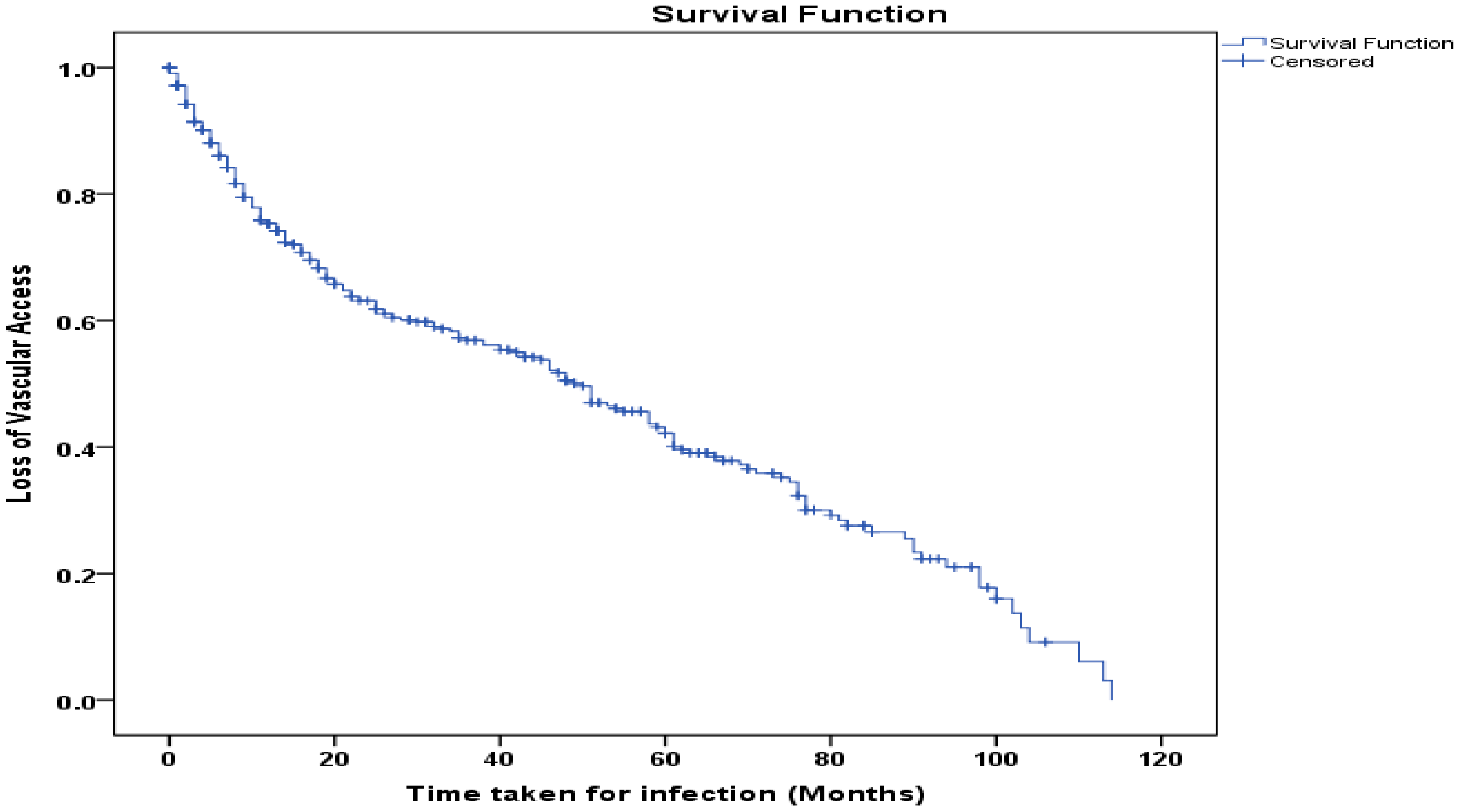
3.6. Survival Function Curve
The Kaplan–Meier Curve (
Figure 4) shows the period in months (beginning of hemodialysis till loss of access) of loss of vascular access due to infection among patients. The probability of maintaining vascular access declines over a period of time. At the median time of 50 months, approximately 50% of the patients retained vascular access. By the end of the observation period (approx. 115 months), less than 10% of patients had retained access. Overall, the curve depicts a progressive decline in vascular access, revealing long-term risks of infection among patients.
4. Discussion
Patients with kidney failure need reliable vascular access for ongoing hemodialysis (HD), which can be established through methods such as arteriovenous fistulas (AVFs), arteriovenous grafts (AVGs), or central venous catheters (CVCs). While the use of central venous catheters is generally limited to reduce associated risks, many hemodialysis patients still depend on CVCs for long-term treatment. In the United States, approximately 20% of patients undergoing hemodialysis utilize tunneled catheters [
5]. These catheters are especially beneficial for individuals who cannot have an AVF or AVG, whether due to small veins or other underlying health issues.
Tunneled catheters can lead to several complications, including thrombosis, central venous stenosis, and catheter-related bloodstream infections (CRBSIs) [
6]. The frequency of CRBSIs varies widely across different regions. This variation is influenced by factors such as geographical location, climate, healthcare systems, patient education, and socio-economic conditions [
7]. The risk of tunneled central line (central venous access device, CVAD) loss due to infection is clinically significant and well-documented in the medical literature. In a large systematic review and meta-analysis of adult ICU patients, the proportion of central venous access devices removed specifically due to suspected infection was 17% (20.4 per 1000 catheter days; 95% CI, 15.7–25.2), although this figure includes all central lines and not exclusively tunneled catheters. The overall central line-associated bloodstream infection (CLABSI) rate was 4.59 per 1000 catheter days, with the highest rates in non-tunneled devices, but infection remains a leading cause of device loss across all types [
8]. The risk increases with catheter dwell time and prior history of tunneled catheter use.
Tunneled central line removal is still frequently caused by infection, which emphasizes the significance of infection prevention measures and close observation. Our research aimed to determine the factors contributing to the loss of vascular access as a result of bloodstream infections, and our findings indicate that most cases were linked to central line-associated bloodstream infections (CLABSI). CLABSI is predominantly caused by bacterial pathogens. Gram-positive organisms, usually the skin flora, are culprits in the majority of cases [
9]. According to Parameswaran R et al., Gram-positive organisms are the main contributors to CLABSIs [
10]. A retrospective analysis of a prospective vascular access database from a large academic dialysis center revealed that nearly 80% of BSIs were caused by Gram-positive pathogens [
11].
Conversely, Gram-negative pathogens have also been reported to be highly prevalent. Balkhy et al. carried out a prospective study from 2008 to 2016 in hospitals under the Ministry of National Guard, and reported that Gram-negative pathogens accounted for 59.1%, Gram-positive pathogens accounted for 27.5%, and fungi accounted for 13.5% of the causative organisms in central line-associated bloodstream infection (CLABSI) [
12]. Typically, the origin of Gram-negative pathogens is urinary or intra-abdominal; however, in chronic hemodialysis patients, additional factors must also be considered. Frequent hospital visits, diabetic foot ulcers, CVC hubs, dialysis water source, injection, medication handling, and environmental reservoirs in patients’ proximity have been identified as potential contributors of Gram-negative bloodstream infection [
13,
14]. The prevalence of Gram-negative pathogen among CLABSI is increasing according to epidemiology reports from eight centers in Italy [
15]. Several factors may have contributed to the increase in Gram-negative pathogens. The increased use of broad-spectrum antibiotics, and prolonged catheter duration, provide a longer timeframe for bacteria to colonize and form biofilms which are often resistant to many antibiotics.
The reasons for the removal of a central venous catheter (CVC) are hemodynamic instability, ongoing fever despite the initiation of IV antibiotics, infected tunnel, or due to multi-resistant organisms or difficult-to-eradicate organisms such as
Staphylococcus aureus,
Pseudomonas species, or fungi [
16]. Additionally, we found that the presence of a concurrent tunnel infection can result in the loss of vascular access, which is difficult to treat with antibiotics alone due to the high likelihood of infection recurrence. In contrast, if infection only occurs at the exit site, it can be addressed with antibiotic treatment [
17,
18].
The patients in our study were split into four groups on the basis of body mass index (BMI). Vascular access was significantly lost in the cohort with a high BMI (>30). Bivariate logistic regression demonstrated this significance. Previous research has noted this pattern, and a meta-analysis published in 2022 showed a significant correlation between obesity and increased risk of bloodstream infection from catheter use. Close attention should be given to the complications and prognosis of obese patients with vascular catheterization in clinical work [
19].
Tunnel infection is a well-recognized predictor of catheter removal in hemodialysis patients. According to the KDOQI Clinical Practice Guideline for Vascular Access, the presence of a tunnel-site infection is a specific indication for removal of a central venous catheter (CVC), including tunneled hemodialysis catheters, due to the risk of persistent or complicated infection and poor response to conservative management. Our study reinforces the importance of tunnel infection, as on multivariate analysis, it stands out significant (p < 0.001), while other variables were not significant.
Finally, our findings indicate that the likelihood of preserving vascular access decreases over time as a result of infection. As noted in the literature, the Kaplan–Meier curve (
Figure 4) shows a gradual decline in vascular access over time. This pattern has been reported in multiple studies. A recently published meta-analysis showed that the incidence of CLABSI increased with increasing duration of CVC [
20].
5. Conclusions
Bloodstream infections remain a major complication of hemodialysis, predominantly linked to central venous catheters. In this study, Gram-negative organisms and tunnel infections emerged as the strongest predictors of vascular access loss, while arteriovenous fistulas demonstrated protective effects. Obesity also contributed to increased risk, although with less independent significance. Despite high access loss rates, short-term mortality was low. These findings underscore the urgent need to minimize catheter dependence and implement strategies focused on preventing tunnel infections and promptly addressing Gram-negative bacteremia.
The use of maximal barrier, safety measures during catheter insertion, effective cutaneous anti-sepsis, and preventive strategies based on preventing microorganisms from the skin or catheter hub from adhering to catheters are some of the measures that have been put in place to lower the risk for CLABSI.
The most significant preventive measures for catheter-related infections were the implementation of continuous quality improvement programs, healthcare worker education, and adherence to standardized protocols for intravascular catheter insertion and maintenance. New technologies for the prevention of infections directed at CVCs, which have been shown to reduce the risk of CLABSI, including catheters and dressings impregnated with antiseptics or antibiotics, new hub models, and antibiotic lock solutions, are in use [
21]. The use of hub devices appears to be associated with a reduction in catheter-related bloodstream infections in the central venous catheter dialysis population [
22]. Bench studies and in vitro simulations have characterized the relationship between needle-free connector (NC) design and backflow phenomena in midline catheters and peripherally inserted central catheters (PICCs). Comparative bench studies have shown that negative-pressure NCs are associated with less catheter failure than neutral-pressure designs, likely due to reduced backflow and blood reflux into the catheter lumen [
23,
24].
Catheters should be removed from patients with CLABSI associated with any local or systemic inflammation or immunocompromised condition. Antibiotic therapy for catheter-related infections is often initiated empirically. The initial choice of antibiotics depends on local infection control policies which are dependent on antibiograms.
Strengths and Limitations
The take-home message from our study is that central venous catheters remain the weakest link in hemodialysis vascular access due to their high infection burden and risk of access loss. Preventive strategies should prioritize reducing tunnel infections, minimizing catheter use, and promoting AVF creation whenever feasible. Early recognition of Gram-negative bacteremia and aggressive management are crucial to preserving vascular access and improving patient outcomes.
This study has a few limitations. First, there are several risk factors for bloodstream infections in cuffed tunneled catheters. This includes previous CLABSI, left-sided internal jugular catheter use, hypoalbuminemia, iron overload, immunosuppression, diabetes mellitus, and recent surgery [
25]. Not every factor was examined in this study. Investigating comprehensive risk predictors for CLABSI via prospective model would be helpful. Second, this study did not assess catheter types and their impact on infections. This resulted from a lack of data because it was impossible to track patients who were affiliated with different primary hospitals. Third, we tracked mortality for only a short period of time (4 weeks). Fourth, the patients’ travel records to identify whether vascular access was used other than regular dialysis centers were difficult to obtain because of the retrospective analysis of the data.