Biomarkers Predicting Major Adverse Cardiovascular Events in End-Stage Kidney Disease: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
- Selection process
- Data collection and analysis
3. Results
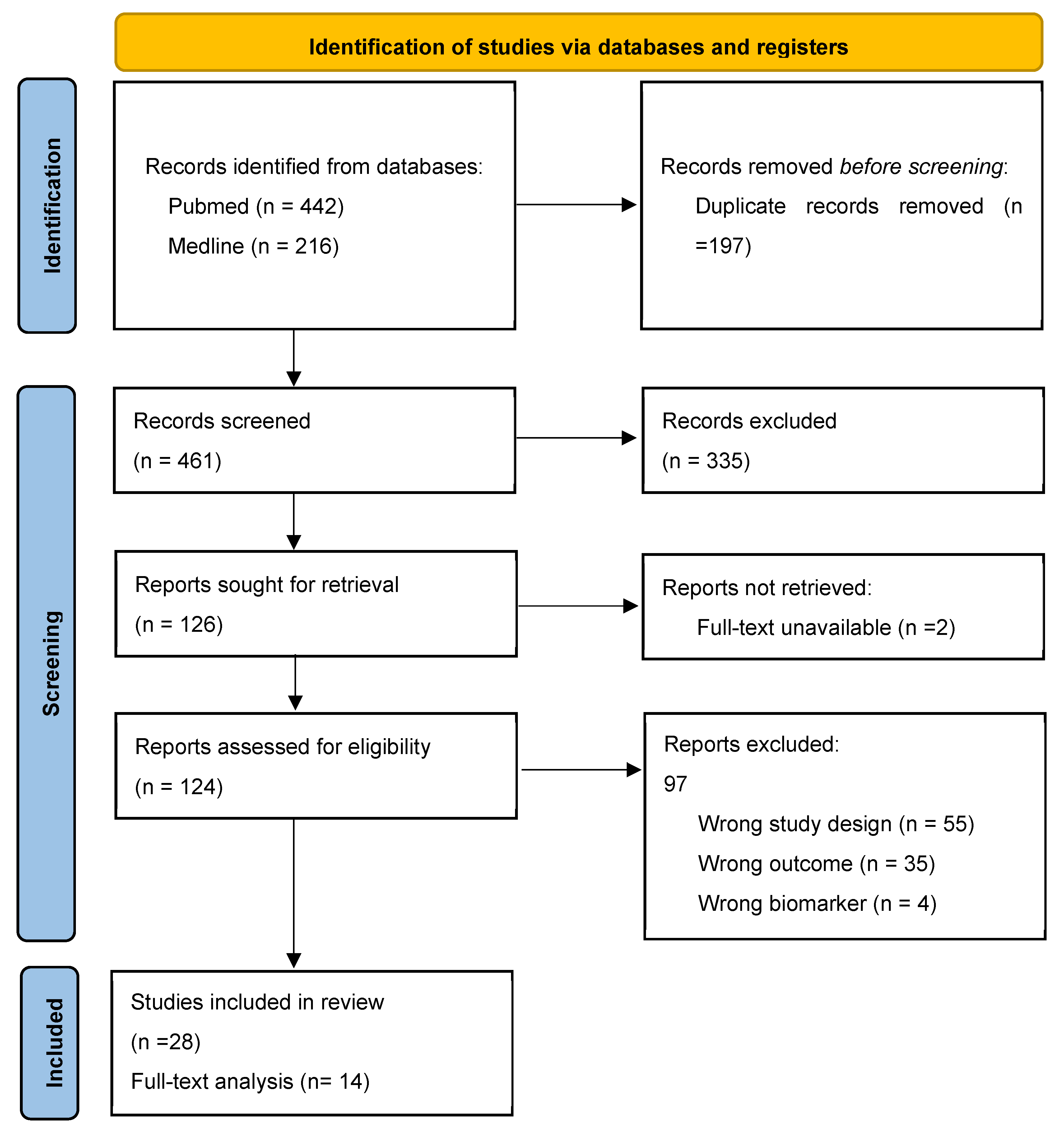
3.1. Data Extraction
3.2. Quality Assessment
3.3. Demographics and Study Design
3.4. Included Biomarkers
- Cardiac Troponin: Troponin I (n = 3), Troponin T (n = 3)
- BNP and NTpro BNP: BNP (n = 2), NTproBNP (n = 7)
- Soluble suppression of tumorigenicity-2 (sST2): (n = 2)
- Soluble receptor for advanced glycation end products (sRAGE): (n = 2)
- Galectin 3 (Gal–3): (n = 4)
4. Discussion
- Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef] [PubMed]
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [PubMed]
- Carney, E.F. The impact of chronic kidney disease on global health. Nat. Rev. Nephrol. 2020, 16, 251. [Google Scholar] [CrossRef]
- Nissenson, A.R.; Collins, A.J.; Hurley, J.; Petersen, H.; Pereira, B.J.G.; Steinberg, E.P. Opportunities for improving the care of patients with chronic renal insufficiency: Current practice patterns. J. Am. Soc. Nephrol. 2001, 12, 1713–1720. [Google Scholar] [CrossRef]
- Stevens, P.E.; O’Donoghue, D.J.; de Lusignan, S.; Van Vlymen, J.; Klebe, B.; Middleton, R.; Hague, N.; New, J.; Farmer, C.K. Chronic kidney disease management in the United Kingdom: NEOERICA project results. Kidney Int. 2007, 72, 92–99. [Google Scholar] [CrossRef]
- Kidney Research UK. Kidney Disease: A UK Public Health Emergency The Health Economics of Kidney Disease to 2033. Available online: https://kidneyresearchuk.org/wp-content/uploads/2023/06/Economics-of-Kidney-Disease-full-report_accessible.pdf (accessed on 3 June 2024).
- Sarnak, M.J.; Levey, A.S.; Schoolwerth, A.C.; Coresh, J.; Culleton, B.; Hamm, L.L.; McCullough, P.A.; Kasiske, B.L.; Kelepouris, E.; Klag, M.J.; et al. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 2003, 108, 2154–2169. [Google Scholar] [CrossRef]
- Matsushita, K.; Coresh, J.; Sang, Y.; Chalmers, J.; Fox, C.; Guallar, E.; Jafar, T.; Jassal, S.K.; Landman, G.W.; Muntner, P.; et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: A collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015, 3, 514–525. [Google Scholar] [CrossRef]
- Matsushita, K.; Ballew, S.H.; Wang, A.Y.; Kalyesubula, R.; Schaeffner, E.; Agarwal, R. Epidemiology and risk of cardiovascular disease in populations with chronic kidney disease. Nat. Rev. Nephrol. 2022, 18, 696–707. [Google Scholar] [CrossRef]
- Levey, A.S.; Eckardt, K.U.; Tsukamoto, Y.; Levin, A.; Coresh, J.; Rossert, J.; De Zeeuw, D.; Hostetter, T.H.; Lameire, N.; Eknoyan, G. Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005, 67, 2089–2100. [Google Scholar] [CrossRef]
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef]
- Vaziri, N.D. Dyslipidemia of chronic renal failure: The nature, mechanisms, and potential consequences. Am. J. Physiol. Ren. Physiol. 2006, 290, F262–F272. [Google Scholar] [CrossRef] [PubMed]
- Wanner, C.; Amann, K.; Shoji, T. The heart and vascular system in dialysis. Lancet 2016, 388, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; van der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; de Jong, P.E.; Coresh, J.; Gansevoort, R.T. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 2010, 375, 2073–2081. [Google Scholar] [CrossRef] [PubMed]
- Kerr, M.; Bray, B.; Medcalf, J.; O’Donoghue, D.J.; Matthews, B. Estimating the financial cost of chronic kidney disease to the NHS in England. Nephrol. Dial. Transplant. 2012, 27 (Suppl. 3), iii73–iii80. [Google Scholar] [CrossRef]
- Darlington, O.; Dickerson, C.; Evans, M.; McEwan, P.; Sörstadius, E.; Sugrue, D.; van Haalen, H.; Garcia Sanchez, J.J. Costs and Healthcare Resource Use Associated with Risk of Cardiovascular Morbidity in Patients with Chronic Kidney Disease: Evidence from a Systematic Literature Review. Adv. Ther. 2021, 38, 994–1010. [Google Scholar] [CrossRef]
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and Other Tools); National Institutes of Health: Bethesda, MD, USA, 2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK453484 (accessed on 3 June 2024).
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef]
- Kula, A.; Bansal, N. Applications of cardiac biomarkers in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2022, 31, 534–540. [Google Scholar] [CrossRef]
- Chazot, C.; Rozes, M.; Vo-Van, C.; Deleaval, P.; Hurot, J.M.; Lorriaux, C.; Mayor, B.; Zaoui, E.; Jean, G. Brain Natriuretic Peptide Is a Marker of Fluid Overload in Incident Hemodialysis Patients. Cardiorenal Med. 2017, 7, 218–226. [Google Scholar] [CrossRef]
- Moura, B.; Aimo, A.; Al-Mohammad, A.; Flammer, A.; Barberis, V.; Bayes-Genis, A.; Brunner-La Rocca, H.P.; Fontes-Carvalho, R.; Grapsa, J.; Hülsmann, M.; et al. Integration of imaging and circulating biomarkers in heart failure: A consensus document by the Biomarkers and Imaging Study Groups of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2021, 23, 1577–1596. [Google Scholar] [CrossRef]
- Aurora, L.; Peterson, E.; Gui, H.; Zeld, N.; McCord, J.; Pinto, Y.; Cook, B.; Sabbah, H.N.; Keoki Williams, L.; Snider, J.; et al. Suppression tumorigenicity 2 (ST2) turbidimetric immunoassay compared to enzyme-linked immunosorbent assay in predicting survival in heart failure patients with reduced ejection fraction. Clin. Chim. Acta 2020, 510, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Drechsler, C.; Delgado, G.; Wanner, C.; Blouin, K.; Pilz, S.; Tomaschitz, A.; Kleber, M.E.; Dressel, A.; Willmes, C.; Krane, V.; et al. Galectin-3, Renal Function, and Clinical Outcomes: Results from the LURIC and 4D Studies. J. Am. Soc. Nephrol. 2015, 26, 2213–2221. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Yang, J.W.; Chai, M.H.; Lee, J.Y.; Park, H.; Kim, Y.; Choi, S.O.; Han, B.G. Copeptin in Hemodialysis Patients with Left Ventricular Dysfunction. Yonsei Med. J. 2015, 56, 976–980. [Google Scholar] [CrossRef] [PubMed]
- Molsted, S.; Eidemak, I.; Sorensen, H.T.; Kristensen, J.H.; Harrison, A.; Andersen, J.L. Myosin heavy-chain isoform distribution, fibre-type composition and fibre size in skeletal muscle of patients on haemodialysis. Scand. J. Urol. Nephrol. 2007, 41, 539–545. [Google Scholar] [CrossRef]
- Cediel, G.; Santiago-Vacas, E.; Bayes-Genis, A. Biomarkers and heart–kidney interaction. Eur. Heart J. Suppl. 2018, 20, G28–G36. [Google Scholar] [CrossRef]
- Zhong, X.; Qian, X.; Chen, G.; Song, X. The role of galectin-3 in heart failure and cardiovascular disease. Clin. Exp. Pharmacol. Physiol. 2019, 46, 197–203. [Google Scholar] [CrossRef]
- Vasan, R.S. Biomarkers of cardiovascular disease: Molecular basis and practical considerations. Circulation 2006, 113, 2335–2362. [Google Scholar] [CrossRef]
- Critical Appraisal Skills Programme. CASP Cohort Study Checklist. Available online: https://casp-uk.net/images/checklist/documents/CASP-Cohort-Study-Checklist/CASP-Cohort-Study-Checklist-2018_fillable_form.pdf (accessed on 6 August 2023).
- Alam, A.; Palumbo, A.; Mucsi, I.; Barré, P.E.; Sniderman, A.D. Elevated troponin I levels but not low grade chronic inflammation is associated with cardiac-specific mortality in stable hemodialysis patients. BMC Nephrol. 2013, 14, 247. [Google Scholar] [CrossRef]
- Jung, E.S.; Chung, W.; Kim, A.J.; Ro, H.; Chang, J.H.; Lee, H.H.; Jung, J.Y. Associations between Soluble Receptor for Advanced Glycation End Products (sRAGE) and S100A12 (EN-RAGE) with Mortality in Long-term Hemodialysis Patients. J. Korean Med. Sci. 2017, 32, 54–59. [Google Scholar] [CrossRef]
- Kawagoe, C.; Sato, Y.; Toida, T.; Nakagawa, H.; Yamashita, Y.; Fukuda, A.; Iwatsubo, S.; Fujimoto, S. N-terminal-pro-B-type-natriuretic peptide associated with 2-year mortality from both cardiovascular and non-cardiovascular origins in prevalent chronic hemodialysis patients. Ren. Fail. 2018, 40, 127–134. [Google Scholar] [CrossRef]
- Kim, A.J.; Ro, H.; Kim, H.; Ko, K.P.; Chang, J.H.; Lee, H.H.; Chung, W.; Jung, J.Y. Elevated levels of soluble ST2 but not galectin-3 are associated with increased risk of mortality in hemodialysis patients. Kidney Res. Clin. Pract. 2021, 40, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Kruzan, R.M.; Herzog, C.A.; Wu, A.; Sang, Y.; Parekh, R.S.; Matsushita, K.; Hwang, S.; Cheng, A.; Coresh, J.; Powe, N.R.; et al. Association of NTproBNP and cTnI with outpatient sudden cardiac death in hemodialysis patients: The Choices for Healthy Outcomes in Caring for ESRD (CHOICE) study. BMC Nephrol. 2016, 17, 18. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wu, Q.; Zhang, S.; Wang, Z.; Liu, H.; Teng, L.; Xiao, P.; Lu, Y.; Wang, X.; Dong, C.; et al. Serum Galectin-3 levels and all-cause and cardiovascular mortality in maintenance hemodialysis patients: A prospective cohort study. BMC Nephrol. 2022, 23, 5. [Google Scholar] [CrossRef] [PubMed]
- Obokata, M.; Sunaga, H.; Ishida, H.; Ito, K.; Ogawa, T.; Ando, Y.; Kurabayashi, M.; Negishi, K. Independent and incremental prognostic value of novel cardiac biomarkers in chronic hemodialysis patients. Am. Heart J. 2016, 179, 29–41. [Google Scholar] [CrossRef]
- Otsuka, K.; Nakanishi, K.; Shimada, K.; Nakamura, H.; Inanami, H.; Nishioka, H.; Fujimoto, K.; Kasayuki, N.; Yoshiyama, M. Ankle-brachial index, arterial stiffness, and biomarkers in the prediction of mortality and outcomes in patients with end-stage kidney disease. Clin. Cardiol. 2019, 42, 656–662. [Google Scholar] [CrossRef]
- Schwermer, K.; Hoppe, K.; Radziszewska, D.; Kłysz, P.; Sawatiuk, P.; Nealis, J.; Kałużna, M.; Kaczmarek, J.; Baum, E.; Lindholm, B.; et al. N-terminal pro-B-type natriuretic peptide as a marker of hypervolemia and predictor of increased mortality in patients on hemodialysis. Pol. Arch. Intern. Med. 2015, 125, 560–569. [Google Scholar] [CrossRef]
- Shafi, T.; Zager, P.G.; Sozio, S.M.; Grams, M.E.; Jaar, B.G.; Christenson, R.H.; Boulware, L.E.; Parekh, R.S.; Powe, N.R.; Coresh, J. Troponin I and NT-proBNP and the association of systolic blood pressure with outcomes in incident hemodialysis patients: The Choices for Healthy Outcomes in Caring for ESRD (CHOICE) Study. Am. J. Kidney Dis. 2014, 64, 443–451. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Zhang, N.; Xie, X.; Ding, M.; Guo, H.; Li, F.; Wang, X. High-Sensitive Cardiac Troponin T for Prediction of Cardiovascular Outcomes in Stable Maintenance Hemodialysis Patients: A 3-Year Prospective Study. Kidney Blood Press. Res. 2021, 46, 484–494. [Google Scholar] [CrossRef]
- Voroneanu, L.; Siriopol, D.; Apetrii, M.; Hogas, S.; Onofriescu, M.; Nistor, I.; Kanbay, M.; Dumea, R.; Cusai, S.; Cianga, P.; et al. Prospective Validation of a Screening Biomarker Approach Combining Amino-Terminal Pro-Brain Natriuretic Peptide With Galectin-3 Predicts Death and Cardiovascular Events in Asymptomatic Hemodialysis Patients. Angiology 2018, 69, 449–455. [Google Scholar] [CrossRef]
- Hayashi, T.; Kimura, T.; Yasuda, K.; Sasaki, K.; Obi, Y.; Rakugi, H.; Isaka, Y. Cardiac troponin T elevation at dialysis initiation is associated with all-cause and cardiovascular mortality on dialysis in patients without diabetic nephropathy. Clin. Exp. Nephrol. 2017, 21, 333–341. [Google Scholar] [CrossRef]
- Dozio, E.; Ambrogi, F.; de Cal, M.; Vianello, E.; Ronco, C.; Corsi Romanelli, M.M. Role of the Soluble Receptor for Advanced Glycation End Products (sRAGE) as a Prognostic Factor for Mortality in Hemodialysis and Peritoneal Dialysis Patients. Mediat. Inflamm. 2018, 2018, 1347432. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Winkler, K.; Wanner, C.; Drechsler, C.; Lilienthal, J.; März, W.; Vera Krane for the German Diabetes and Dialysis Study Investigators. Change in N-terminal-pro-B-type-natriuretic-peptide and the risk of sudden death, stroke, myocardial infarction, and all-cause mortality in diabetic dialysis patients. Eur. Heart J. 2008, 29, 2092–2099. [Google Scholar] [CrossRef]
- Weinberg, E.O.; Shimpo, M.; De Keulenaer, G.W.; MacGillivray, C.; Tominaga, S.; Solomon, S.D.; Rouleau, J.L.; Lee, R.T. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation 2002, 106, 2961–2966. [Google Scholar] [CrossRef] [PubMed]
- Ip, C.; Luk, K.S.; Yuen, V.L.C.; Chiang, L.; Chan, C.K.; Ho, K.; Gong, M.; Lee, T.T.L.; Leung, K.S.K.; Roever, L.; et al. Soluble suppression of tumorigenicity 2 (sST2) for predicting disease severity or mortality outcomes in cardiovascular diseases: A systematic review and meta-analysis. Int. J. Cardiol. Heart Vasc. 2021, 37, 100887. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.V.; Januzzi, J.L., Jr. ST2: A novel remodeling biomarker in acute and chronic heart failure. Curr. Heart Fail. Rep. 2010, 7, 9–14. [Google Scholar] [CrossRef]
- Reichert, S.; Triebert, U.; Santos, A.N.; Hofmann, B.; Schaller, H.G.; Schlitt, A.; Schulz, S. Soluble form of receptor for advanced glycation end products and incidence of new cardiovascular events among patients with cardiovascular disease. Atherosclerosis 2017, 266, 234–239. [Google Scholar] [CrossRef]
- Delrue, C.; Delanghe, J.R.; Speeckaert, M.M. The role of sRAGE in cardiovascular diseases. Adv. Clin. Chem. 2023, 117, 53–102. [Google Scholar] [CrossRef]
- Sciacchitano, S.; Lavra, L.; Morgante, A.; Ulivieri, A.; Magi, F.; De Francesco, G.P.; Bellotti, C.; Salehi, L.B.; Ricci, A. Galectin-3: One Molecule for an Alphabet of Diseases, from A to Z. Int. J. Mol. Sci. 2018, 19, 379. [Google Scholar] [CrossRef]
- Blanda, V.; Bracale, U.M.; Di Taranto, M.D.; Fortunato, G. Galectin-3 in Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 9232. [Google Scholar] [CrossRef]
- Sygitowicz, G.; Maciejak-Jastrzębska, A.; Sitkiewicz, D. The Diagnostic and Therapeutic Potential of Galectin-3 in Cardiovascular Diseases. Biomolecules 2021, 12, 46. [Google Scholar] [CrossRef]
- Camp, R.L.; Dolled-Filhart, M.; Rimm, D.L. X-tile: A new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin. Cancer Res. 2004, 10, 7252–7259. [Google Scholar] [CrossRef] [PubMed]
- Hall, C. NT-ProBNP: The mechanism behind the marker. J. Card. Fail. 2005, 11, S81–S83. [Google Scholar] [CrossRef]
- Bayes-Genis, A.; Docherty, K.F.; Petrie, M.C.; Januzzi, J.L.; Mueller, C.; Anderson, L.; Bozkurt, B.; Butler, J.; Chioncel, O.; Cleland, J.G.F.; et al. Practical algorithms for early diagnosis of heart failure and heart stress using NT-proBNP: A clinical consensus statement from the Heart Failure Association of the ESC. Eur. J. Heart Fail. 2023, 25, 1891–1898. [Google Scholar] [CrossRef]
- Jaffe, A.S.; Babuin, L.; Apple, F.S. Biomarkers in acute cardiac disease: The present and the future. J. Am. Coll. Cardiol. 2006, 48, 1–11. [Google Scholar] [CrossRef]
- Takase, H.; Dohi, Y. Kidney function crucially affects B-type natriuretic peptide (BNP), N-terminal proBNP and their relationship. Eur. J. Clin. Investig. 2014, 44, 303–308. [Google Scholar] [CrossRef]
- Kraus, D.; von Jeinsen, B.; Tzikas, S.; Palapies, L.; Zeller, T.; Bickel, C.; Fette, G.; Lackner, K.J.; Drechsler, C.; Neumann, J.T.; et al. Cardiac Troponins for the Diagnosis of Acute Myocardial Infarction in Chronic Kidney Disease. J. Am. Heart Assoc. 2018, 7, e008032. [Google Scholar] [CrossRef]
- Twerenbold, R.; Wildi, K.; Jaeger, C.; Gimenez, M.R.; Reiter, M.; Reichlin, T.; Walukiewicz, A.; Gugala, M.; Krivoshei, L.; Marti, N.; et al. Optimal Cutoff Levels of More Sensitive Cardiac Troponin Assays for the Early Diagnosis of Myocardial Infarction in Patients with Renal Dysfunction. Circulation 2015, 131, 2041–2050. [Google Scholar] [CrossRef]
- Kanderian, A.S.; Francis, G.S. Cardiac troponins and chronic kidney disease. Kidney Int. 2006, 69, 1112–1114. [Google Scholar] [CrossRef]
- Wu, A.H.; Jaffe, A.S.; Apple, F.S.; Jesse, R.L.; Francis, G.L.; Morrow, D.A.; Newby, L.K.; Ravkilde, J.; Tang, W.H.; Christenson, R.H.; et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines: Use of cardiac troponin and B-type natriuretic peptide or N-terminal proB-type natriuretic peptide for etiologies other than acute coronary syndromes and heart failure. Clin. Chem. 2007, 53, 2086–2096. [Google Scholar] [CrossRef]
- Lidgard, B.; Zelnick, L.R.; Go, A.; O’Brien, K.D.; Bansal, N. Framingham and American College of Cardiology/American Heart Association Pooled Cohort Equations, High-Sensitivity Troponin T, and N-Terminal Pro-Brain-Type Natriuretic Peptide for Predicting Atherosclerotic Cardiovascular Events Across the Spectrum of Kidney Dysfunction. J. Am. Heart Assoc. 2022, 11, e024913. [Google Scholar] [CrossRef] [PubMed]
- Bundy, J.D.; Rahman, M.; Matsushita, K.; Jaeger, B.C.; Cohen, J.B.; Chen, J.; Deo, R.; Dobre, M.A.; Feldman, H.I.; Flack, J.; et al. Risk Prediction Models for Atherosclerotic Cardiovascular Disease in Patients with Chronic Kidney Disease: The CRIC Study. J. Am. Soc. Nephrol. 2022, 33, 601–611. [Google Scholar] [CrossRef]
- Kattan, M.W. Evaluating a new marker’s predictive contribution. Clin. Cancer Res. 2004, 10, 822–824. [Google Scholar] [CrossRef] [PubMed]
- Lukowsky, L.R.; Kheifets, L.; Arah, O.A.; Nissenson, A.R.; Kalantar-Zadeh, K. Patterns and predictors of early mortality in incident hemodialysis patients: New insights. Am. J. Nephrol. 2012, 35, 548–558. [Google Scholar] [CrossRef]
- Eckardt, K.U.; Gillespie, I.A.; Kronenberg, F.; Richards, S.; Stenvinkel, P.; Anker, S.D.; Wheeler, D.C.; de Francisco, A.L.; Marcelli, D.; Froissart, M.; et al. High cardiovascular event rates occur within the first weeks of starting hemodialysis. Kidney Int. 2015, 88, 1117–1125. [Google Scholar] [CrossRef]
- Matsushita, K.; Jassal, S.K.; Sang, Y.; Ballew, S.H.; Grams, M.E.; Surapaneni, A.; Arnlov, J.; Bansal, N.; Bozic, M.; Brenner, H.; et al. Incorporating kidney disease measures into cardiovascular risk prediction: Development and validation in 9 million adults from 72 datasets. EClinicalMedicine 2020, 27, 100552. [Google Scholar] [CrossRef]
- Anderson, R.T.; Cleek, H.; Pajouhi, A.S.; Bellolio, M.F.; Mayukha, A.; Hart, A.; Hickson, L.J.; Feely, M.A.; Wilson, M.E.; Giddings Connolly, R.M.; et al. Prediction of Risk of Death for Patients Starting Dialysis: A Systematic Review and Meta-Analysis. Clin. J. Am. Soc. Nephrol. 2019, 14, 1213–1227. [Google Scholar] [CrossRef]
- Hemmelgarn, B.R.; Manns, B.J.; Quan, H.; Ghali, W.A. Adapting the Charlson Comorbidity Index for use in patients with ESRD. Am. J. Kidney Dis. 2003, 42, 125–132. [Google Scholar] [CrossRef]
- Couchoud, C.; Labeeuw, M.; Moranne, O.; Allot, V.; Esnault, V.; Frimat, L.; Stengel, B. A clinical score to predict 6-month prognosis in elderly patients starting dialysis for end-stage renal disease. Nephrol. Dial. Transpl. 2009, 24, 1553–1561. [Google Scholar] [CrossRef]
- Khan, I.H.; Catto, G.R.; Edward, N.; Fleming, L.W.; Henderson, I.S.; MacLeod, A.M. Influence of coexisting disease on survival on renal-replacement therapy. Lancet 1993, 341, 415–418. [Google Scholar] [CrossRef]
- Floege, J.; Gillespie, I.A.; Kronenberg, F.; Anker, S.D.; Gioni, I.; Richards, S.; Pisoni, R.L.; Robinson, B.M.; Marcelli, D.; Froissart, M.; et al. Development and validation of a predictive mortality risk score from a European hemodialysis cohort. Kidney Int. 2015, 87, 996–1008. [Google Scholar] [CrossRef]
- Nowak, C.; Ärnlöv, J. Kidney Disease Biomarkers Improve Heart Failure Risk Prediction in the General Population. Circ. Heart Fail. 2020, 13, e006904. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.K.; Chambless, L.E.; Ballantyne, C.M.; Astor, B.; Bertoni, A.G.; Chang, P.P.; Folsom, A.R.; He, M.; Hoogeveen, R.C.; Ni, H.; et al. Prediction of incident heart failure in general practice: The Atherosclerosis Risk in Communities (ARIC) Study. Circ. Heart Fail. 2012, 5, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Gregg, L.P.; Adams-Huet, B.; Li, X.; Colbert, G.; Jain, N.; de Lemos, J.A.; Hedayati, S.S. Effect Modification of Chronic Kidney Disease on the Association of Circulating and Imaging Cardiac Biomarkers with Outcomes. J. Am. Heart Assoc. 2017, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Staude, H.; Jeske, S.; Schmitz, K.; Warncke, G.; Fischer, D.C. Cardiovascular risk and mineral bone disorder in patients with chronic kidney disease. Kidney Blood Press. Res. 2013, 37, 68–83. [Google Scholar] [CrossRef]
- Dimkovic, N.; Schlieper, G.; Jankovic, A.; Djuric, Z.; Ketteler, M.; Damjanovic, T.; Djuric, P.; Marinkovic, J.; Radojcic, Z.; Markovic, N.; et al. Prognostic value of cardiovascular calcifications in hemodialysis patients: A longitudinal study. Int. Urol. Nephrol. 2018, 50, 939–946. [Google Scholar] [CrossRef]
- Bosco, E.; Hsueh, L.; McConeghy, K.W.; Gravenstein, S.; Saade, E. Major adverse cardiovascular event definitions used in observational analysis of administrative databases: A systematic review. BMC Med. Res. Methodol. 2021, 21, 241. [Google Scholar] [CrossRef]
- Bhatty, A.; Wilkinson, C.; Sydes, M.; Gale, C.P. Defining the need for cardiovascular event definitions. Eur. Heart J. Qual. Care Clin. Outcomes 2024, 10, 105–107. [Google Scholar] [CrossRef]
- Gershlick, A.H.; Khan, J.N.; Kelly, D.J.; Greenwood, J.P.; Sasikaran, T.; Curzen, N.; Blackman, D.J.; Dalby, M.; Fairbrother, K.L.; Banya, W.; et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: The CvLPRIT trial. J. Am. Coll. Cardiol. 2015, 65, 963–972. [Google Scholar] [CrossRef]
- Bolignano, D.; Greco, M.; Arcidiacono, V.; Tripolino, O.; Vita, C.; Provenzano, M.; Donato, C.; Chiarella, S.; Fuiano, G.; De Sarro, G.; et al. Cathepsin-K is a potential cardiovascular risk biomarker in prevalent hemodialysis patients. Int. Urol. Nephrol. 2021, 53, 171–175. [Google Scholar] [CrossRef]
- Claus, R.; Berliner, D.; Bavendiek, U.; Vodovar, N.; Lichtinghagen, R.; David, S.; Patecki, M.; Launay, J.M.; Bauersachs, J.; Haller, H.; et al. Soluble neprilysin, NT-proBNP, and growth differentiation factor-15 as biomarkers for heart failure in dialysis patients (SONGBIRD). Clin. Res. Cardiol. 2020, 109, 1035–1047. [Google Scholar] [CrossRef]
- de Gonzalo-Calvo, D.; Martínez-Camblor, P.; Bär, C.; Duarte, K.; Girerd, N.; Fellström, B.; Schmieder, R.E.; Jardine, A.G.; Massy, Z.A.; Holdaas, H.; et al. Improved cardiovascular risk prediction in patients with end-stage renal disease on hemodialysis using machine learning modeling and circulating microribonucleic acids. Theranostics 2020, 10, 8665–8676. [Google Scholar] [CrossRef]
- Hutchison, C.A.; Burmeister, A.; Harding, S.J.; Basnayake, K.; Church, H.; Jesky, M.D.; White, K.; Green, C.E.; Stringer, S.J.; Bassett, P.; et al. Serum polyclonal immunoglobulin free light chain levels predict mortality in people with chronic kidney disease. Mayo Clin. Proc. 2014, 89, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.C.; Jiang, M.Y.; Lu, Y.H.; Wang, C.T. Precedent fluctuation of serum hs-CRP to albumin ratios and mortality risk of clinically stable hemodialysis patients. PLoS ONE 2015, 10, e0120266. [Google Scholar] [CrossRef] [PubMed]
- Jaroszyński, A.; Jaroszyńska, A.; Zaborowski, T.; Drelich-Zbroja, A.; Zapolski, T.; Dąbrowski, W. Serum heat shock protein 27 levels predict cardiac mortality in hemodialysis patients. BMC Nephrol. 2018, 19, 359. [Google Scholar] [CrossRef]
- Krzanowski, M.; Krzanowska, K.; Gajda, M.; Dumnicka, P.; Dziewierz, A.; Woziwodzka, K.; Litwin, J.A.; Sułowicz, W. Pentraxin 3 as a new indicator of cardiovascular-related death in patients with advanced chronic kidney disease. Pol. Arch. Intern. Med. 2017, 127, 170–177. [Google Scholar] [CrossRef]
- Liu, S.; Qiu, P.; Luo, L.; Jiang, L.; Chen, Y.; Yan, C.; Zhan, X. Serum C-reactive protein to albumin ratio and mortality associated with peritoneal dialysis. Ren. Fail. 2020, 42, 600–606. [Google Scholar] [CrossRef]
- Mavrakanas, T.A.; Sniderman, A.D.; Barré, P.E.; Alam, A. Serial versus single troponin measurements for the prediction of cardiovascular events and mortality in stable chronic haemodialysis patients. Nephrology 2018, 23, 69–74. [Google Scholar] [CrossRef]
- Neuen, B.L.; Leather, N.; Greenwood, A.M.; Gunnarsson, R.; Cho, Y.; Mantha, M.L. Neutrophil-lymphocyte ratio predicts cardiovascular and all-cause mortality in hemodialysis patients. Ren. Fail. 2016, 38, 70–76. [Google Scholar] [CrossRef]
- Rusu, C.C.; Racasan, S.; Kacso, I.M.; Ghervan, L.; Moldovan, D.; Potra, A.; Patiu, I.M.; Bondor, C.; Caprioara, M.G. The association of high sCD163/sTWEAK ratio with cardiovascular disease in hemodialysis patients. Int. Urol. Nephrol. 2015, 47, 2023–2030. [Google Scholar] [CrossRef]
- Wlazeł, R.N.; Szadkowska, I.; Bartnicki, P.; Rośniak-Bąk, K.; Rysz, J. Clinical and prognostic usefulness of soluble urokinase plasminogen activator receptor in hemodialysis patients. Int. Urol. Nephrol. 2018, 50, 339–345. [Google Scholar] [CrossRef]
- Yang, F.J.; Hsieh, C.Y.; Shu, K.H.; Chen, I.Y.; Pan, S.Y.; Chuang, Y.F.; Chiu, Y.L.; Yang, W.S. Plasma Leucine-Rich α-2-Glycoprotein 1 Predicts Cardiovascular Disease Risk in End-Stage Renal Disease. Sci. Rep. 2020, 10, 5988. [Google Scholar] [CrossRef]
| First Author Country Year | Study Design | Dialysis Modality | Participants (n) | Biomarker | Biomarker Threshold | Outcome Measure | Number with Outcome | Hazard Ratio or Odds Ratio with [95% Confidence Interval] | |
|---|---|---|---|---|---|---|---|---|---|
| Alam et al. [31] Canada 2013 | Single Centre Prospective | HD a | 133 | Troponin I | 0.06 μg/L | All-cause mortality | 23 | 2.83 [1.49–5.37] | |
| Cardiac specific mortality (AMI b, CCF c, fatal arrhythmia) | 15 | 4.04 [1.46–11.2] | |||||||
| Hayashi et al. [43] Japan 2017 | Single Centre Prospective | HD PD d | 248 220 HD 28 PD | Troponin T | 0.01 ng/mL | All-cause mortality | 51 | 1.47 [1.15–1.88] | |
| Cardiac specific mortality (Not specified) | 10 | 1.479 [0.93–2.36] | |||||||
| Kawagoe et al. [33] Japan 2018 | Multi-centre Prospective | HD | 1310 | NTproBNP | Continuous pg/mL | All-cause mortality | 144 | 4.62 [3.48–6.14] | |
| Cardiac specific mortality (ischemic or haemorrhagic stroke, AMI, CCF, or rupture of an aortic aneurysm) | 54 | 4.95 [3.11–7.89] | |||||||
| Schwermer et al. [39] Poland 2015 | Multi-centre Prospective | HD | 321 | NT-proBNP | Continuous pg/mL | All-cause mortality | 97 | 1.41 [1.17–1.70] | |
| Dozio et al. [44] Italy 2018 | Single–centre Prospective | HD PD | 123 56 HD 67 PD | sRAGE e | Continuous pg/mL | All-cause mortality | 23 | 1.04 [1.01–1.08] (ODDS RATIO) | |
| Jung et al. [32] Korea 2017 | Single- centre Prospective | HD | 199 | sRAGEs | Continuous ng/mL | All-cause mortality | 27 | 1.074 [0.59–1.97] | |
| Kruzan et al. [35] USA 2016 | Multi-centre Prospective SECONDARY DATA | HD | 503 | NTproBNP | (ng/mL) Continuous and tertiles | Sudden cardiac death (out-of-hospital deaths) | 75 | 1.33 [1.21–1.46] | |
| 59–1710 | Reference | ||||||||
| 1728–7269 | 1.99 [1.25–3.14] | ||||||||
| 7350–273,502 | 4.49 [2.61–7.71] | ||||||||
| Troponin I | (pg/mL) Continuous and tertiles | 1.19 [1.06–1.32] | |||||||
| <0.0015 | Reference | ||||||||
| 0.015–0.039 | 1.82 [1.06–3.10] | ||||||||
| 0.040–3.09 | 2.14 [1.46–3.13] | ||||||||
| Otsuka et al. [38] Japan 2019 | Single centre Prospective | HD | 104 | hs Troponin T | Continuous (ng/Ml) | MACE (all-cause death, AMI requiring coronary revascularisation, and stroke) | 51 | 3.12 [1.79–5.44] | |
| BNP | Continuous (pg/mL) | 1.90 [1.09–3.32] | |||||||
| Shafi et al. [40] USA 2014 | Multi-centre Prospective | HD | 446 | Troponin I | ≥0.1 ng/mL | All-cause mortality | 323 | 1.75 [1.37–2.24] | |
| NT-proBNP | ≥9252 pg/mL | Cardiac specific mortality | 143 | 2.29 [1.55–3.38] | |||||
| (All outcome measured for one of the biomarkers above threshold and not demonstrated separately) | First CVE | 271 | 1.67 [1.32–2.10] | ||||||
| Sun et al. [41] China 2021 | Single centre Prospective | HD | 180 | hs-Troponin T | 14 pg/mL | All-cause mortality | 37 | hs-Troponin T | BNP |
| 3.32 [1.93–5.71] | 2.24 [1.47–3.43] | ||||||||
| BNP | Continuous pg/mL | First fatal or non-fatal CVE | 84 | 3.02 [2.11–4.31] | 2.36 [1.83–3.04] | ||||
| MACE (non-fatal AMI; non-fatal CVA h; CCF; Cardiac specific mortality | 78 | 3.37 [2.32–4.89] | 2.22 [1.43–3.34] | ||||||
| Voroneanu et al. [42] Romania 2018 | Multi-centre Prospective | HD | 173 | Galectin 3 (Gal–3) | median levels | MACE (Death and CV events (AMI; SCD i; non-fatal CVA) | 47 | ||
| 28.1 ng/mL | |||||||||
| NT-proBNP | 4234 pg/mL | low NT-proBNP–low Gal-3 | reference | ||||||
| low NT-proBNP–high Gal-3 | 2.1 [0.79–5.63] | ||||||||
| high NT-proBNP–low Gal-3 | 1.98 [0.73–5.35] | ||||||||
| high NT-proBNP–high Gal-3 | 3.65 [1.45–9.21] | ||||||||
| Liu et al. [36] China 2022 | Single centre Prospective | HD | 506 | Gal–3 | 8.65 ng/mL | All-cause mortality | 188 | 1.92 [1.17–3.17] | |
| Cardiac specific mortality | 125 | 2.47 [1.25–4.87] | |||||||
| Kim et al. [34] Korea 2021 | Single centre Prospective | HD | 296 | Gal–3 | Continuous ng/mL | All-cause mortality | 36 | Gal–3 | sST2 |
| 1.35 [0.93–1.97] | 1.81 [1.24–2.65] | ||||||||
| Serum-soluble suppression of tumorigenicity-2 (sST2) | MACE (Unstable angina pectoris, AMI, TIA j, CVA, and CCF) | 69 | 1.04 [0.82–1.33] | 1.100 [0.855–1.414] | |||||
| Obokata et al. [37] Japan 2016 | Single centre Prospective | HD | 423 | Gal–3 | Continuous ng/mL | All-cause mortality | 48 | 23.7 [6.45–86.9] | |
| <8.1 | Reference | ||||||||
| 8.1–15.2 | 2.89 [1.04–8.02] | ||||||||
| >15.2 | 6.51 [2.52–16.8] | ||||||||
| sST2 | Continuous ng/mL | 10.6 [4.98–22.5] | |||||||
| <0.237 | Reference | ||||||||
| 0.237–0.299 | 1.12 [0.43–2.91] | ||||||||
| >0.299 | 4.15 [1.91–9.03] | ||||||||
| NTproBNP | Continuous pg/mL | 3.85 [2.22–6.68] | |||||||
| <2440 | Reference | ||||||||
| 2440–8220 | 1.55 [0.60–4] | ||||||||
| >8220 | 4.7 [2.07–10.7] | ||||||||
| Gal–3 | Continuous ng/mL | Composite-all cause death and MACE (non-fatal AMI; CCF hospitalisation; non-fatal CVA) | 78 | 50.1 [16.7–151] | |||||
| <8.1 | Reference | ||||||||
| 8.1–15.2 | 2.13 [0.96–4.73] | ||||||||
| >15.2 | 7.06 [3.47–14.4] | ||||||||
| sST2 | Continuous ng/mL | 8.87 [4.73–16.6] | |||||||
| <0.237 | Reference | ||||||||
| 0.237–0.299 | 0.93 [0.46–1.88] | ||||||||
| >0.299 | 3.21 [1.82–5.66] | ||||||||
| NTproBNP | Continuous pg/mL | 3.31 [2.02–4.83 | |||||||
| <2440 | Reference | ||||||||
| 2440–8220 | 0.91 [0.47–1.77] | ||||||||
| >8220 | 2.71 [1.57–4.71] | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davies, E.M.; Ezenwekere, M.; Chetwynd, A.J.; Oni, L.; McDowell, G.; Rao, A. Biomarkers Predicting Major Adverse Cardiovascular Events in End-Stage Kidney Disease: A Systematic Review. Kidney Dial. 2025, 5, 39. https://doi.org/10.3390/kidneydial5030039
Davies EM, Ezenwekere M, Chetwynd AJ, Oni L, McDowell G, Rao A. Biomarkers Predicting Major Adverse Cardiovascular Events in End-Stage Kidney Disease: A Systematic Review. Kidney and Dialysis. 2025; 5(3):39. https://doi.org/10.3390/kidneydial5030039
Chicago/Turabian StyleDavies, Elin Mitford, Morka Ezenwekere, Andrew J. Chetwynd, Louise Oni, Garry McDowell, and Anirudh Rao. 2025. "Biomarkers Predicting Major Adverse Cardiovascular Events in End-Stage Kidney Disease: A Systematic Review" Kidney and Dialysis 5, no. 3: 39. https://doi.org/10.3390/kidneydial5030039
APA StyleDavies, E. M., Ezenwekere, M., Chetwynd, A. J., Oni, L., McDowell, G., & Rao, A. (2025). Biomarkers Predicting Major Adverse Cardiovascular Events in End-Stage Kidney Disease: A Systematic Review. Kidney and Dialysis, 5(3), 39. https://doi.org/10.3390/kidneydial5030039








